2. 大洋渔业资源可持续开发省部共建教育部重点实验室 上海 201306;
3. 国家远洋渔业工程技术研究中心 上海 201306;
4. 农业农村部大洋渔业开发重点实验室 上海 201306
2. Key Laboratory of Sustainable Exploitation of Oceanic Fishery Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China;
3. National Distant-Water Fisheries Engineering Research Center, Shanghai Ocean University, Shanghai 201306, China;
4. Key Laboratory of Oceanic Fisheries Exploration, Ministry of Agriculture and Rural Affairs, Shanghai 201306, China
头足类是高等级的海洋软体动物,广泛分布于世界各大洋和各海域(除波罗的海和黑海外)(陈新军等, 2019)。据估计,头足类分布在大陆架和大陆坡海域的资源蕴藏量为1 000万t,而大洋中分布的头足类资源蕴藏量则可能更高(陈新军, 2019),被认为是最具开发潜力的渔业资源种类之一,已成为全球渔业国家和地区的主要捕捞对象(FAO, 2022; 章贤成等, 2022)。同时,头足类在海洋生态系统中发挥着重要作用,既是贪婪的海洋捕食者,也是许多高营养级海洋动物的重要饵料生物,成为链接海洋食物网中低营养层级与高营养层级的重要媒介(Boyle et al, 2005; Xavier et al, 2015)。
阿根廷滑柔鱼(Illex argentinus)是典型的大洋性头足类,分布在西南大西洋22°~55°S的大陆架和大陆坡海域(Rodhouse et al, 2013)。阿根廷滑柔鱼具有与其他头足类相一致的生活史特性——生长快、寿命短(Schwarz et al, 2010; Lu et al, 2012; 林东明等, 2020),营间歇性终端产卵策略,即配子批次发育成熟、批次产卵,产卵结束后不久便死去(Rocha et al, 2001; Lin et al, 2017a)。通常,阿根廷滑柔鱼的生活史过程可划分为2个关键阶段,即个体生长阶段和生殖发育阶段(Rodhouse et al, 2013; Schwarz et al, 2013),2个阶段均表现出对能量需求大的特点(Rodhouse et al, 1996; 林东明等, 2017)。为了满足生长发育的较大能量需求,阿根廷滑柔鱼食性贪婪、食物种类繁多,并且具有较快的食物消化与吸收速率(Rodhouse et al, 1996、2013)。同时,阿根廷滑柔鱼是机会主义型捕食者,食物组成因栖息海域不同存在差异,在不同海域的食物网结构中,其营养生态位也会有所不同(Arkhipkin, 2013)。然而,该种类对环境波动变化敏感,组织能量积累与栖息海域环境密切相关(Lin et al, 2017b; 刘伟等, 2022);并且阿根廷滑柔鱼的生殖能量投入为收入–资本混合型,即生殖能量以现场摄食转化利用为主、肌肉存储能量投入为辅(Lin et al, 2015、2019; 林东明等, 2017)。能量积累及其对生殖发育的投入分配影响着生物个体自身的繁殖潜能和后代存活率,随之决定补充群体的大小(McBride et al, 2015; Karjalainen et al, 2016)。为系统掌握阿根廷滑柔鱼的能量积累策略,本文对其组织能量积累特点及积累过程中的食物组成,以及作为西南大西洋生态系统重要的能量传输者等进行扼要阐述。同时,对阿根廷滑柔鱼能量积累需求与洄游、摄食策略选择,以及在面对全球气候变化、渔业活动下作为海洋生物食物网能量传输者的角色进行了展望分析,以期为科学认识和评估该种类资源提供参考资料。
1 能量积累特点及食物组成 1.1 肌肉和性腺组织的能量积累特点在自然界中,生物个体在其能量积累变化过程中具有进化适应性特征(Vance, 1992; Johns et al, 2018)。研究发现,阿根廷滑柔鱼在生活史前期体型生长迅速,在性腺组织开始发育之后,体型生长速度减缓,生长发育重心从体型生长转向性腺组织发育成熟(Arkhipkin, 1993; Schwarz et al, 2010)。林东明等(2017)利用氧弹热量仪测定阿根廷滑柔鱼的胴体、足腕、性腺等组织的能量密度值并计算组织能量积累,发现阿根廷滑柔鱼的胴体与足腕等肌肉组织的能量积累在个体生长阶段(Ⅰ~Ⅲ期)迅速增大,随后趋于稳定,而性腺组织在未发育时的能量积累相对缓慢,随后增长迅速。Hatfield等(1992)和Rodhouse等(1992)对不同发育时期的胴体组织厚度分析的结果也印证了这一点,随着性腺发育的成熟,胴体的纤维组织厚度变薄,且胴体重量的增长速率与增长比例相较于性腺组织也呈下降趋势。茎柔鱼(Dosidicus gigas)和鸢乌贼(Sthenoteuthis oualaniensis)的肌肉和性腺组织能量积累也表现出相似的特点,即性腺发育前,肌肉组织能量积累迅速,性腺组织能量积累缓慢;性腺发育开始之后,性腺组织能量积累增加明显,而肌肉组织能量积累趋于稳定或呈下降趋势(韩飞等, 2020; 连晋欣等, 2022; 朱凯等, 2019、2020)。Pascual等(2020)分析玛雅蛸(Octopus maya)的能量存储时也发现,性腺和肌肉组织的脂肪、糖原和热量值等均与生长发育密切相关,肌肉组织的脂肪在性腺成熟前期积累最为丰富,性腺组织的脂肪、糖原等则在性腺功能性成熟期时达到最高值。此外,Sieiro等(2020)研究发现,真蛸(Octopus vulgaris)肌肉中的蛋白质含量在性腺发育期积累显著,在繁殖期则逐渐下降,性腺组织则以积累脂肪物质为主。可见肌肉和性腺组织能量积累因生长发育阶段不同而有所差别可能是头足类的共有特点,与其终生一次繁殖产卵的生活史密切相关。
阿根廷滑柔鱼性腺发育滞后于个体生长,性腺发育开始后,性腺组织能量积累迅速增大,所需的能量积累主要来源于对饵料生物摄食的吸收转化(Clarke et al, 1994; Lin et al, 2019)。但是,随着性腺发育阿根廷滑柔鱼摄食强度逐渐下降(Arkhipkin, 1993),在生殖能量需求较大的阶段转化部分肌肉组织的存储能量(Lin et al, 2015、2019; 林东明等, 2017)。阿根廷滑柔鱼的这种生殖投入与其他营间歇性终端产卵策略的头足类相似,如福氏枪乌贼(Loligo forbesi)随着性腺发育,肌肉组织的生长逐步停止,性腺组织生长逐步加强(Collins et al, 1995)。然而,这种生殖能量投入方式与采取其他产卵策略的头足类有较大差异。比如,茎柔鱼作为多次产卵者,其生殖投入来源于饵料摄食,并在性腺发育后持续进食以及继续体细胞的生长,在能量积累上逐渐增加对生殖的投入分配,但在繁殖产卵期间可以保持较好的肌肉组织状态(Nigmatullin et al, 2009; 韩飞等, 2019; 连晋欣等, 2022)。作为瞬时终端产卵者的强壮桑椹乌贼(Moroteuthis ingens),其生殖能量则主要来源于肌肉组织等存储能量的转化,随着性腺发育,强壮桑椹乌贼的肌肉组织逐渐萎缩衰败(Jackson et al, 2004)。
此外,性腺指数作为衡量性腺发育过程中生物个体的能量资源在性腺和肌肉组织之间的分配比例,可表征对性腺组织的能量投入水平(Chen et al, 2022; Patrick et al, 2022)。根据性腺指数数据,阿根廷滑柔鱼雌性成熟个体的性腺组织能量为体重的13%~32% (Santos et al, 1997b; Schwarz et al, 2013; 林东明等, 2014);雄性成熟个体的性腺组织能量较低,为体重的5%~13% (Perez et al, 2009; 林东明等, 2014)。头足类瞬时终端产卵者的性腺组织能量则为体重的35%~ 50% (Pecl, 2001; Jackson et al, 2004),多次产卵者的性腺组织能量多为体重的6%~12% (McGrath et al, 2002; Beasley et al, 2018),雄性个体的性腺组织能量积累则更低,为2%~7% (Salman et al, 2004; Laptikhovsky et al, 2014)。可见阿根廷滑柔鱼性腺组织的能量积累介于头足类瞬时终端产卵者和多次产卵者之间。最近基于组织能量密度技术计算的肌肉、消化腺和性腺等组织能量积累占比的结果也显示,阿根廷滑柔鱼雌性成熟个体的性腺组织能量积累占比为15%~30% (Song et al, 2023)。
1.2 能量积累过程中的食物组成生物个体的能量积累与其食物来源、食性转变等密切相关(Stephens et al, 2009)。一般认为,甲壳类作为饵料生物所提供的能量次于头足类(Ciancio et al, 2007),而头足类的能量价值又低于鱼类(Dessier et al, 2018; Schaafsma et al, 2018)。头足类作为机会主义型海洋捕食者,在能量积累过程中,更倾向于捕食能量高、体型适中的鱼类资源(Rodhouse et al, 1996)。相类似的,阿根廷滑柔鱼在能量积累过程中也具有食性转变,倾向于进食鱼类、头足类等高能量饵料生物(Ivanovic et al, 1994; Santos et al, 1997a; Crespi-Abril et al, 2011)。总体上,阿根廷滑柔鱼能量积累的食物组成存在海域间以及生长发育期和体型间的差异。
1.2.1 海域间的食物组成差异阿根廷滑柔鱼种群结构复杂,分布具有一定的海域特殊性,主要集中分布在5个海域(Rodhouse et al, 2013):26°~34°S的巴西南部海域,34°~40°S的布宜诺斯艾利斯海域,42°S的浅海水域圣马蒂亚斯湾,45°~46°S的福克兰群岛以及45~55°S的巴塔哥尼亚大陆架附近海域。类似于其他大洋性柔鱼类,阿根廷滑柔鱼食物组成包括鱼类、头足类和甲壳类(Rodhouse et al, 2013)。
在不同栖息海域,阿根廷滑柔鱼食物组成中的鱼类、头足类、甲壳类等的占比存在较大差异(表 1)。其中,在巴西南部海域,阿根廷滑柔鱼的食物组成以鱼类为主,在胃含物中的出现频率达43.8%,阿根廷无须鳕(Merluccius hubbsi)幼体、杜氏眶灯鱼(Diaphus dumerilii)等小型鱼类是主要的食物对象(Santos et al, 1997a)。头足类也是较为重要的食物来源,并且同类相食现象较多(Ivanovic et al, 1994; Santos et al, 1997a)。食物组成中的甲壳类则以磷虾类(Euphausia sp.)为主(Santos et al, 1997a)。类似地,在阿根廷沿海水域圣马蒂亚斯湾,阿根廷滑柔鱼的摄食种类也以鱼类为主要捕食对象,鱼类在其食物组成中高达79%,头足类和甲壳类的摄食比例较低(Crespi-Abril et al, 2011)。在圣马蒂亚斯湾,阿根廷滑柔鱼捕食的鱼类种类主要为尼氏裸灯鱼(Gymnoscopelus nicholsi)和阿根廷鳀鱼(Engraulis anchoita)等(Crespi-Abril et al, 2011)。
|
|
表 1 不同海域阿根廷滑柔鱼食物组成情况/% Tab.1 Prey composition of I. argentinus from different waters/% |
在福克兰群岛、巴塔哥尼亚大陆架、布宜诺斯艾利斯等海域,阿根廷滑柔鱼的食物组成结构基本一致,但与巴西南部海域和圣马蒂亚斯湾的食物组成存在差异(表 1)。在福克兰群岛海域和巴塔哥尼亚大陆架海域,阿根廷滑柔鱼均以捕食甲壳类为主,所捕食的甲壳类在其食物组成中的占比分别为72.1%和85.3%,其次为头足类,鱼类的占比则很低(Ivanovic et al, 1994; Mouat et al, 2001)。在布宜诺斯艾利斯海域,阿根廷滑柔鱼的食物结构依然以甲壳类为主要饵料生物,但占比有所下降,为56.9%;鱼类和头足类的占比则有所增加,分别为29.4%和13.6%。其中,摄食的鱼类以灯笼鱼为主,也摄食少量阿根廷鳀鱼,头足类则以阿根廷滑柔鱼幼体为主(Ivanovic et al, 1994)。
1.2.2 生长发育期及体型间的食物组成差异随着生长发育和体型增大,阿根廷滑柔鱼的能量积累较迅速地增加(林东明等, 2017),食物组成也会有所差异(Rodhouse et al, 2013)。通常,在生活史早期,阿根廷滑柔鱼以捕食甲壳类等浮游动物为主,随着生长发育逐渐转向捕食营养层级较高的小型鱼类和头足类等(Rodhouse et al, 2013)。这种食性转换与阿根廷滑柔鱼生长发育的能量积累需求是相一致的,在能量积累需求较大的成鱼期,转向捕食营养物质较大、能量较高的鱼类(Crespi-Abril et al, 2011; Lin et al, 2022; Song et al, 2022)。比如在巴西南部海域,阿根廷滑柔鱼稚鱼和亚成鱼主要捕食甲壳类,而且同类相食现象较多(Santos et al, 1997a);成鱼则以捕食阿根廷无须鳕及中深层鱼类等为主,食物组成中的鱼类比例明显增加(Santos et al, 1997a; Haimovici et al, 1994)。在圣马蒂亚斯湾,体型较大的阿根廷滑柔鱼食物组成中,头足类的出现比例也显著增加(Crespi-Abril et al, 2011)。在巴塔哥尼亚海域,阿根廷滑柔鱼内壳所对应的稚鱼期、成鱼期等不同分段上的碳氮稳定同位素值存在显著差异(Rosas-Luis et al, 2017)。Queirós等(2019)分析阿根廷滑柔鱼角质颚上的稳定同位素,结果也显示,成鱼期的氮稳定同位素值比稚鱼期的高一个营养级,且具有较宽的同位素生态位宽度。值得注意的是,阿根廷滑柔鱼的食物组成差异也往往存在一个体型参考点。比如,在布宜诺斯艾利斯海域和巴塔哥尼亚大陆架海域,阿根廷滑柔鱼的食性变化均可以以胴长200 mm为参考点,小于200 mm的个体以甲壳类为主要食物来源,大于200 mm的则以鱼类和头足类为主(Ivanovic et al, 1994)。而在福克兰群岛附近海域,阿根廷滑柔鱼的胴长≥220 mm时,其食物组成中的头足类占比明显增大,甲壳类占比则显著减少(Mouat et al, 2001)。
2 作为重要的生态系统能量传输者在西南大西洋,阿根廷滑柔鱼较大量地捕食甲壳类、头足类和鱼类等以满足生长发育对能量积累的需求(如上所述);同时,该种类因海域分布广、资源量丰富,也是许多大型海洋生物的重要饵料生物(图 1)。碳氮稳定同位素分析结果显示,阿根廷滑柔鱼在食物网中的营养层级处于3.5~4.7之间(Rosas-Luis et al, 2016、2017),并且生态位宽度会随个体生长发育而增大(Rosas-Luis et al, 2017; Queirós et al, 2019),在西南大西洋生态系统中扮演着链接营养及能量传输的重要生态角色(Arkhipkin, 2013)。已有研究发现,阿根廷滑柔鱼的主要捕食者有大型鱼类、哺乳类动物及海鸟等(表 2)。
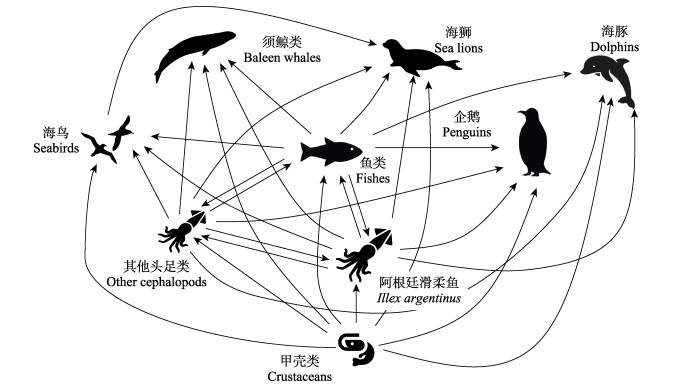
|
图 1 阿根廷滑柔鱼的食物组成及主要捕食者网络 Fig.1 Diagrammatic scheme of prey composition and main predators for I. argentinus |
|
|
表 2 阿根廷滑柔鱼在捕食者胃含物中的出现频率 Tab.2 The frequency of occurrence of I. argentinus in predator´s stomach content |
阿根廷滑柔鱼既是中上层鱼类的重要饵料生物,也是底栖鱼类和鲨鱼的重要捕食对象,起着链接栖息海域的中上层至深层水域之间能量传输的枢纽作用。在巴西南部中上层水域,它是大眼金枪鱼(Thunnus obesus)、剑鱼(Xiphias gladius)等大型游泳生物的重要食物,在二者胃含物中的出现频率分别为49.3%和31.3% (Santos et al, 2000);而在大陆坡上斜坡海域,阿根廷滑柔鱼也是乌拉圭猫鲨(Scyliorhinus besnardi)和美洲多锯鲈(Polyprion americanus)等底栖生物的捕食对象,在食物组成中的占比分别为62.5%和26.7% (Santos et al, 2000) (表 2)。
然而,鱼类捕食者对阿根廷滑柔鱼的捕食存在生长发育差异性。比如,在巴塔哥尼亚中北部海域(41°~47°S),鳐鱼(Dipturus chilensis)是阿根廷滑柔鱼的主要捕食者之一(Alonso et al, 2001)。但是,鳐鱼在生长发育前期(体长 < 35 cm)主要捕食甲壳类;在生长发育中后期,阿根廷滑柔鱼在其食物组成中的比例逐渐增加,并与阿根廷无须鳕、拉氏南美南极鱼(Patagonotothen ramsayi)等成为主要的捕食对象(Lucifora et al, 2000; Alonso et al, 2001)。
同时,阿根廷滑柔鱼在鱼类捕食者食物组成中的重要性存在季节性差异,与阿根廷滑柔鱼海域分布的季节性变化密切相关。比如,在福克兰群岛附近海域,当夏季到来时,阿根廷滑柔鱼大量聚集,成为该海域澳洲犁齿鳕(Salilota australis) (Arkhipkin et al, 2001)和阿根廷无须鳕(Belleggia et al, 2014)的主要捕食对象,后者的食物组成中阿根廷滑柔鱼的出现频率高达43.1% (表 2)。在其他季节,巴塔哥尼亚枪乌贼(Loligo gahi)成为福克兰群岛附近海域主要分布的头足类种类,并成为澳洲犁齿鳕的主要捕食对象,而阿根廷滑柔鱼资源量偏低,在澳洲犁齿鳕胃含物中出现的频率也较低(Arkhipkin et al, 2001)。
2.2 海洋哺乳类捕食者在西南大西洋生态系统中,阿根廷滑柔鱼是海豚、海狮、小须鲸等海洋哺乳动物的重要饵料生物。其中,在巴塔哥尼亚海域,暗色斑纹海豚(Lagenorhynchus obscurus)主要捕食阿根廷滑柔鱼,在其胃含物中,阿根廷滑柔鱼的出现频率高达68% (Alonso et al, 1998; Romero et al, 2012) (表 2)。然而,阿根廷滑柔鱼在暗色斑纹海豚食物组成结构中的比重存在海域差异性。Castro等(2016)对不同海岸搁浅的暗色斑纹海豚胃含物组成研究发现,来自Golfo San Matías海岸和Golfo San Jorge海岸的暗色斑纹海豚胃含物中,阿根廷滑柔鱼的占比分别为19%和32%;而来自Golfo San José海岸和Golfo Nuevo海岸上的暗色斑纹海豚胃含物中,阿根廷滑柔鱼的比例很低,取而代之的是巴塔哥尼亚枪乌贼。巴塔哥尼亚北部和中部海域的南美海狮(Otaria byronia)也以捕食阿根廷滑柔鱼为主,但阿根廷滑柔鱼在其食物组成结构中存在一定的雌雄差异,雌性海狮因需要喂养、保护幼崽等,捕食地点相较于雄性海狮离海岸更近、捕食水层也较浅(Crespo et al, 1997; Alonso et al, 2000)。因此,阿根廷滑柔鱼在雄性海狮食物组成中的出现频率达54.5%,在雌性海狮食物组成中则为38.5% (表 2)。同时,栖息在圣马蒂亚斯湾的南美海狮尽管偏好于捕食特维尔切蛸(Octopus tehuelchus)、巴塔哥尼亚枪乌贼等头足类,但阿根廷滑柔鱼也是其重要的捕食对象(Bustos et al, 2019)。
此外,巴西外海的侏儒小须鲸(Balaenoptera acutorostrata subsp.)也主要捕食阿根廷滑柔鱼,从搁浅的侏儒小须鲸胃含物中发现其食物仅有阿根廷滑柔鱼(Milmann et al, 2019)。在火地岛附近海域,花斑喙头海豚(Cephalorhynchus commersonii)和皮尔海豚(Lagenorhynchus australis)也常以捕食阿根廷滑柔鱼为主(Riccialdelli et al, 2013)。
2.3 海鸟类捕食者在西南大西洋栖息的许多海鸟也以阿根廷滑柔鱼为重要的饵料生物。其中,企鹅是最具代表性的捕食者之一。在巴西南部海域、圣马蒂亚斯湾海域和巴塔哥尼亚海域,阿根廷滑柔鱼是麦哲伦企鹅(Spheniscus magellanicus)的主要捕食对象之一(Alonso et al, 2000; Yorio et al, 2017; Castillo et al, 2019; Fernandez et al, 2019) (表 2)。在福克兰群岛附近海域,帝企鹅(Aptenodytes patagonicus)在冬季主要以头足类为食,甚至可以前往距离繁殖地几百公里外的海域捕食阿根廷滑柔鱼(Piatkowski et al, 2001)。
同时,在南大洋觅食的黑眉信天翁(Thalassarche melanophris)、灰头信天翁(Thalassarche chrysostoma)、漂泊信天翁(Diomedea exulans)、白颊刻风鸌(Procellaria aequinoctialis)也以阿根廷滑柔鱼为重要捕食对象。利用稳定同位素技术,分析这些海鸟胃含物中留存的阿根廷滑柔鱼角质颚得知,它们所捕食的阿根廷滑柔鱼均来自于巴塔哥尼亚海域(Berrow et al, 2000; Seco et al, 2015; Queirós et al, 2019)。
3 总结与展望 3.1 能量积累需求与洄游阿根廷滑柔鱼作为一年生的大洋性头足类,有效地获得能量积累对其生长发育及资源量维持至关重要。研究表明,在能量积累过程中,阿根廷滑柔鱼的食物组成存在生长发育期差异性,也与体型大小密切相关。值得注意的是,在短暂的生命周期里,阿根廷滑柔鱼沿着巴塔哥尼亚大陆架200~300 m等深线附近海域进行长距离的南向索饵洄游和北向产卵洄游,或者进行巴塔哥尼亚大陆坡折水域与阿根廷近海水域之间短距离的离岸索饵洄游和向岸产卵洄游(Rodhouse et al, 2013)。同时,西南大西洋海域的饵料生物存在空间异质性。比如,巴西南部海域的饵料生物群落以甲壳类为主(Santos et al, 1997a),巴塔哥尼亚大陆架海域至马尔维纳斯群岛附近海域则栖息着丰富的鱼类及头足类资源(Arkhipkin, 2013)。鱼类、头足类拥有比甲壳类更高的营养和能量价值(Ciancio et al, 2007; Dessier et al, 2018; Schaafsma et al, 2018)。阿根廷滑柔鱼的洄游过程也是其生长发育的过程,能量积累需求也随之增加;那么阿根廷滑柔鱼的索饵洄游习性应该是能量积累所驱动,以满足个体生长和后代存活的能量需求。然而,现有研究结果尚未报道洄游与能量积累的相关性,后续可以从能量需求角度研究分析阿根廷滑柔鱼的洄游行为,可为揭示其洄游的内在机理及能量积累需求提供更深层的解释。
3.2 能量积累需求与摄食策略在能量积累过程中,阿根廷滑柔鱼的食物组成由甲壳类转向鱼类和头足类。虽然已有研究认为,这是一种捕食能力提升的结果,然而,体型较大的阿根廷滑柔鱼仍捕食营养层级较低的甲壳类饵料生物,并且在福克兰群岛附近海域甲壳类是主要的食物来源(Ivanovic et al, 1994; Mouat et al, 2001)。最近的研究结果也发现,阿根廷滑柔鱼的生态位随着能量积累需求的增加而升高,但它们的生态位宽度也随之增大(Lin et al, 2022)。一方面,营养层级较高的生物种类具有较好的避敌能力(Burger et al, 2019),必将增加捕食者在捕食过程中的能量消耗,使得捕食者的能量净收入有所降低。另一方面,捕食者也可能较大量地捕食营养层级较低的饵料生物以获得较好的能量净收入,但频繁地捕食也会增加它们被捕食的风险(Bhat et al, 2020)。因此,阿根廷滑柔鱼在能量积累过程中可能存在较为灵活的摄食策略,以实现能量净收入的最大化。这种摄食策略可能是在生活史后期倾向于进食鱼类、头足类等高能量物种,从而满足生殖能量的高需求;或者捕食容易捕获的低营养层级、低能量物种的甲壳类饵料生物,通过增大摄食量来达到能量的收支平衡;又或者二者兼有。同时,阿根廷滑柔鱼为聚群性海洋捕食者,个体之间的食物竞争也较为突出(Rodhouse et al, 2013)。为此,阿根廷滑柔鱼能量积累需求的摄食策略选择,以及摄食策略选择是否具有个体特殊性等仍有待深入研究,以认知该种类食性转变的生态习性和能量积累最优化的生活史特性。
3.3 作为海洋食物网能量传输者角色作用当前,全球日趋显著的气候变化,包括全球变暖、海水酸化、南极海冰消融等,已对阿根廷滑柔鱼的产卵孵化场及索饵育肥场造成不同程度的影响(刘赫威等, 2020)。比如,全球气候变化引起的海水温度变化,不仅影响着阿根廷滑柔鱼的栖息海域和资源补充量(Sacau et al, 2005; Queirós et al, 2019),也影响着仔稚鱼的生长速率、索饵洄游路线及其索饵时长(Moustahfid et al, 2021),并进一步影响着能量积累以及食性变化过程(Yalçınkaya et al, 2019)。海水酸化则影响体内钙质耳石的形成和胚胎发育及随后的孵化率(Rodhouse et al, 2013)。值得注意的是,频繁的渔业活动也增加了阿根廷滑柔鱼作为饵料生物对西南大西洋生态系统的影响。在西南大西洋35°~55°S海域,白斑角鲨(Squalus acanthias)在近50年间摄食饵料生物的种类变化印证了这一点。白斑角鲨捕食的鱼类和底栖生物的数量逐渐减少,而阿根廷滑柔鱼和水母的比例呈上升趋势(Belleggia et al, 2012)。在20世纪80年代,阿根廷无须鳕是白斑角鲨的主要食物,相对重要指数为65.46;阿根廷滑柔鱼是第2种主要饵料生物,相对重要指数为10.15。而到21世纪初,阿根廷滑柔鱼已成为白斑角鲨的主要食物,相对重要指数高达88.92。这一变化是由于商业过度捕捞阿根廷无须鳕所致,使得白斑角鲨转向摄食阿根廷滑柔鱼等较低营养层级的饵料生物种类(Belleggia et al, 2012)。Alonso等(2002)对巴塔哥尼亚海域41°~46°S的白斑角鲨进行胃含物分析时也得出相同的结果,发现白斑角鲨的食物结构已从鳕鱼转变为阿根廷滑柔鱼。为此,深入阐述气候变化、渔业活动等对阿根廷滑柔鱼生命过程的影响机制与原理,包括探究阿根廷滑柔鱼生态食物网中营养层级变化、资源量补充过程、个体对栖息环境变化的适应能力以及在海洋食物网中能量传输的生态角色等,必将能够更科学地掌握阿根廷滑柔鱼响应气候变化的适应性过程,也为持续开发该种类资源提供资料参考。
ALONSO M K, CRESPO E A, PEDRAZA S N, et al. Food habits of the south American sea lion, Otaria flavescens, off Patagonia, Argentina. Fishery Bulletin, 2000, 92(8): 250-263 |
ALONSO M K, CRESPO E A, GARCÍA N A, et al. Fishery and ontogenetic driven changes in the diet of the spiny dogfish, Squalus acanthias, in Patagonian waters, Argentina. Environmental Biology of Fishes, 2002, 63(2): 193-202 DOI:10.1023/A:1014229432375 |
ALONSO M K, CRESPO E A, GARCÍA N A, et al. Food habits of Dipturus chilensis (Pisces: Rajidae) off Patagonia, Argentina. ICES Journal of Marine Science, 2001, 58(1): 288-297 DOI:10.1006/jmsc.2000.1010 |
ALONSO M K, CRESPO E A, GARCÍA N, et al. Diet of dusky dolphins, Lagenorhynchus obscurus, in waters off Patagonia, Argentina. Fishery Bulletin, 1998, 96: 366-374 |
ARKHIPKIN A I. Squid as nutrient vectors linking Southwest Atlantic marine ecosystems. Deep-Sea Research Part Ⅱ: Topical Studies in Oceanography, 2013, 95: 7-20 DOI:10.1016/j.dsr2.2012.07.003 |
ARKHIPKIN A, BRICKLE P, LAPTIKHOVSKY V, et al. Variation in the diet of the red cod with size and season around the Falkland Islands (south-west Atlantic). Journal of the Marine Biological Association of the United Kingdom, 2001, 81(6): 1035-1040 DOI:10.1017/S0025315401005021 |
ARKHIPKIN A. Age, growth, stock structure and migratory rate of pre-spawning short-finned squid Illex argentinus based on statolith ageing investigations. Fisheries Research, 1993, 16(4): 313-338 DOI:10.1016/0165-7836(93)90144-V |
BEASLEY A L, HALL K C, LATELLA C I, et al. Reproductive characteristics of three small-bodied cuttlefish in subtropical waters. Marine and Freshwater Research, 2018, 69(3): 403-417 DOI:10.1071/MF17169 |
BELLEGGIA M, FIGUEROA D E, SÁNCHEZ F, et al. Long-term changes in the spiny dogfish (Squalus acanthias) trophic role in the southwestern Atlantic. Hydrobiologia, 2012, 684: 57-67 DOI:10.1007/s10750-011-0967-y |
BELLEGGIA M, FIGUEROA D E, IRUSTA G, et al. Spatio-temporal and ontogenetic changes in the diet of the Argentine hake Merluccius hubbsi. Journal of the Marine Biological Association of the United Kingdom, 2014, 94(8): 1701-1710 DOI:10.1017/S0025315414000629 |
BERROW S D, WOOD A G, PRINCE P A. Foraging location and range of white-chinned petrels Procellaria aequinoctialis breeding in the South Atlantic. Journal of Avian Biology, 2000, 31(3): 303-311 DOI:10.1034/j.1600-048X.2000.310305.x |
BHAT U, KEMPES C P, YEAKEL J D. Scaling the risk landscape drives optimal life-history strategies and the evolution of grazing. Proceedings of the National Academy of Sciences, 2020, 117(3): 1580-1586 DOI:10.1073/pnas.1907998117 |
BOYLE P, RODHOUSE P. Cephalopods: Ecology and fisheries. Oxford, UK: Wiley-Blackwell, 2005
|
BURGER J R, HOU C, BROWN J H. Toward a metabolic theory of life history. Proceedings of the National Academy of Sciences, 2019, 116(52): 26653-26661 DOI:10.1073/pnas.1907702116 |
BUSTOS R L, DANERI G A, VARELA E A, et al. South American sea lions Otaria byronia as biological samplers of local cephalopod fauna in the Patagonian shelf marine ecosystem. Journal of the Marine Biological Association of the United Kingdom, 2019, 99(6): 1459-1463 DOI:10.1017/S0025315419000432 |
CASTILLO J, YORIO P, GATTO A. Shared dietary niche between sexes in Magellanic penguins. Austral Ecology, 2019, 44(4): 635-647 DOI:10.1111/aec.12706 |
CASTRO R L D, SAPORITI F, VALES D G, et al. Feeding ecology of dusky dolphins Lagenorhynchus obscurus: Evidence from stable isotopes. Journal of Mammalogy, 2016, 97(1): 310-320 DOI:10.1093/jmammal/gyv180 |
CHEN X J, LIU B L, FANG Z, et al. Cephalopod. Beijing: China Ocean Press, 2019: 49-77 [陈新军, 刘必林, 方舟, 等. 头足纲. 北京: 海洋出版社, 2019: 49-77]
|
CHEN X J, LIU B L, LIN D. Sexual maturation, reproductive habits, and fecundity of fish. In CHEN X J, LIU B L (eds) Biology of fishery resources. Singapore: Springer Singapore, 2022, 113–142
|
CHEN X J. Development status of world cephalopod fisheries and suggestions for squid jigging fishery in China. Journal of Shanghai Ocean University, 2019, 28(3): 321-330 [陈新军. 世界头足类资源开发现状及我国远洋鱿钓渔业发展对策. 上海海洋大学学报, 2019, 28(3): 321-330] |
CIANCIO J E, PASCUAL M A, BEAUCHAMP D A. Energy density of patagonian aquatic organisms and empirical predictions based on water content. Transactions of the American Fisheries Society, 2007, 136(5): 1415-1422 DOI:10.1577/T06-173.1 |
CLARKE A, RODHOUSE P G, GORE D J. Biochemical composition in relation to the energetics of growth and sexual maturation in the Ommastrephid squid Illex argentinus. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 1994, 344(1308): 201-212 DOI:10.1098/rstb.1994.0061 |
COLLINS M A, BURNELL G M, RODHOUSE P G. Reproductive strategies of male and female Loligo forbesi (Cephalopoda: Loliginidae). Journal of the Marine Biological Association of the United Kingdom, 1995, 75(3): 621-634 DOI:10.1017/S0025315400039059 |
CRESPI-ABRIL A C, TRIVELLINI M M. Diet of spring and summer spawning groups of Illex argentinus inhabiting coastal waters in San Matias Gulf (northern Patagonia, Argentina). Aquatic Biology, 2011, 14(1): 99-103 DOI:10.3354/ab00386 |
CRESPO E A, PEDRAZA S N, DANS S L, et al. Direct and indirect effects of highseas fisheries on the marine mammal populations in the northern and central Patagonian coast. Journal of Northwest Atlantic Fishery Science, 1997, 22: 189-207 DOI:10.2960/J.v22.a15 |
DESSIER A, DUPUY C, KERRIC A, et al. Variability of energy density among mesozooplankton community: New insights in functional diversity to forage fish. Progress in Oceanography, 2018, 166: 121-128 DOI:10.1016/j.pocean.2017.10.009 |
FAO. The state of world fisheries and aquaculture 2022. Towards Blue Transformation. Rome, Italy: FAO, 2022
|
FERNANDEZ S J, YORIO P, CIANCIO J E. Diet composition of expanding breeding populations of the Magellanic penguin. Marine Biology Research, 2019, 15(1): 84-96 DOI:10.1080/17451000.2019.1596286 |
HAIMOVICI M, MARTINS A S, FIGUEIREDO J L D, et al. Demersal bony fish of the outer shelf and upper slope of the southern Brazil subtropical convergence ecosystem. Marine Ecology Progress Series, 1994, 108: 59-77 DOI:10.3354/meps108059 |
HAN F, CHEN X J, LIN D M, et al. The body condition and reproductive investment of Dosidicus gigas in the equatorial waters of eastern Pacific Ocean. Journal of Fisheries of China, 2019, 43(12): 2511-2522 [韩飞, 陈新军, 林东明, 等. 东太平洋赤道海域茎柔鱼体征生长及生殖投入. 水产学报, 2019, 43(12): 2511-2522 DOI:10.11964/jfc.20180611323] |
HAN F, CHEN X J, LIN D M. Energy accumulation and its relation to sea surface environments in Dosidicus gigas from the equatorial water of the eastern Pacific. Journal of Fishery Sciences of China, 2020, 27(4): 427-437 [韩飞, 陈新军, 林东明. 东太平洋赤道海域茎柔鱼组织能量积累及其与海表面环境因子的关系. 中国水产科学, 2020, 27(4): 427-437] |
HATFIELD E, RODHOUSE P, BARBER D. Production of soma and gonad in maturing female Illex argentinus (Mollusca: Cephalopoda). Journal of the Marine Biological Association of the United Kingdom, 1992, 72: 281-291 DOI:10.1017/S0025315400037693 |
IVANOVIC M, BRUNETTI N E. Food and feeding of Illex argentinus. Antarctic Science, 1994, 6(2): 185-193 DOI:10.1017/S0954102094000295 |
JACKSON G D, SEMMENS J M, PHILLIPS K L, et al. Reproduction in the deepwater squid Moroteuthis ingens, what does it cost?. Marine Biology, 2004, 145(5): 905-916 DOI:10.1007/s00227-004-1375-x |
JOHNS M E, WARZYBOK P, BRADLEY R W, et al. Increased reproductive investment associated with greater survival and longevity in Cassin's auklets. Proceedings of the Royal Society B: Biological Sciences, 2018, 285(1885): 20181464 DOI:10.1098/rspb.2018.1464 |
KARJALAINEN J, URPANEN O, KESKINEN T, et al. Phenotypic plasticity in growth and fecundity induced by strong population fluctuations affects reproductive traits of female fish. Ecology and Evolution, 2016, 6(3): 779-790 DOI:10.1002/ece3.1936 |
LAPTIKHOVSKY V, SALMAN A, ÖNSOY B, et al. Reproduction in rare bathyal octopods Pteroctopus tetracirrhus and Scaeurgus unicirrhus (Cephalopoda: Octopoda) in the east Mediterranean as an apparent response to extremely oligotrophic deep seas. Deep-Sea Research Part Ⅰ: Oceanographic Research Papers, 2014, 92: 85-92 DOI:10.1016/j.dsr.2014.06.009 |
LIAN J X, FENG Y X, LIN D M. Relative energy accumulation in soma and gonad tissues of female Dosidicus gigas and relation to environmental effects. South China Fisheries Science, 2022, 18(4): 34-43 [连晋欣, 冯艺璇, 林东明. 茎柔鱼肌肉和性腺组织相对能量积累及环境效应关系研究. 南方水产科学, 2022, 18(4): 34-43] |
LIN D M, CHEN X J, CHEN Y, et al. Ovarian development in Argentinean shortfin squid Illex argentinus: Group-synchrony for corroboration of intermittent spawning strategy. Hydrobiologia, 2017a, 795(1): 327-339 DOI:10.1007/s10750-017-3154-y |
LIN D M, CHEN X J, WEI Y R, et al. The energy accumulation of somatic tissue and reproductive organs in post-recruit female Illex argentinus and the relationship with sea surface oceanography. Fisheries Research, 2017b, 185: 102-114 DOI:10.1016/j.fishres.2016.09.023 |
LIN D M, CHEN X J, CHEN Y, et al. Sex-specific reproductive investment of summer spawners of Illex argentinus in the southwest Atlantic. Invertebrate Biology, 2015, 134(3): 203-213 DOI:10.1111/ivb.12088 |
LIN D M, CHEN X J, FANG Z. Preliminary study on reproductive biology of summer spawning stock of Illex argentinus in the southwestern Atlantic Ocean. Journal of Fisheries of China, 2014, 38(6): 843-852 [林东明, 陈新军, 方舟. 西南大西洋阿根廷滑柔鱼夏季产卵种群繁殖生物学的初步研究. 水产学报, 2014, 38(6): 843-852] |
LIN D M, CHEN X J, WEI Y R, et al. Energy accumulation of both somatic and reproductive tissuesand its allocation to reproduction in Argentinean short-fin squid (Illex argentinus). Journal of Fisheries of China, 2017, 41(1): 70-80 [林东明, 陈新军, 魏嫣然, 等. 阿根廷滑柔鱼雌性个体肌肉和性腺组织能量积累及其生殖投入. 水产学报, 2017, 41(1): 70-80] |
LIN D M, HAN F, XUAN S, et al. Fatty acid composition and the evidence for mixed income-capital breeding in female Argentinean short-fin squid Illex argentinus. Marine Biology, 2019, 166(7): 90-102 DOI:10.1007/s00227-019-3534-0 |
LIN D M, HAN F, ZHU K, et al. Effects of hatching season on the growth and development in Illex argentinus. Journal of Shanghai Ocean University, 2020, 29(3): 374-384 [林东明, 韩飞, 朱凯, 等. 孵化季节对阿根廷滑柔鱼生长发育的影响. 上海海洋大学学报, 2020, 29(3): 374-384] |
LIN D M, ZANG N, ZHU K, et al. Energy acquisition strategy for reproduction in a semelparous squid. Frontiers in Zoology, 2022, 19(1): 28 DOI:10.1186/s12983-022-00473-w |
LIU H W, YU W, CHEN X J. A review of Illex argentinus resources and the responses to environmental variability in the southwest Atlantic Ocean. Journal of Fishery Sciences of China, 2020, 27(10): 1254–1265 [刘赫威, 余为, 陈新军. 西南大西洋阿根廷滑柔鱼资源及其对环境响应的研究进展. 中国水产科学, 27(10): 1254–1265]
|
LIU W, FENG Y X, SONG W. Body condition and reproductive investment of mature male argentinean shortfin squid Illex argentinu. Progress in Fishery Sciences, 2022, 43(5): 11-20 [刘伟, 冯艺璇, 宋维, 等. 阿根廷滑柔鱼雄性成熟个体的体征和生殖投入. 渔业科学进展, 2022, 43(5): 11-20] |
LU H J, CHEN X J. Age, growth and population structure of Illex argentinus based on statolith microstructure in Southwest Atlantic Ocean. Journal of Fisheries of China, 2012, 36(7): 1049-1056 DOI:10.3724/SP.J.1231.2012.27654 |
LUCIFORA L O, VALERO J L, BREMEC C S, et al. Feeding habits and prey selection by the skate Dipturus chilensis (Elasmobranchii: Rajidae) from the south-western Atlantic. Journal of the Marine Biological Association of the United Kingdom, 2000, 80(5): 953-954 DOI:10.1017/S002531540000299X |
MCBRIDE R S, SOMARAKIS S, FITZHUGH G R, et al. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish and Fisheries, 2015, 16(1): 23-57 DOI:10.1111/faf.12043 |
MCGRATH B, JACKSON G. Egg production in the arrow squid Nototodarus gouldi (Cephalopoda: Ommastrephidae), fast and furious or slow and steady?. Marine Biology, 2002, 141(4): 699-706 DOI:10.1007/s00227-002-0864-z |
MILMANN L, MACHADO R, SUCUNZA F, et al. New trophic link and potential feeding area of dwarf minke whale (Balaenoptera acutorostrata subsp.) in mid latitude waters of the southwestern Atlantic Ocean. Mammalia, 2019, 83(1): 49-52 |
MOUAT B, COLLINS M A, POMPERT J. Patterns in the diet of Illex argentinus (Cephalopoda: Ommastrephidae) from the Falkland Islands jigging fishery. Fisheries Research, 2001, 52(1): 41-49 |
MOUSTAHFID H, HENDRICKSON L C, ARKHIPKIN A, et al. Ecological-fishery forecasting of squid stock dynamics under climate variability and change: review, challenges, and recommendations. Reviews in Fisheries Science and Aquaculture, 2021, 29(4): 682-705 DOI:10.1080/23308249.2020.1864720 |
NIGMATULLIN C M, MARKAIDA U. Oocyte development, fecundity and spawning strategy of large sized jumbo squid Dosidicus gigas (Oegopsida: Ommastrephinae). Journal of the Marine Biological Association of the United Kingdom, 2009, 89(4): 789-801 DOI:10.1017/S0025315408002853 |
PASCUAL C, CRUZ-LOPEZ H, MASCARÓ M, et al. Changes in biochemical composition and energy reserves associated with sexual maturation of Octopus maya. Frontiers in Physiology, 2020, 11: 22 DOI:10.3389/fphys.2020.00022 |
PATRICK S C, RÉALE D, POTTS J R, et al. Differences in the temporal scale of reproductive investment across the slow-fast continuum in a passerine. Ecology Letters, 2022, 25(5): 1139-1151 DOI:10.1111/ele.13982 |
PECL G. Flexible reproductive strategies in tropical and temperate Sepioteuthis squids. Marine Biology, 2001, 138(1): 93-101 DOI:10.1007/s002270000452 |
PEREZ J A A, SILVA T N, SCHROEDER R, et al. Biological patterns of the Argentine shortfin squid Illex argentinus in the slope trawl fishery off Brazil. Latin American Journal of Aquatic Research, 2009, 37(3): 409-428 DOI:10.3856/vol37-issue3-fulltext-11 |
PIATKOWSKI U, PÜTZ K, HEINEMANN H. Cephalopod prey of king penguins (Aptenodytes patagonicus) breeding at Volunteer Beach, Falkland Islands, during austral winter 1996. Fisheries Research, 2001, 52(1/2): 79-90 |
QUEIRÓS J P, PHILLIPS R A, BAETA A, et al. Habitat, trophic levels and migration patterns of the short-finned squid Illex argentinus from stable isotope analysis of beak regions. Polar Biology, 2019, 42(12): 2299-2304 DOI:10.1007/s00300-019-02598-x |
RICCIALDELLI L, NEWSOME S D, DELLABIANCA N A, et al. Ontogenetic diet shift in Commerson´s dolphin (Cephalorhynchus commersonii commersonii) off Tierra del Fuego. Polar Biology, 2013, 36(5): 617-627 DOI:10.1007/s00300-013-1289-5 |
ROCHA F, GUERRA Á, GONZÁLEZ Á F. A review of reproductive strategies in cephalopods. Biological Reviews, 2001, 76(3): 291-304 DOI:10.1017/S1464793101005681 |
RODHOUSE P G, ARKHIPKIN A I, LAPTIKHOVSKY V, et al. Illex argentinus, Argentine shortfin squid. In ROSA R G PIERCE R O'DOR (eds) Advances in squid biology, ecology and fisheries Part Ⅱ- Oegopsid squids. New York: Nova Science Publishers, 2013, 109–148
|
RODHOUSE P G, HATFIELD E M C. Production of soma and gonad in maturing male Illex argentinus (Mollusca: Cephalopoda). Journal of the Marine Biological Association of the United Kingdom, 1992, 72(2): 293-300 DOI:10.1017/S002531540003770X |
RODHOUSE P G, NIGMATULLIN C M. Role as consumers. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 1996, 351(1343): 1003-1022 DOI:10.1098/rstb.1996.0090 |
ROMERO M A, DANS S L, GARCÍA N, et al. Feeding habits of two sympatric dolphin species off North Patagonia, Argentina. Marine Mammal Science, 2012, 28(2): 364-377 DOI:10.1111/j.1748-7692.2011.00477.x |
ROSAS-LUIS R, NAVARRO J, MARTÍNEZ-BAENA F, et al. Differences in the trophic niche along the gladius of the squids Illex argentinus and Doryteuthis gahi based on their isotopic values. Regional Studies in Marine Science, 2017, 1117-1122 |
ROSAS-LUIS R, NAVARRO J, SÁNCHEZ P, et al. Assessing the trophic ecology of three sympatric squid in the marine ecosystem off the Patagonian shelf by combining stomach content and stable isotopic analyses. Marine Biology Research, 2016, 12(4): 402-411 DOI:10.1080/17451000.2016.1142094 |
SACAU M, PIERCE G J, WANG J, et al. The spatio-temporal pattern of Argentine shortfin squid Illex argentinus abundance in the southwest Atlantic. Aquatic Living Resources, 2005, 18(4): 361-372 DOI:10.1051/alr:2005039 |
SALMAN A, ÖNSOY B. Analysis of fecundity of some bobtail squid of the genus Sepiola (Cephalopoda: Sepiolida) in the Aegean Sea (eastern Mediterranean). Journal of the Marine Biological Association of the United Kingdom, 2004, 84(4): 781-782 |
SANTOS R A, HAIMOVICI M. Food and feeding of the short-finned squid Illex argentinus (Cephalopoda: Ommastrephidae) off southern Brazil. Fisheries Research, 1997a, 33(1): 139-147 |
SANTOS R A, HAIMOVICI M. Reproductive biology of the winter-spring spawners of Illex argentinus (Cephalopoda; Ommastrephidae) off southern Brazil. Scientia Marina, 1997b, 61(1): 53-64 |
SANTOS R A, HAIMOVICI M. The Argentine short-finned squid Illex argentinus in the food webs of southern Brazil. Sarsia, 2000, 85(1): 49-60 |
SCHAAFSMA F L, CHEREL Y, FLORES H, et al. Review: The energetic value of zooplankton and nekton species of the Southern Ocean. Marine Biology, 2018, 165(8): 129-164 |
SCHWARZ R, PEREZ J A A. Age structure and life cycles of the Argentine shortfin squid Illex argentinus (Cephalopoda: Ommastrephidae) in southern Brazil. Journal of the Marine Biological Association of the United Kingdom, 2013, 93(2): 557-565 |
SCHWARZ R, PEREZ J A A. Growth model identification of short-finned squid Illex argentinus (Cephalopoda: Ommastrephidae) off southern Brazil using statoliths. Fisheries Research, 2010, 106(2): 177-184 |
SECO J, DANERI G A, CEIA F R, et al. Distribution of short-finned squid Illex argentinus (Cephalopoda: Ommastrephidae) inferred from the diets of Southern Ocean albatrosses using stable isotope analyses. Journal of the Marine Biological Association of the United Kingdom, 2015, 96(6): 1211-1215 |
SIEIRO P, OTERO J, AUBOURG S P. Biochemical composition and energy strategy along the reproductive cycle of female Octopus vulgaris in Galician waters (NW Spain). Frontiers in Physiology, 2020, 11: 760 |
SONG W, FENG Y, LIN D, et al. Stable isotope evidence for a shift in diet with increasing energy demand for reproduction in squid Illex argentinus. Hydrobiologia, 2023, 850: 489-502 |
STEPHENS P A, BOYD I L, MCNAMARA J M, et al. Capital breeding and income breeding: Their meaning, measurement, and worth. Ecology, 2009, 90(8): 2057-2067 |
VANCE R R. Optimal somatic growth and reproduction in a limited, constant environment: The general case. Journal of Theoretical Biology, 1992, 157(1): 51-70 |
XAVIER J C, ALLCOCK A L, CHEREL Y, et al. Future challenges in cephalopod research. Journal of the Marine Biological Association of the United Kingdom, 2015, 95(5): 999-1015 |
YALÇINKAYA B H, YILMAZ B, ÖZILGEN M. Thermodynamic assessment of information transmission in squid's giant axon may explain why squid populations thrive with global warming. International Journal of Global Warming, 2019, 19(3): 233-250 |
YORIO P, GONZÁLEZ-ZEVALLOS D, GATTO A, et al. Relevance of forage fish in the diet of Magellanic penguins breeding in northern Patagonia, Argentina. Marine Biology Research, 2017, 13(6): 603-617 |
ZHANG X C, WANG J T, CHEN X J. CPUE standardization of Illex argentinus based on BP neural network. Progress in Fishery Sciences, 2022, 43(2): 11-20 [章贤成, 汪金涛, 陈新军. 基于BP神经网络的阿根廷滑柔鱼资源CPUE标准化研究. 渔业科学进展, 2022, 43(2): 11-20] |
ZHU K, ZHANG L C, CHEN X J, et al. Energy accumulation and allocation of somatic and reproductive tissues in medium form of male Sthenoteuthis oualaniensis in the South China Sea. Journal of Tropical Oceanography, 2019, 38(4): 41-51 [朱凯, 张立川, 陈新军, 等. 南海鸢乌贼中型群雄性个体肌肉和性腺组织能量积累及其分配. 热带海洋学报, 2019, 38(4): 41-51] |
ZHU K, YAO J X, CHEN X J, et al. Energy accumulation of both somatic and reproductive tissues and the allocation to reproduction in the dwarf form individuals of Sthenoteuthis oualaniensis in the South China Sea. Journal of Shanghai Ocean University, 2020, 29(6): 910-920 [朱凯, 姚吉祥, 陈新军, 等. 南海鸢乌贼微型群肌肉和性腺组织能量积累及其分配. 上海海洋大学学报, 2020, 29(6): 910-920] |


