2. 海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
3. 中国农业科学院研究生院 北京 100081
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao 266071, China;
3. Graduate school of Chinese Academy of Agricultural Sciences, Beijing 100081, China
凡纳滨对虾(Litopenaeus vannamei)具有营养丰富、养殖周期短、盐度适应范围广和抗病性强等特点(冯旭等, 2023),已成为目前国内外主要的对虾养殖品种。2021年我国凡纳滨对虾海水养殖产量达127.36万t,占甲壳类海水养殖总产量的68.66% (农业农村部渔业渔政管理局等, 2022)。近年来,在山东地区出现新兴的“渔盐一体化”养殖模式,将凡纳滨对虾养殖与盐业生产相结合,海水经养虾后再蒸发晒盐。这种“一水多用”的养殖模式整个生产过程实现了废水零排放,有效利用了海水资源(刘振鲁等, 2022)。通过控制养殖密度和投喂鲜活动物饵料等措施,高盐水体养殖的凡纳滨对虾肉质紧实,味道鲜美。但该养殖模式目前生产工艺滞后、饵料利用率低、水体环境不稳定等导致的高死亡率问题制约了产业可持续发展。因此,亟待了解养殖水环境的变化特性及其与养殖生产活动的关系,为大水面凡纳滨对虾高盐养殖提供技术支持。
浮游植物在水产养殖生态系统中具有不可替代的生态功能,除了作为养殖系统的生产者,同时也是对虾养殖前期的直接饵料和中后期的间接饵料,为浮游动物、无脊椎动物和对虾提供食物(Zebek et al, 2017)。浮游植物会对水体环境的变化做出迅速反应,因此可作为评价水体营养健康状况的生物学指标(阎喜武等, 1997; Bosak et al, 2012; 柴然等, 2020)。De la Rey等(2004)研究表明,当浮游植物的种类、数量和组成结构发生改变时,对水生态系统中其他生物也会产生影响,甚至可影响到整个生态系统的平衡。不同粒级的浮游植物因被摄食压力和生理功能的不同,对池塘食物链具有不同的生态学意义(Brewin et al, 2019)。目前,国内外对Chl-a的粒径结构和影响因素的研究多集中于海域(吴文广等, 2015; 孙越峰等, 2020; Delgadillo-Hinojosa et al, 2020; Ağirbas et al, 2022; Wei et al, 2022),而对大水面养殖水体中浮游植物Chl-a的变化特征报道较少(Montecino et al, 2000; Iriarte et al, 2004)。由于池塘水体面积相对较小,缓冲能力弱,其水体理化性质会因降雨、光照、水温、进水和排水等因素在一天内变化剧烈,浮游植物生物量也会随之变化,因此,研究池塘水体中分级Chl-a的日变化特性具有一定的意义。国内对加州鲈(Micropterus salmoides) (卫鹏等, 2022)、轮虫(Rotifer) (赵文等, 2004)、三疣梭子蟹(Portunus trituberculatus) (孙忠等, 2012)、刺参(Apostichopus japonicus)(张义伟等, 2009; 姜森颢等, 2014)和对虾(刘国才等, 2001)养殖池塘的分级Chl-a进行过报道,但对凡纳滨对虾高盐养殖水体分级Chl-a浓度的变化特性研究未见报道。
本研究选取滨州不同盐度的凡纳滨对虾大水面养殖池塘,在养殖的初期、中期和收获期测定水体的Chl-a浓度、粒径结构及其他生态环境因子,分析大水面对虾养殖水体中Chl-a的变化规律及其影响因素,以期为凡纳滨对虾大水面养殖的健康可持续发展提供参考。
1 材料与方法 1.1 研究池塘概况实验点位于山东省滨州市,取样时间为2021年5、6、7月,即从放苗到收获的一个养殖周期。选取6口养殖池塘,于每月的26—30日进行取样。池塘分为高盐组(39.89~62.29)和对照组(29.40~34.60),每组3个平行。2个盐度组在“渔盐一体化”养殖模式下自然形成。实验虾池面积约为20 hm2,养殖池塘为泥底,水深在0.5~1.5 m之间。5月1日开始投放虾苗,7月15日开始收获,放苗密度约为30万尾/hm2,虾苗体长在1.2 cm以上。养殖初期投喂卤虫(Artemia)作为对虾的开口饵料,凡纳滨对虾长到6~7 cm后,只投喂对虾配合饲料,投喂量为对虾体重的5%~6%。
1.2 采样及测定方法 1.2.1 Chl-a样品的采集与测定每次分别在08:00、12:00、15:00和17:00共4个时间点按五点取样法进行取样。使用有机玻璃采水器采集上层0.5 m海水样品各1 L,充分混匀,经100目的筛网过滤去除大型浮游生物。按照Cermeño等(2006)的方法,取500 mL采集并处理过的水样,经20 μm筛绢过滤,分离大于20 μm的小型浮游植物(micro Chl-a),再经过2 μm的玻璃纤维滤膜,分离2~20 μm的微型浮游植物(nano Chl-a),最后,通过0.45 μm的玻璃纤维滤膜,截留0.45~2 μm的微微型浮游植物(pico Chl-a)。滤膜置于–20 ℃冷冻保存,带回实验室后使用美国Turner-Designs Trilogy荧光仪测定Chl-a浓度(Agawin et al, 2000)。总Chl-a浓度为3个粒级Chl-a浓度的和。同时采取水样,用于营养盐含量的测定。
1.2.2 理化环境因子的测定使用多参数水质分析仪(美国YSI 6600)测定池塘的水温、溶氧、盐度和pH。使用全自动营养盐分析仪(QuAAtro型,SEAL,德国)测定水体中的氮、磷、硅营养盐浓度。其中,硝酸盐(NO3–-N)和总氮(TN)采用锌镉还原法,亚硝酸盐(NO2–-N)采用盐酸萘乙二胺比色法,氨盐(NH4+-N)采用次溴酸钠氧化法、磷酸盐(PO43–-P)采用抗坏血酸还原磷钼蓝法,总磷(TP)采用钼酸铵分光光度法,硅酸盐(SiO32–-Si)采用硅钼黄法。溶解无机氮(DIN)浓度为NO3–-N、NO2–-N和NH4+-N浓度之和,溶解有机氮(DON)浓度为TN与DIN的差值。溶解有机磷(DOP)浓度为TP与PO43–-P的差值。
1.3 数据处理与统计分析实验数据采用平均值±标准差(Mean±SD)表示,符合正态分布后采用R语言进行单因素方差分析(one-way ANOVA)和皮尔逊相关性分析,设置显著性水平为P<0.05。使用Canoco5软件进行冗余分析(redundancy analysis, RDA)。
2 结果与分析 2.1 池塘环境因子的变化特性高盐组和对照组水体环境指标变化见图 1。高盐组和对照组的水温无显著性差异(P>0.05)。高盐组5月和6月的溶解氧浓度均显著低于对照组(P<0.05),6月溶解氧出现最低值,其平均浓度仅为(4.77±0.11) mg/L。高盐组的硅酸盐、硝酸盐、亚硝酸盐及DIN浓度均显著低于对照组(P<0.05)。活性磷酸盐浓度的峰值都出现在5月,高盐组浓度逐渐降低,在7月浓度为(0.34±0.04) μmol/L,而对照组6月最低。2个盐度组的氨盐都呈倒V型变化,6月份浓度最高。高盐组的DON浓度3个月均显著高于对照组(P<0.05),高盐组5月和6月DOP浓度显著高于对照组(P<0.05),7月2个盐度组无显著性差异(P>0.05)。
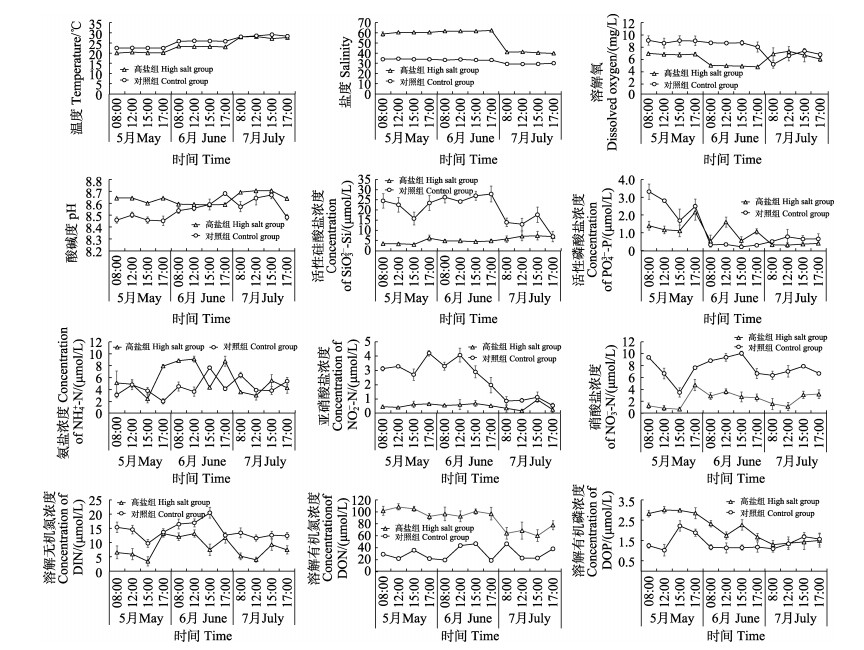
|
图 1 养殖水体环境因子的变化 Fig.1 Variation of pond environmental factors |
温度、盐度和pH的日变化较小,无显著性差异(P>0.05)。对照组的活性硅酸盐浓度3个月的日变化均较显著(P<0.05),而高盐组波动较平稳,无显著性差异(P<0.05)。5月活性磷酸盐浓度日变化显著(P<0.05),6月只有高盐组差异显著(P<0.05)。两组3个月的氨盐和硝酸盐浓度均具有显著日变化(P<0.05)。对照组的亚硝酸盐浓度日变化差异显著(P<0.05),高盐组仅在7月波动较大,存在显著性差异(P<0.05)。
2.2 Chl-a浓度的变化池塘总Chl-a浓度和分级叶绿素的日变化和月变化情况见图 2。高盐池塘3个月的总Chl-a浓度分别为11.11、7.13和35.60 μg/L,对照组的总Chl-a浓度分别为5.79、5.28和45.01 μg/L。2个盐度组总Chl-a浓度在7月存在显著性差异(P<0.05)。
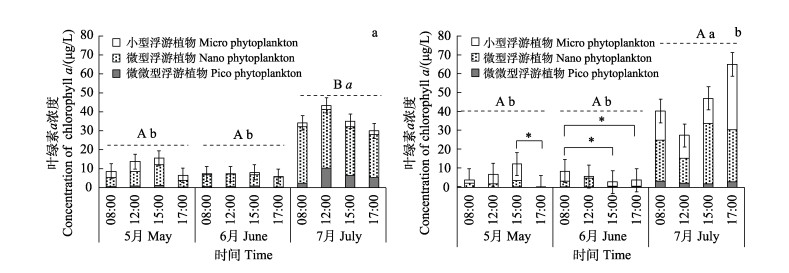
|
图 2 实验池塘中Chl-a浓度的月份变化 Fig.2 Monthly variation of chlorophyll-a concentration in experimental ponds a:高盐组;b:对照组;*表示日变化差异显著,不同大写字母表示不同盐度间差异显著,不同小写字母表示不同月份间差异显著(P<0.05)。 a: High salt group; b: Control group; * indicates significant diurnal variation, different capital letters indicate significant differences between salinities, and different lowercase letters indicate significant differences between months (P < 0.05). |
Chl-a浓度的日变化情况:高盐组总Chl-a浓度无显著日变化(P>0.05),而对照组的总Chl-a浓度在5月和6月均存在显著的日变化(P<0.05),5月的最高值出现在15:00点,6月的高值出现在08:00。对于分级叶绿素,在7月,高盐组的pico Chl-a浓度存在显著的日变化(P<0.05),高值出现在12:00;对照组的micro Chl-a浓度在5、6、7月均存在显著的日变化(P<0.05),6月对照组nano Chl-a浓度的日变化显著(P<0.05)。
Chl-a浓度的月变化情况:2个盐度组的总Chl-a浓度最低值和最高值都分别出现在6月和7月,且7月的总Chl-a浓度显著高于5月和6月(P<0.05)。对于分级叶绿素,高盐组的pico Chl-a和nano Chl-a浓度7月显著高于5月和6月(P<0.05),且5月和6月之间无显著性差异(P>0.05);对照组7月的pico Chl-a、nano Chl-a和micro Chl-a浓度均显著高于5月和6月(P<0.05),且5月和6月之间无显著性差异(P>0.05)。
2.3 分级Chl-a贡献率高盐组和对照组池塘浮游植物不同粒径的贡献率如图 3所示。高盐组micro Chl-a、nano Chl-a和pico Chl-a对总Chl-a的贡献率分别为(15.64±0.16)%、(73.81±0.13)%和(10.55±0.06)%,5、6、7月均以nano Chl-a占主要优势,7月份pico Chl-a贡献率由5月的6.43%提高至7月的16.81%,并超过micro Chl-a的贡献率。
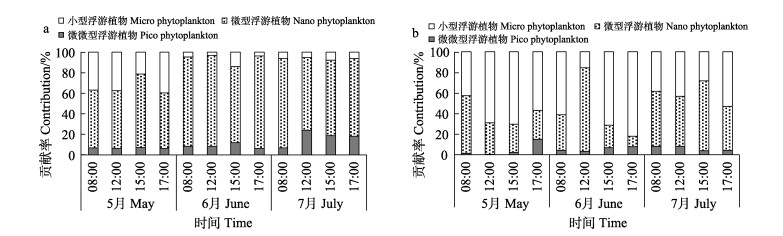
|
图 3 实验池塘不同粒级Chl-a的贡献率 Fig.3 The contribution of size-fractionated Chl-a of pico, nano and micro to total Chl-a in experimental pond a:高盐组;b:对照组 a: High salt group; b: Control group |
对照组micro Chl-a、nano Chl-a和pico Chl-a对总Chl-a的贡献率分别为(52.29±0.10)%、(41.82± 0.10)%和(5.59±0.01)%。5月和6月micro Chl-a占主要优势,分别占59.64%和57.49%,其次是nano Chl-a,分别占35.46%和36.90%,7月nano Chl-a占主要优势,贡献率达53.09%。
2.4 Chl-a浓度变化与环境因子相关性分析 2.4.1 Chl-a浓度日变化与环境因子的关系将环境因子(包括SiO32–-Si、PO43–-P、NH4+-N、NO3–-N、NO2–-N、水温、盐度、DON和DOP)与总Chl-a浓度和分级Chl-a浓度进行皮尔逊相关性分析。结果显示,对于高盐池塘,5月和6月,Chl-a浓度日变化与这9个环境因子均无显著性关系,仅在养殖的末期(7月),nano Chl-a和总Chl-a浓度与NO3–-N浓度显著负相关,micro Chl-a和总Chl-a浓度与SiO32–-Si显著正相关(P<0.05)(表 1)。对照组,5月nano Chl-a与水温显著正相关,总Chl-a浓度和PO43–-P显著负相关;7月份pico Chl-a与NO3–-N浓度显著负相关(P<0.05)(表 2)。
|
|
表 1 高盐组Chl-a浓度与环境因子的相关系数 Tab.1 Correlation coefficient between chlorophyll-a concentration and environmental factors in high salt group |
|
|
表 2 对照组Chl-a浓度与环境因子的相关系数 Tab.2 Correlation coefficient between chlorophyll-a concentration and environmental factors in control group |
变量,9个环境因子(包括SiO32–-Si、PO43–-P、NH4+-N、NO3–-N、NO2–-N、水温、盐度、DON和DOP)作为解释变量进行RDA分析。当夹角为锐角时为正相关,当夹角大于90°时为负相关,夹角越小,相关性越强。RDA排序图显示(图 4),高盐组的pico Chl-a、nano Chl-a、总Chl-a与SiO32–-Si、水温显著正相关,与PO43–-P、盐度、DON、DOP显著负相关,micro Chl-a与NO3–-N显著负相关。对照组所有粒径浮游植物Chl-a浓度与水温、DON显著正相关,与SiO32–-Si、NO2–-N、盐度显著负相关。
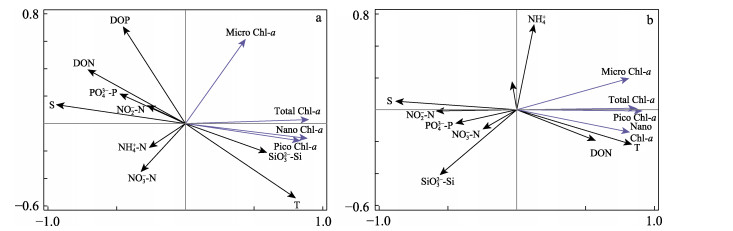
|
图 4 Chl-a浓度与环境因子RDA排序图
Fig.4 RDA ordination of chlorophyll-a and environmental factors
a:高盐组;b:对照组 S:盐度;T:温度;NO2–-N:亚硝酸盐;NO3–-N:硝酸盐;PO43–-P:活性磷酸盐;SiO32–-Si:活性硅酸盐;NH4+-N:氨盐;DOP:溶解有机磷;DON:溶解有机氮。 a: High salt group; b: Control group S: Salinity; T: Temperature; NO2–-N: Nitrite; NO3–-N: Nitrate; PO43–-P: Active phosphate; SiO32–-Si: Active silicate; NH4+-N: Ammonia nitrogen nutrient; DOP: Dissolved organic phosphorus; DON: Dissolved organic nitrogen. |
浮游植物的生长和繁殖通常受温度、营养盐和光照等环境因子的影响,另外,还受次级生长者(如浮游动物、滤食性贝类等)的摄食调控(Liu et al, 2003; Popovich et al, 2008)。在一定温度范围内,通常随着水温的升高,浮游植物的光合作用增强,生长速度加快。本研究发现,2种类型的水体,不论是总Chl-a浓度还是各分级Chl-a浓度(高盐组micro Chl-a除外),都与水温显著正相关(见图 4)。可见,水温是池塘中浮游植物生长的关键控制因子。
营养盐是浮游植物生长所必须的基础物质。浮游植物的生长不仅受营养盐绝对浓度的影响,而且,受不同营养盐比值即相对浓度的影响。虽然水体营养盐浓度较高,处于富营养化状态,但是从N、P、Si的比值来看,存在营养盐相对限制的情况。根据营养盐相对限制法计算N、P、Si之间的比例关系,若Si/P>22和N/P>22,则PO43–-P为浮游植物生长限制因子,若N/P<10和Si/N>1,则为DIN为限制因子,若Si/P<10和Si/N<1,则Si为限制因子(Justic et al, 1995)。据此来评估,5月和6月高盐组的浮游植物生长属于Si相对限制状态。冗余分析结果也显示,高盐组的pico Chl-a、nano Chl-a和总Chl-a浓度与SiO32–-Si呈显著正相关,这说明高盐组水体浮游植物生长受SiO32–-Si的限制。而对照组从养殖开始至收获,SiO32–-Si一直处于较高的浓度(12.95~26.47 μmol/L),显著高于高盐组(见图 1)。SiO32–-Si不是对照组浮游植物生长的限制因子,对照组水体浮游植物生长受DON含量的影响较大(Chl-a浓度与DON含量显著正相关,见图 4b)。是否是由于高盐限制了沉积物中SiO32–-Si的溶出释放导致了高盐组与对照组的SiO32–-Si含量显著不同,还有待进一步的研究。
本研究发现,2种类型水体Chl-a的粒径结构是不同的,在养殖初期(5月),高盐组以nano Chl-a为主[占(73.81±0.13)%],对照组以micro Chl-a为主[占(52.29±0.10)%],水体浮游植物粒级结构的变化可能与水体中NH4+-N含量的差异有关。高盐组因养殖前的肥水(投入有机肥),使得DON浓度显著高于对照
组。张建乐等(2020)研究显示,在DON含量较高的水体中,对有机氮营养物质利用能力强的微型及微微型浮游植物更有可能成为优势种,从而导致浮游植物群落结构发生变化,水体中的小型浮游植物向微型和微微型浮游植物演替。据报道,DON能直接被浮游植物利用12%~72% (Stepanauskas et al, 1999)。有机氮(如尿素)往往能刺激微型浮游植物的暴发,如近些年我国海域微小原甲藻(Prorocentrum minimum)和抑食金球藻(Aureococcus anophagefferens)赤潮与DON浓度过高有关(Mulholland et al, 2009; Gobler et al, 2012)。其次,SiO32–-Si是硅藻类浮游植物必须的营养元素。本研究镜检结果显示,小型浮游植物中硅藻占主导地位(待发表数据)。在5月和6月高盐组都出现SiO32–-Si限制,这将影响粒径大于20 μm的硅藻的生长。另外,高盐组浮游植物粒径小于对照组,也可能与投喂的卤虫摄食压力有关。通常卤虫在高盐水体中的摄食行为更为活跃(Sura et al, 2017)。虽然高盐组和对照组水体浮游植物的粒径结构不同,但本研究发现,随着养殖的进行(5—7月),2个实验组的浮游植物都有小型化的趋势(见图 2),这种变化特性可能与水温升高促进微型和微微型浮游植物生长有关。Mousing等(2014)研究发现,小粒径的浮游植物细胞在群落中的重要性随着温度的增加而增加,较大细胞的贡献率随温度升高而降低,产生这种现象与养分独立效应和养分共享效应有关(Moran et al, 2010)。夏滨等(2001)研究发现,冷水中较大粒级的浮游植物占比较高。也有研究表明,随着温度的升高,群落结构向较小细胞占主要优势转变(Sin et al, 2000; Coello-Camba et al, 2014)。
高盐组和对照组的产量分别为870和675 kg/hm2,产值分别为34 950和27 000元/hm2。从Chl-a浓度和粒径结构来看,高盐组的日变化较小(总Chl-a、micro Chl-a、nano Chl-a浓度在5—7月的3次调查中均无显著的日变化)(表 1),即高盐水体浮游植物相对稳定,有利于凡纳滨对虾的存活和生长。
浮游植物对水质调控具有重要作用,能够调节池塘水体透明度、酸碱度、溶解氧和营养盐水平(乔玲等, 2022)。细胞的粒径大小也是影响藻类新陈代谢的关键因素,有研究表明,不同细胞大小的藻类生长速率和对营养物质的吸收效率都不同,微型浮游植物在大多数水域中占主要优势是因为中等细胞大小的藻类代谢率最快(李胜男等, 2020)。所以,浮游植物群落粒级结构的变化在一定程度上也反映了水质营养的变化状况,可作为调节水环境的重要指示参数。
4 结论综上所述,高盐组相较于对照组Chl-a浓度日变化较小,水体中浮游植物生物量相对稳定。且随着养殖进行,高盐组5、6、7月nano Chl-a占主要优势,对照组5月和6月micro Chl-a为主,7月nano Chl-a占主要优势。高盐组和对照组浮游植物粒级结构都具有小型化趋势,这一现象可能与较高的温度和DON浓度有关。
AGAWIN N S R, DUARTE C M, AGUSTÍ S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnology and Oceanography, 2000, 45(3): 591-600 DOI:10.4319/lo.2000.45.3.0591 |
AĞIRBAS E, BAKIRCI M. Size-fractionated primary production in the south-eastern Black Sea. Oceanologia, 2022, 64(2): 244-266 DOI:10.1016/j.oceano.2021.11.002 |
BOSAK S, ŠILOVIĆ T, LJUBEŠIĆ Z, et al. Phytoplankton size structure and species composition as an indicator of trophic status in transitional ecosystems: The case study of a Mediterranean fjord-like karstic bay. Oceanologia, 2012, 54(2): 255-286 DOI:10.5697/oc.54-2.255 |
BREWIN R J W, MORAN X A G, RAITSOS D E, et al. Factors regulating the relationship between total and size-fractionated chlorophyll-a in coastal waters of the Red Sea. Frontiers in Microbiology, 2019, 10: 1964 DOI:10.3389/fmicb.2019.01964 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2022. Beijing: China Agriculture Press, 2022 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2022中国渔业统计年鉴. 北京: 中国农业出版社, 2022]
|
CERMEÑO P, MARAÑÓN E, PÉREZ V, et al. Phytoplankton size structure and primary production in a highly dynamic coastal ecosystem (Ría de Vigo, NW-Spain): Seasonal and short-time scale variability. Estuarine, Coastal and Shelf Science, 2006, 67(1/2): 251-266 |
CHAI R, FENG J, CHEN B J, et al. Seasonal variations in the phytoplankton community structure and their environmental impact factors in the offshore area of Laoshan, Qingdao. Progress in Fishery Sciences, 2020, 41(1): 21-30 [柴然, 冯娟, 陈碧鹃, 等. 崂山近岸浮游植物群落结构季节变化及其环境影响因素. 渔业科学进展, 2020, 41(1): 21-30] |
COELLO-CAMBA A, AGUSTÍ S, HOLDING J, et al. Interactive effect of temperature and CO2 increase in Arctic phytoplankton. Frontiers in Marine Science, 2014, 1: 49 |
DE LA REY P A, TAYLOR J C, LAAS A, et al. Determining the possible application value of diatoms as indicators of general water quality: A comparison with SASS 5. Water SA, 2004, 30(3): 325-332 |
DELGADILLO-HINOJOSA F, FÉLIX-BERMÚDEZ A, TORRES-DELGADO E V, et al. Impacts of the 2014–2015 warm-water anomalies on nutrients, chlorophyll-a and hydrographic conditions in the coastal zone of Northern Baja California. Journal of Geophysical Research-Oceans, 2020, 125(12): e2020JC016473 DOI:10.1029/2020JC016473 |
FENG X, WU W G, LIU Y, et al. Effect of temperature on individual energy budget of Litopenaeus vannamei families. Progress in Fishery Sciences, 2023, 44(3): 133-143 [冯旭, 吴文广, 刘毅, 等. 温度对不同家系凡纳滨对虾个体能量收支的影响. 渔业科学进展, 2023, 44(3): 133-143] |
GOBLER C J, SUNDA W G. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae, 2012, 14: 36-45 DOI:10.1016/j.hal.2011.10.013 |
IRIARTE J L, GONZÁLEZ H E. Phytoplankton size structure during and after the 1997/98 El Niño in a coastal upwelling area of the northern Humboldt Current System. Marine Ecology Progress Series, 2004, 269: 83-90 DOI:10.3354/meps269083 |
JIANG S H, ZHOU Y B, TANG B P, et al. Annual variations of the primary productivity and its size-fractioned structure in culture ponds of Apostichopus japonicus Selenka. Acta Ecologica Sinica, 2014, 34(7): 1698-1706 [姜森颢, 周一兵, 唐伯平, 等. 刺参养殖池塘初级生产力及其粒级结构周年变化. 生态学报, 2014, 34(7): 1698-1706] |
JUSTIC D, RABALAIS N N, TURNER R E, et al. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuarine, Coastal and Shelf Science, 1995, 40(3): 339-356 DOI:10.1016/S0272-7714(05)80014-9 |
LI S N, XIONG L P, PENG H, et al. Size-structure of phytoplankton biomass and driving factors in east Lake Dongting. Journal of Lake Sciences, 2020, 32(5): 1508-1518 [李胜男, 熊丽萍, 彭华, 等. 东洞庭湖浮游藻类粒级结构组成及其关键影响因子. 湖泊科学, 2020, 32(5): 1508-1518] |
LIU G C, LI D S, DONG S L. Size-fractionated plankton respiration and phytoplankton production rates in shrimp culture ecosystems. Acta Oceanologica Sinica, 2001, 23(6): 101-107 [刘国才, 李德尚, 董双林. 对虾养殖生态系不同粒级浮游生物呼吸率和初级生产率测定. 海洋学报, 2001, 23(6): 101-107] |
LIU H B, DAGG M. Interactions between nutrients, phytoplankton growth, and micro-and mesozooplankton grazing in the plume of the Mississippi River. Marine Ecology Progress Series, 2003, 258: 31-42 DOI:10.3354/meps258031 |
LIU Z L, GAO Z M, ZHAO Y J, et al. Explore the path of green and efficient development of Penaeus shrimp industry in Binzhou. China Fisheries, 2022(6): 69-71 [刘振鲁, 高兆明, 赵玉静, 等. 探索滨州对虾产业绿色高效发展的路径. 中国水产, 2022(6): 69-71] |
MONTECINO V, QUIROZ D. Specific primary production and phytoplankton cell size structure in an upwelling area off the coast of Chile (30°S). Aquatic Sciences, 2000, 62(4): 364-380 DOI:10.1007/PL00001341 |
MORAN X A G, LÓPEZ-URRUTIA Á, CALVO-DÍAZ A, et al. Increasing importance of small phytoplankton in a warmer ocean. Global Change Biology, 2010, 16(3): 1137-1144 DOI:10.1111/j.1365-2486.2009.01960.x |
MOUSING E A, ELLEGAARD M, RICHARDSON K. Global patterns in phytoplankton community size structure-evidence for a direct temperature effect. Marine Ecology Progress Series, 2014, 497: 25-38 DOI:10.3354/meps10583 |
MULHOLLAND M R, BONEILLO G E, BERNHARDT P W, et al. Comparison of nutrient and microbial dynamics over a seasonal cycle in a mid-Atlantic coastal lagoon prone to Aureococcus anophagefferens (brown tide) blooms. Estuaries and Coasts, 2009, 32(6): 1176-1194 DOI:10.1007/s12237-009-9218-0 |
POPOVICH C A, MARCOVECCHIO J E. Spatial and temporal variability of phytoplankton and environmental factors in a temperate estuary of South America (Atlantic coast, Argentina). Continental Shelf Research, 2008, 28(2): 236-244 DOI:10.1016/j.csr.2007.08.001 |
QIAO L, CHANG Z Q, LI J, et al. Comparison of phytoplankton community diversity in the ecological aquaculture system of a marine pond using morphological analysis and high-throughput sequencing. Progress in Fishery Sciences, 2022, 43(2): 32-43 [乔玲, 常志强, 李健, 等. 基于形态学和高通量测序的海水池塘生态养殖系统中浮游植物多样性比较. 渔业科学进展, 2022, 43(2): 32-43] |
SIN Y, WETZEL R L, ANDERSON I C. Seasonal variations of size-fractionated phytoplankton along the salinity gradient in the York River estuary, Virginia (USA). Journal of Plankton Research, 2000, 22(10): 1945-1960 DOI:10.1093/plankt/22.10.1945 |
STEPANAUSKAS R, EDLING H, TRANVIK L J. Differential dissolved organic nitrogen availability and bacterial aminopeptidase activity in limnic and marine waters. Microbial Ecology, 1999, 38(3): 264-272 DOI:10.1007/s002489900176 |
SUN Y F, QIN Y J, LI H B, et al. Spatial and temporal distribution characteristics and influencing factors of chlorophyll a in the Liaohe Estuary. Environmental Protection Science, 2020, 46(2): 44-48 [孙越峰, 秦艳杰, 李洪波, 等. 辽河口海域叶绿素a的时空分布特征及其影响因素. 环境保护科学, 2020, 46(2): 44-48] |
SUN Z, WANG Y B, LU J X. On the variation and size-fractionated chlorophyll a in ponds with Portunus trituberculatus cultured in different models. Marine Fisheries, 2012, 34(2): 177-182 [孙忠, 王跃斌, 陆建学. 三疣梭子蟹不同养殖模式池塘叶绿素a的变化特征及粒级结构. 海洋渔业, 2012, 34(2): 177-182] |
SURA S A, HERLIHY N S, MAHON H K, et al. Environmental impacts on grazing of different brine shrimp (Artemia franciscana) life stages. Hydrobiologia, 2017, 792(1): 97-104 DOI:10.1007/s10750-016-3047-5 |
WEI P, BI X D, DAI W, et al. Community structure composition of nanophytoplankton and picophytoplankton in freshwater culture ponds. Journal of Dalian Ocean University, 2022, 37(1): 113-119 [卫鹏, 毕相东, 戴伟, 等. 淡水养殖池塘微型和超微型浮游植物的群落结构组成. 大连海洋大学学报, 2022, 37(1): 113-119] |
WEI Y Q, CUI Z G, WANG X Z, et al. Comparative analysis of total and size-fractionated chlorophyll a in the Yellow Sea and Western Pacific. Frontiers in Microbiology, 2022, 13: 903159 DOI:10.3389/fmicb.2022.903159 |
WU W G, ZHANG J H, WANG W, et al. Variation of Chl. a concentration and its control factors pre-and-post-the-harvest of kelp in Sanggou Bay. Journal of Fisheries of China, 2015, 39(8): 1178-1186 [吴文广, 张继红, 王巍, 等. 桑沟湾水域叶绿素a在海带收获前后的变化及其影响因素. 水产学报, 2015, 39(8): 1178-1186] |
XIA B, LÜ R H, SUN P X. Spatial distibutions and size compositions of chlorophyll-a in the typical areas of the Yellow Sea and East China Sea in the autumn of 2000. Journal of Oceanograpgy of Huanghai & Bohai Seas, 2001, 19(4): 37-42 [夏滨, 吕瑞华, 孙丕喜. 2000年秋季黄、东海典型海区叶绿素a的时空分布及其粒径组成特征. 黄渤海海洋, 2001, 19(4): 37-42] |
YAN X W, HE Z H. Studies on primary production of phytoplankton in shrimp ponds. Journal of Fisheries of China, 1997, 21(3): 288-295 [阎喜武, 何志辉. 虾池浮游植物初级生产力的研究. 水产学报, 1997, 21(3): 288-295 DOI:10.3321/j.issn:1000-0615.1997.03.011] |
ZEBEK E, SZYMANSKA U. Abundance, biomass and community structure of pond phytoplankton related to the catchment characteristics. Knowledge and Management of Aquatic Ecosystems, 2017, 418: 45 |
ZHANG J L, WANG Q Y, ZHANG Y F, et al. Characteristics of seawater nutrients during the occurrence of brown tide in the coastal area of Qinhuangdao. Chinese Journal of Applied Ecology, 2020, 31(1): 282-292 [张建乐, 王全颖, 张永丰, 等. 秦皇岛海域褐潮生消过程中营养盐特征. 应用生态学报, 2020, 31(1): 282-292] |
ZHANG Y W, ZHAO W, YU X H. Respiration and production of size-fractionated plankton in ponds with sea cucumber Apostichopus japonicus. Journal of Dalian Fisheries University, 2009, 24(Suppl): 103-106 [张义伟, 赵文, 于晓辉. 刺参养殖池塘不同粒级浮游生物呼吸率和初级生产率的研究. 大连水产学院学报, 2009, 24(Suppl): 103-106] |
ZHAO W, LI X D, XU J J. The contribution of size fractionated algae to biomass and primary production of phytoplankton in rotifer culturing ponds. Journal of Fisheries of China, 2004, 28(2): 167-174 [赵文, 李晓东, 徐纪军. 轮虫培育池不同粒级藻类对浮游植物生物量和生产量的贡献. 水产学报, 2004, 28(2): 167-174] |



