2. 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 崂山实验室海洋渔业科学与食物产出过程功能实验室 山东 青岛 266237
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao 266237, China
类胰岛素生长因子系统(insulin-like growth factors, IGFs)在鱼类和其他脊椎动物的生长、繁殖、早期发育、细胞分化和增殖等生物过程中发挥着关键性调控作用(Canosa et al, 2020; Hasanpoor et al, 2020; Qin et al, 2020; Triantaphyllopoulos et al, 2020)。在哺乳动物的研究中发现,IGFs家族由IGF1和IGF2 2个亚型组成(Annunziata et al, 2011)。Wang等(2008)在斑马鱼(Danio rerio)和尼罗罗非鱼(Oreochromis niloticus)中首次发现了1个新的IGFs家族基因IGF3。随后,在斜带石斑鱼(Epinephelus coioides) (Yang et al, 2015)、翘嘴鲌(Culter alburnus) (郑建波等, 2021)和双棘黄姑鱼(Protonibea diacanthus) (林权卓等, 2016)等多种鱼类中也陆续证实了该基因存在。IGF3具有B、C、A和D 4个IGFs家族特异性结构域,尽管同IGF1和IGF2序列之间同源性较低,但都有着相似的三级结构(Li J et al, 2021)。IGF3在鱼类的性腺发育过程中扮演着重要的角色,显著影响性别分化。在斑马鱼和尼罗罗非鱼中发现,igf3仅在性腺(精巢和卵巢)特异性表达,且在精巢和卵巢发育过程中呈现出不同的表达规律(Wang et al, 2008; Berishvili et al, 2010)。银鱼(Pampus argenteus)的性腺发育过程中,IGF3参与调控卵黄发生和精原细胞增殖(Gu et al, 2021)。此外,伴随着斑马鱼卵母细胞成熟,igf3转录水平在卵巢中呈显著上升趋势,重组IGF3可修复促黄体生成素β亚型(luteinizing hormone β, LHβ)缺失的卵母细胞成熟缺陷(Li et al, 2011; Li et al, 2015),且IGF3抗体蛋白可显著抑制人绒毛膜促性腺激素(human chorionic gonadtrophin, HCG)诱导的排卵过程(Li et al, 2018)。以上研究表明,IGF3除了参与调控鱼类性别决定和分化过程外,可能也参与调控卵母细胞生长和成熟,进而影响卵巢发育。
大菱鲆(Scophthalmus maximus)自1992年由中国水产科学院黄海水产研究所雷霁霖院士引进中国以来,经过30年的发展,养殖年产量稳定在5万t左右,约占大菱鲆世界总养殖产量的80%,成为海水鱼类良种引进典范(国家海水鱼产业技术体系年度报告2021; 谢婷等, 2023)。在工厂化种苗繁殖过程中,大菱鲆不能自发性产卵,经过光温调控、激素催熟后,需要通过人工挤卵的方式获取成熟卵子进行体外受精孵化,因此,深入研究和系统阐明其卵母细胞发育调控机制将为批量获取高质量卵子提供重要理论依据。目前,对大菱鲆雌性亲鱼营养需求(Lavens et al, 1999; 李庆华等, 2013)、激素诱导(Alvariño et al, 2001; Huang et al, 2019)、光温调控(Imsland et al, 2003; Polat et al, 2021)、卵质评价(Jia et al, 2015a、b)和下丘脑–垂体–性腺(hypothalamus-pituitary-gonad, HPG) 轴关键基因功能(Jia et al, 2014、2016、2019; Hu et al, 2018; Gao et al, 2019)进行了较为系统的研究。IGFs作为重要的局域性内分泌因子,参与调控大菱鲆早期发育和繁殖过程。Duval等(2002)首次克隆获取了大菱鲆IGF1和IGF2的全长cDNA序列,并证实其重组活性肽可显著抑制垂体细胞生长激素的分泌。IGF1和IGF2在大菱鲆胚胎发育和早期变态发育过程中发挥重要功能(Wen et al, 2015; Meng et al, 2016)。igf3可调控大菱鲆性别决定相关基因的表达(赵珊珊, 2022),但IGF3是否影响其卵巢发育尚不明确。本课题组的前期研究检测了目前已知的大菱鲆IGF系统家族成员(IGF1、IGF2、IGFBP1、IGFBP2和IGF-1R)在卵巢发育过程中的表达,发现上述家族成员通过复杂的内分泌、自分泌和旁分泌的方式参与调节卵母细胞的生长、成熟和排卵(Jia et al, 2019)。基于此,本研究克隆获取大菱鲆IGF3的全长cDNA序列,并利用生物信息学工具初步预测其功能,分析其在不同组织和卵巢发育过程中的表达变化,旨在为后续研究IGF3在大菱鲆性腺分化和卵母细胞成熟过程中的作用机制提供基础数据支撑。
1 材料与方法 1.1 实验材料不同规格雌性大菱鲆各10尾[10月龄,(800±150) g;15月龄,(1 500±200) g;20月龄,(2 000± 200) g;24月龄,(2 500±200) g;26月龄,(3 000±200) g]和雄性大菱鲆10尾[10月龄,(700±150) g]由烟台开发区天源水产有限公司提供。实验鱼分别置于6个循环水养殖桶中(10尾/桶,50 L)暂养2周。暂养期间,保持循环水温度(15.0±0.5) ℃,溶解氧(DO) > (4.5± 2.0) mg/L,pH为(7.9±0.3),盐度为(30.0±1.0),每日08:00和16:00进行饱食投喂商品饲料(三通生物工程有限公司)。RNA提取试剂盒(SteadyPure Universal RNA extraction kit)和qRT-PCR试剂盒(SYBR Green Premix Pro Taq HS qPCR KitⅡ)购自艾科瑞生物科技有限公司。反转录试剂盒(Prime Script RT reagent kit)、PCR高保真酶(PrimeSTAR® Max DNA Polymerase)、RACE克隆试剂盒(SMARTer® RACE 5′/3′ kit)和凝胶电泳Marker (DL2000 Marker)购自宝生物工程(大连)有限公司(TaKaRa),引物由生工生物工程(上海)有限公司合成。
1.2 样品采集和组织形态学观察取样前禁食24 h,分别从每个养殖水桶中随机挑选6尾实验用鱼,MS-222 (100 mg/L)深度麻醉后,迅速解剖取出心、肝、脾、肾、胃、肠、鳃、性腺(卵巢和精巢)、脑和垂体组织保存于液氮,用于基因克隆和表达分析。另取部分性腺(卵巢和精巢)组织于4%多聚甲醛中固定,用于组织形态学观察,确定不同规格大菱鲆卵巢和精巢发育阶段。性腺组织固定24 h后取出,经酒精梯度脱水(50%、75%、85%、95%和100%)和二甲苯透明后进行石蜡包埋,然后在切片机(湖北阔海医疗有限公司,KH-Q 330)上进行连续切片(5 μm),再经展片、烘干、脱蜡后进行苏木素–伊红(HE)染色、中性树脂胶封片。组织切片在显微镜(徕卡,DM 500)下进行形态学观察。
1.3 igf3全长cDNA序列克隆使用RNA提取试剂盒获取组织总RNA,用NanoDrop 2000 (Thermofisher,美国)测定RNA浓度。取1 μg总RNA使用反转录试剂盒合成cDNA,根据已知鱼类IGF3保守序列,利用CodeHop原理(Roseet al, 2003)设计简并引物(IGF3-F0和IGF3-R0),使用高保真酶进行PCR,获得IGF3部分CDS序列。扩增体系:12.5 μL PrimeSTAR Max DNA Polymerase,8.5 μL ddH2O,2 μL cDNA,上下游引物各1 μL。反应条件:94 ℃ 5 min;94 ℃ 30 s,55.5 ℃ 40 s,72 ℃ 40 s,30个循环;72 ℃ 10 min。根据保守序列利用Primer Premier 5设计特异性GSP引物(5′IGF3 race、5′IGF3 nest、3′IGF3 race和3′IGF3 nest),利用RACE试剂盒进行全长基因克隆。本实验所用引物见表 1。
|
|
表 1 本研究所用引物 Tab.1 Primers used in the present study |
克隆所得全长cDNA序列在NCBI数据库中进行BLAST (http://blast.ncbi.nlm.nih.gov)比对和同源性分析,利用ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/)推断开放阅读框和氨基酸序列。ExPASy (https://web.expasy.org/protparam/)预测蛋白理化性质。SMART (smart.embl-heidelberg.de)预测序列信号肽结构和保守结构域。DNAMAN对氨基酸序列进行多重比对。采用邻近法(Neighbor-joining, NJ),通过MEGA5.0软件构建系统发育树。其他鱼类IGFs氨基酸序列下载自NCBI (表 2),使用SWISS-MODEL (https://swissmodel.expasy.org/interactive)构建三级结构模型。IGF1R (code 5U8R)和IGF2R (code 6UM2)三级结构从Protein Data Bank (PDB) (https://www.rcsb.org/)下载获得。使用Vakser Lab (https://vakserlab.ku.edu/)预测IGF3和IGFRs的结合位点,并采用Discovery Studio 4.5软件进行可视化修饰。
|
|
表 2 不同物种IGF分子氨基酸序列GenBank登录号 Tab.2 The GenBank number of different species IGFs |
使用实时荧光定量PCR (qRT-PCR)检测igf3在大菱鲆不同组织和卵巢发育过程中的表达。根据克隆所得全长CDS序列,使用Primer Premier5软件设计特异性引物(IGF3-F和IGF3-R),参考本实验室之前研究,选用β-actin为内参基因(Gao et al, 2020),利用ABI7500实时荧光定量PCR仪(Applied Biosystems, 美国)进行qRT-PCR。采用2–ΔΔCt法(Livak et al, 2001)计算igf3在不同组织和卵巢发育中的相对表达水平。每个样品进行3次重复实验。所得数据采用SPSS 22.0软件进行单因素方差分析(one-way ANOVA),用Duncan法进行组间多重比较,所有结果用平均值±标准误(Mean±SE)的方式表示,P < 0.05表示差异显著。
2 结果与分析 2.1 大菱鲆IGF3全长cDNA克隆与序列分析通过RACE克隆技术获得大菱鲆IGF3全长cDNA序列,共1 255 bp,其中,780 bp (12~791)为CDS区,编码259个氨基酸,N末端有33个氨基酸组成的信号肽序列,随后是59个氨基酸(35~93)组成的IGF特异性结构域,序列已上传NCBI,序列登录号为OP028637 (图 1)。物理化学性质预测结果显示,IGF3分子式为C1133H1799N349O356S12,分子量为26.39 kDa,总平均亲水性为–0.368,疏水等电点为8.31。
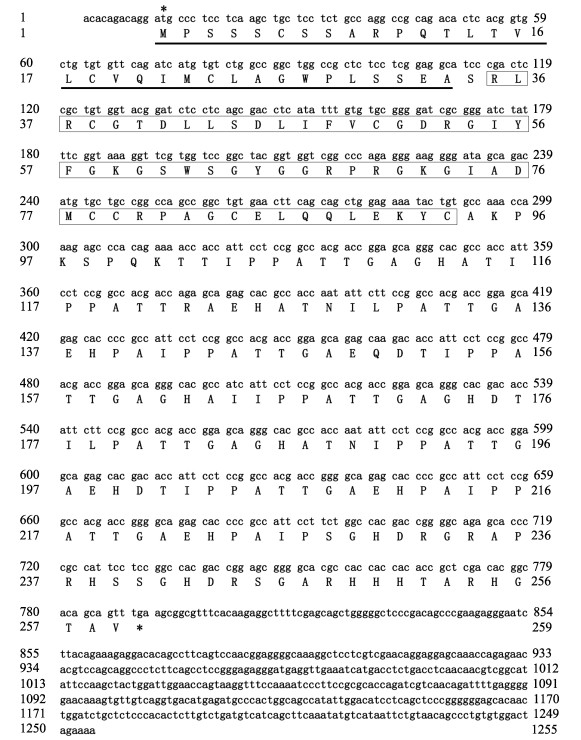
|
图 1 大菱鲆igf3 cDNA全长序列及其编码氨基酸序列
Fig.1 The turbot igf3 cDNA full-length sequence and its encoding amino acid sequence
*:起始密码子和终止密码子;_______:信号肽序列; :IGF特异性结构域
*: Start codon and stop codon; _______: Signal peptide sequence; :IGF特异性结构域
*: Start codon and stop codon; _______: Signal peptide sequence;  : IGF-specific domain : IGF-specific domain
|
通过DNAMAN对大菱鲆IGF3氨基酸序列与其他鱼类已知序列进行比对,并根据NJ法构建IGFs系统发育树。序列对比结果显示,所有鱼类IGF3在氨基端有一个高度保守的结构域,可划分为B、C、A和D四部分,大菱鲆与所有鱼类在该结构域高度同源,其中,大菱鲆与斑马鱼、翘嘴鲌、青鳉(Oryzias latipes)、鲻(Mugil cephalus)、大西洋鲑(Salmo salar)、尼罗罗非鱼、鲤鱼(Cyprinus carpio)和狭鳞庸鲽(Hippoglossus stenolepis)IGF3序列相似度分别为17.04%、16.73%、25.66%、30.19%、16.67%、28.30%、17.45%和37.45% (图 2)。系统发育树分析发现,不同IGFs家族成员分为IGF1,IGF2和IGF3三个分支簇,其中, IGF3分支又可分为2个分支,大西洋鲑、斑马鱼、鲤鱼和翘嘴鲌同属一支,而青鳉、尼罗罗非鱼、鲻、大菱鲆和狭鳞庸鲽同属一支,其中,大菱鲆同狭鳞庸鲽同源性最高(图 3)。
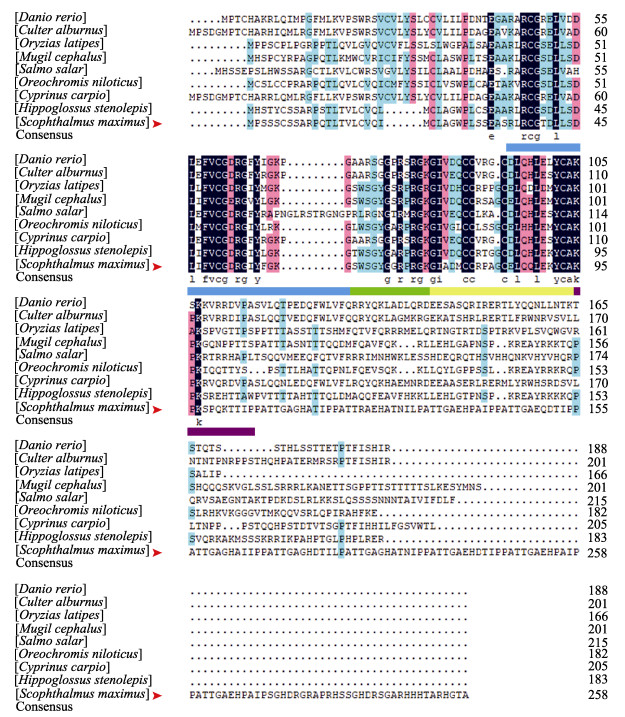
|
图 2 大菱鲆与其他物种IGF3氨基酸序列比对 Fig.2 Comparison of IGF3 amino acid sequences of turbot and other species 洋红色表示匹配度 > 70%,深蓝色表示序列完全匹配。大菱鲆IGF3氨基酸序列用红色箭头标注。蓝色下划线为结构域B,绿色下划线为结构域C,黄色下划线为结构域A,紫色下划线为结构域D。 Position with > 70% similarity are shaded in magenta, while completely conserved positions are shaded in navy. Turbot IGF3 is marked with a red arrow. Domain B is underlined in blue, domain C is underlined in green, domain A is underlined in yellow, and domain D is underlined in purple. |
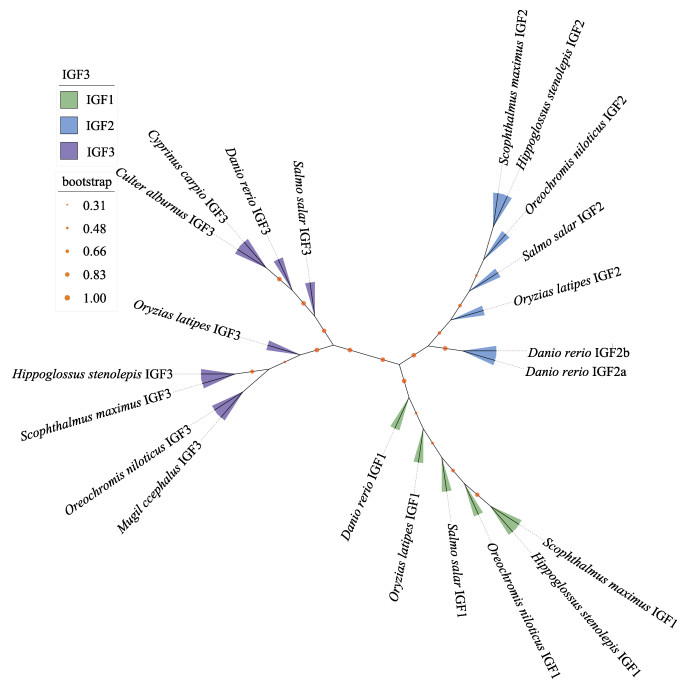
|
图 3 IGF3氨基酸序列NJ系统进化树 Fig.3 The NJ phylogenetic tree of IGF3 amino acid sequence 橙色节点代表 1 000自展(Bootstrap)检验置信值;红色箭头为大菱鲆。 Orange node indicates the percentage of 1 000 bootstrap replicates; Red arrow: Turbot. |
根据大菱鲆IGF3序列利用SWISS-MODEL构建三级结构模型,结果发现,大菱鲆IGF3由3个α螺旋串联而成,并且通过弯曲盘绕使得3个α螺旋区空间位置相互靠近(图 4)。在PDB数据库下载IGF1R和IGF2R三级结构,利用Vakser Lab预测IGF3与受体的对接模型。结果显示,大菱鲆IGF3的α螺旋区可同IGF-1R和IGF-2R结合,且有多个连接键位,其中,与IGF-1R末端结构域结合,与IGF-2R中心区域多个结构域结合(图 4)。
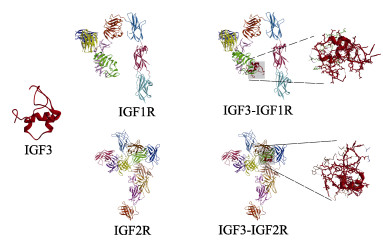
|
图 4 大菱鲆IGF3三级结构以及受体结合预测 Fig.4 Tertiary structure of turbot IGF3 and docking prediction with IGF1R and IGF2R IGF-Rs不同结构域用不同颜色标注,大菱鲆IGF3用红色标记。 Different domains of IGF-Rs are marked with different colors, and turbot IGF3 is marked in red. |
通过观察雌性大菱鲆[10月龄,(800±150) g]和雄性大菱鲆[10月龄,(700±150) g]性腺组织形态学结构,确定其发育阶段,利用qRT-PCR技术检测不同性别大菱鲆各组织igf3相对表达水平。组织形态学观察结果显示,大菱鲆精巢由精小囊组成,精小囊内主要为初级精母细胞和次级精母细胞;卵巢内卵母细胞紧密排列,核仁位于生发泡周围;表明大菱鲆处于性腺发育早期(图 5B)。组织相对表达分析发现,igf3在不同性别大菱鲆中表达水平不同,在肝、肾、胃、性腺和脑中雄性大菱鲆中表达量显著高于雌性(P < 0.05),在心、肠和垂体中,雌性大菱鲆表达量显著高于雄性(P < 0.05);在脾中不同性别大菱鲆igf3表达量差异不显著(P > 0.05);在雄性大菱鲆各组织中,igf3在脑中表达量最高,且显著高于其他组织(P < 0.05),在雌性大菱鲆各组织中,igf3在脑和肠中表达量最高,显著大于其他组织(P < 0.05) (图 5A)。
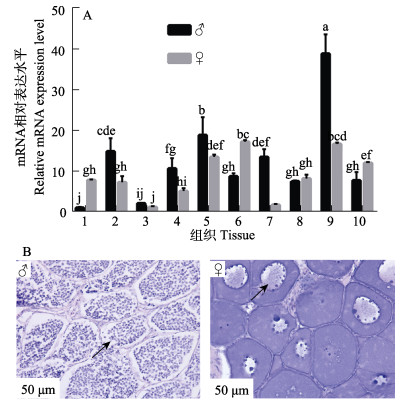
|
图 5 大菱鲆igf3 mRNA在不同组织中相对表达(A)及性腺组织结构(B)
Fig.5 The relative expression of igf3 mRNA in different tissues (A) and the histologic of gonadal (B) of turbot
1:心;2:肝;3:脾;4:肾;5:胃;6:肠;7:性腺;8:鳃;9:脑;10:垂体。 ♂:雄性,箭头指示精小囊内初级精母细胞和次级精母细胞;♀:雌性,箭头指示卵母细胞核位于生发泡外围。 不同小写字母表示差异显著(P < 0.05)。 1: Heart; 2: Liver; 3: Spleen; 4: Kidney; 5: Stomach; 6: Intestine; 7: Gonad; 8: Gills; 9: Brain; 10: Pituitary. ♂: Male, arrows indicate that the seminal vesicles are dominated by primary spermatoblasts and secondary spermatoblasts; ♀: Female, the arrow indicates the nucleoli are on the periphery of the germinal vesicle. Different lowercase letters indicate significant differences (P < 0.05). |
组织形态学观察确定不同规格雌性大菱鲆卵巢发育阶段,组织表达分析igf3在卵巢发育过程中的mRNA水平变化。结果显示,大菱鲆卵巢分为5个不同时期,卵黄生成前期,核仁位于生发泡外围;卵黄生成早期,卵黄颗粒在中部累积,细胞质出现滤泡;卵黄生成晚期,卵黄颗粒几乎充满卵母细胞,细胞核还未开始转移;核迁移期,卵黄充满整个卵母细胞,细胞核不明显;闭锁期,卵母细胞开始收缩和塌陷(图 6B)。mRNA相对表达分析发现,大菱鲆igf3表达量随着卵巢发育过程变化,在卵黄生成后期显著上升(P < 0.05),在核迁移期继续升高且达到最大值,在闭锁期显著降低(P < 0.05) (图 6A)。
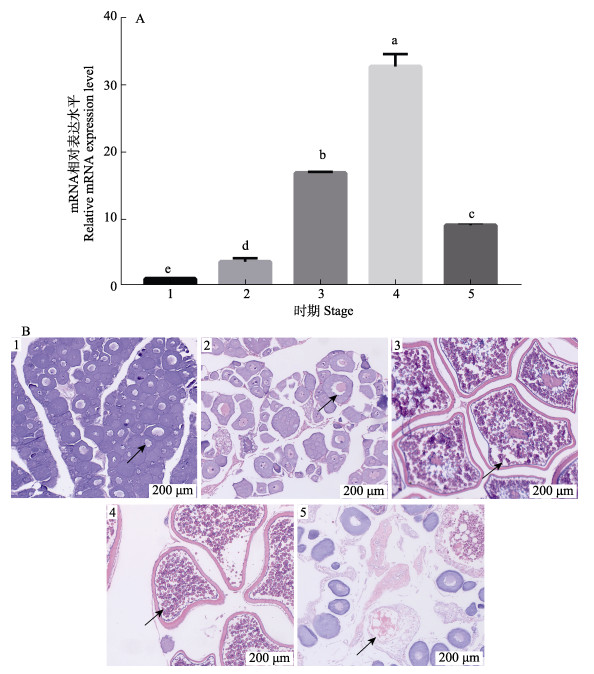
|
图 6 大菱鲆igf3 mRNA在卵巢发育过程中相对表达(A)及卵巢组织学变化(B) Fig.6 The relative expression of igf3 mRNA (A) and the histologic of ovarian (B) during turbot ovarian development 1:卵黄生成前期,箭头指示核仁位于生发泡外围;2:卵黄生成早期,细胞质出现滤泡,卵黄开始积累;3:卵黄生成晚期,箭头指示卵黄颗粒几乎充满细胞,细胞核还未迁移;4:核迁移期,卵黄颗粒充满细胞,细胞核已经迁移;5:闭锁期,箭头指示卵母细胞收缩塌陷。不同小写字母表示差异显著(P < 0.05)。 1: Previtellogenesis, arrows indicate the nucleoli are on the periphery of the germinal vesicle; 2: Early vitellogenesis, arrows indicate gradually accumulations of yolk granules in the central region 3: Late vitellogenesis, arrows indicate the yolk granules almost fill the ooplasm, and the nucleus has not yet begun to migrate peripherally; 4: Migratory nucleus, arrows indicate the yolk granules have attained their maximum size just prior to spawning, and the nucleus is not evident; 5: Atresia, arrows indicate the oocyte shrinkage and collapse. Different lowercase letters indicate significant differences (P < 0.05). |
IGFs是一类和胰岛素高度同源的生长因子蛋白,由5个保守结构域B、C、A、D和E组成(Sachdev et al, 2001),作为分泌性蛋白,经过转录翻译修饰后需利用信号肽转运到细胞外,通过内分泌、旁分泌和自分泌的方式参与调控生命活动(Laviola et al, 2007; Vincent et al, 2002)。本研究结果显示,大菱鲆IGF3总平均亲水系数为–0.368,N末端有33个氨基酸组成的信号肽序列。亲水性有利于IGF3在体液中运输,而信号肽序列是蛋白翻译修饰后转运到胞外所必需的结构(Izard et al, 1995)。序列比较结果显示,大菱鲆IGF3与其他鱼类只在B、C、A和D 4个保守结构域相似度较高,而总体相似度较低,这可能是因为目前已知IGF3序列来自与大菱鲆亲缘性较远的物种,且保守结构域在整个蛋白中占比较低。IGFs分子的结构域E会在形成成熟肽时被切除,因此,同源性较低,难以根据氨基酸序列划分,高同源性的IGF特异性结构域也表明,这是IGFs分子发挥功能必备的结构(Li J et al, 2021)。物种发育树结果显示,所有鱼类IGF3单独聚为一支,且根据物种亲缘性可划分为多个小分支,大菱鲆IGF3与狭鳞庸鲽同源性最高。可见IGF3系统进化关系同物种分类高度一致,大菱鲆在进化关系上与狭鳞庸鲽最接近。以上结果表明,大菱鲆IGF3具有典型的IGFs家族结构特征,为高度保守的新型大菱鲆IGF分子,作为分泌型蛋白发挥其生理调控功能。
IGFs主要通过与其特异性受体(IGF-1R, IGF-2R)结合,诱导细胞内信号级联反应,发挥其生理调控功能(Denley et al, 2005)。IGF-1R属于酪氨酸激酶受体,与配体IGFs结合后可激活PI3信号通路和MAPK信号通路(Chitnis et al, 2008)参与多种细胞功能调节。IGF-2R属于甘露糖6-磷酸受体,被认为是生长抑制因子,主要与IGF2结合发挥功能,并且参与溶酶体的形成和细胞内吞作用(Brown et al, 2008)。蛋白质在转录翻译后还需要折叠成正确的三级结构才能发挥特定的生物学功能,通过预测蛋白质的三级结构以及其对接模型有助于探究其作用机制(Eswar et al, 2008; Bonvin, 2006)。本研究发现,大菱鲆IGF3的三级结构由3个α螺旋串联而成,其α螺旋结构域可分别与IGF-1R的末端结构域以及IGF-2R的中心区域结合,且有多个连接位点。人类IGF1的三级结构已被解析,与上述结果相似(Vajdos et al, 2001)。此外,人类IGF1与IGF-1R的对接结果(Xu et al, 2018)以及牛的IGF2与IGF-2R对接结果(Wang et al, 2020)也与大菱鲆IGF3对接预测位置类似。以上结果表明,大菱鲆IGF3与其他IGFs分子高度同源,拥有相似的三级结构,可通过IGF信号系统发挥相应生物学功能。
在斑马鱼和尼罗罗非鱼的研究中发现,IGF3为性腺特异性表达蛋白,参与性别决定和分化过程(Wang et al, 2008),同时,igf3在斜带石斑鱼(Yang et al, 2015)、鲤鱼(Song et al, 2016)、圆尾金翅鲷(Chrysiptera cyanea) (Li Z et al, 2021)和金钱鱼(Scatophagus argus)(Rizky et al, 2020)性腺外多个组织广泛分布。本研究发现,igf3在性腺发育早期雌、雄大菱鲆各组织中均有表达,且呈现不同表达规律,但均在脑中表达最高,这表明与大多数鱼类一样,大菱鲆IGF3具有广泛的组织分布,在脑中的高水平表达预示其可能参与了更为复杂的神经内分泌调控。脑是控制中心,通过下丘脑–垂体–性腺(HPG)轴与GH (growth hormone)/IGF轴调控脊椎动物的生长和繁殖过程(Servili et al, 2020; 徐永江等, 2017)。在哺乳动物脑中发现IGF1与雌激素协同参与生殖功能调节(Quesada et al, 2002)。此外,Margaret等(2002)发现,外源GH可以提高幼年鲤鱼脑中igf1和igf2的表达水平。在草鱼中发现,igf3在下丘脑和小脑中高表达,可能参与生长摄食调控(Yang et al, 2022)。林权卓等(2016)发现,随着性腺发育,igf3在不同性别双棘黄姑鱼脑中的表达变化规律不同,脑可能参与了igf3在性腺表达的反馈调节。此外,本研究还发现,IGF3分别在雌、雄大菱鲆肠组织和胃组织中高表达;对罗非鱼和金头鲷(Sparus aurata)的研究发现,IGF1和IGF2在肠道渗透调节以及消化道早期发育过程中发挥重要功能(Link et al, 2010; Perrot et al, 1999)。IGF3作为新型IGFs分子,可能在大菱鲆肠道中也扮演重要角色。以上结果表明,IGF3在性腺发育早期大菱鲆组织中广泛分布,存在显著性别二态性差异,可能通过HPG轴参与调控生长繁殖过程,同时作为重要的局域性内分泌因子,在维系胃肠道正常生理功能过程中发挥重要作用。
IGF3作为鱼类性腺特异性分子,在卵母细胞成熟过程中扮演着重要角色(Li J et al, 2021)。在斑马鱼的研究中已经证明IGF3受到LH的调控,并且通过IGF-1R信号系统参与调控卵母细胞成熟过程(Li et al, 2011; Li et al, 2015)。大多数鱼类的卵母细胞会在第一次减数分裂前期的双线期停滞,直到细胞内积累足够的卵黄后,随着体内促性腺激素水平的上调,恢复减数分裂过程(Patiño et al, 2002)。根据卵母细胞组织形态可将大菱鲆卵巢划分为5个不同发育时期:卵黄生成前期、卵黄生成早期、卵黄生成晚期、核迁移期和闭锁期(Jia et al, 2014)。本研究发现,igf3表达水平在大菱鲆卵巢卵黄生成后期显著上升,在核迁移期继续上升达到最大值,随后在闭锁期降低。在金钱鱼和圆尾金翅雀鲷的卵巢发育过程中也发现igf3转录水平在卵黄生成后期显著上调(Rizky et al, 2020; Li Z et al, 2021)。对青鳉的研究发现,igf3转录水平随着卵母细胞成熟逐渐上升,并且原位杂交实验结果证实其主要分布于卵黄生成后期和核迁移期卵巢内的颗粒细胞和膜细胞中(Xie et al, 2020)。大菱鲆igf3在卵黄生成后期和核迁移期卵巢内显著高表达,预示其在恢复卵母细胞减数分裂、促进卵母细胞成熟过程中发挥重要功能。在一些养殖鱼类中已经发现,IGF1和IGF2的转录水平会随着卵子老化而显著下调,可以作为评估卵子质量的指标(Sullivan et al, 2015; Aegerter et al, 2005)。IGF3作为参与调控卵母细胞成熟的重要IGFs家族成员,进一步探明其作用机制,可为评估卵子质量提供新的研究思路。
4 结论综上所述,本研究首次克隆了大菱鲆IGF3全长cDNA序列,通过生物信息学分析验证了其序列保守性,并初步预测了其编码蛋白的功能、三级结构以及与IGF受体的结合模型,同时检测了其在性腺发育早期大菱鲆各组织中的分布和卵巢各发育阶段的表达水平。结果发现,大菱鲆IGF3 cDNA全长序列为1 255 bp,编码259个氨基酸,同狭鳞庸鲽同源性最高;蛋白质三级结构域由3个α螺旋串联而成,可同IGF-1R和IGF-2R紧密结合;igf3在大菱鲆各个组织中均有表达分布,且呈显著性别二态性;在卵巢发育过程中,igf3表达水平随着卵母细胞成熟逐渐升高,在排卵后下降。相关结果为研究IGF3在大菱鲆卵巢发育和卵母细胞成熟过程中的作用机制奠定了基础,同时也为探究鱼类IGF3功能提供了重要参考。
AEGERTER S, JALABERT B, BOBE J. Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post-ovulatory ageing. Molecular Reproduction and Development: Incorporating Gamete Research, 2005, 72(3): 377-385 |
ALVARIÑO J M R, REBOLLAR P G, OLMEDO M, et al. Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquaculture International, 2001, 9(6): 477-487 DOI:10.1023/A:1020590111031 |
ANNUNZIATA M, GRANATA R, GHIGO E. The IGF system. Acta Diabetologica, 2011, 48(1): 1-9 DOI:10.1007/s00592-010-0227-z |
BERISHVILI G, BAROILLER J F, EPPLER E, et al. Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: Development and regulation of gene expression by growth hormone (GH) and 17 α-ethinylestradiol (EE2). General and Comparative Endocrinology, 2010, 167(1): 128-134 DOI:10.1016/j.ygcen.2010.01.023 |
BONVIN A M J J. Flexible protein-protein docking. Current Opinion in Structural Biology, 2006, 16(2): 194-200 DOI:10.1016/j.sbi.2006.02.002 |
BROWN J, DELAINE C, ZACCHEO O J, et al. Structure and functional analysis of the IGF-Ⅱ/IGF2R interaction. The EMBO Journal, 2008, 27(1): 265-276 DOI:10.1038/sj.emboj.7601938 |
CANOSA L F, BERTUCCI J I. Nutrient regulation of somatic growth in teleost fish: The interaction between somatic growth, feeding and metabolism. Molecular and Cellular Endocrinology, 2020, 518(1): 1-18 |
CHITNIS M M, YUEN J S P, PROTHEROE A S, et al. The type 1 insulin-like growth factor receptor pathway. Clinical Cancer Research, 2008, 14(20): 6364-6370 DOI:10.1158/1078-0432.CCR-07-4879 |
DUVAL H, ROUSSEAU K, ELIES G, et al. Cloning, characterization, and comparative activity of turbot IGF-Ⅰ and IGF-Ⅱ. General and Comparative Endocrinology, 2002, 126(3): 269-278 DOI:10.1016/S0016-6480(02)00002-3 |
DENLEY A, COSGROVE L J, BOOKER G W, et al. Molecular interactions of the IGF system. Cytokine and Growth Factor Reviews, 2005, 16(4/5): 421-439 |
ESWAR N, ERAMIAN D, WEBB B, et al. Protein structure modeling with MODELLER structural proteomics. Humana Press, 2008, 145-159 |
GAO Y H, GAO Y T, HUANG B, et al. Reference gene validation for quantification of gene expression during ovarian development of turbot (Scophthalmus maximus). Scientific Reports, 2020, 10(1): 1-10 DOI:10.1038/s41598-019-56847-4 |
GAO Y H, JING Q Q, HUANG B, et al. Molecular cloning, characterization, and mRNA expression of gonadotropins during larval development in turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 2019, 45(5): 1697-1707 DOI:10.1007/s10695-019-00656-z |
GU W W, YANG Y, NING C, et al. Identification and characteristics of insulin-like growth factor system in the brain, liver, and gonad during development of a seasonal breeding teleost, Pampus argenteus. General and Comparative Endocrinology, 2021, 11(12): 1-10 |
HASANPOOR S, GHOMI MARZDASHTI M R, VAHABZADEH ROODSARI H, et al. IGF-Ⅰ gene expression in liver and white muscles confirming promotion effect of dietary NaCl on growth indices of giant sturgeon (Huso huso) juveniles. Iranian Journal of Fisheries Sciences, 2020, 19(5): 2634-2648 |
HU P, MENG Z, JIA Y D. Molecular characterization and quantification of estrogen receptors in turbot (Scophthalmus maximus). General and Comparative Endocrinology, 2018, 257: 38-49 DOI:10.1016/j.ygcen.2017.01.003 |
HUANG B, WANG N, WANG L, et al. Vitamin E stimulates the expression of gonadotropin hormones in primary pituitary cells of turbot (Scophthalmus maximus). Aquaculture, 2019, 509: 47-51 DOI:10.1016/j.aquaculture.2019.05.023 |
IMSLAND A K, DRAGSNES M, STEFANSSON S O. Exposure to continuous light inhibits maturation in turbot (Scophthalmus maximus). Aquaculture, 2003, 219(1/2/3/4): 911-919 |
IZARD J W, DOUGHTY M B, KENDALL D A. Physical and conformational properties of synthetic idealized signal sequences parallel their biological function. Biochemistry, 1995, 34(31): 9904-9912 DOI:10.1021/bi00031a012 |
JIA Y D, JING Q Q, GAO Y H, et al. Involvement and expression of growth hormone/insulin-like growth factor member mRNAs in the ovarian development of turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 2019, 45(3): 955-964 DOI:10.1007/s10695-018-0604-z |
JIA Y D, MENG Z, LIU X, et al. Molecular components related to egg quality during the reproductive season of turbot (Scophthalmus maximus). Aquaculture Research, 2015a, 46(10): 2565-2572 DOI:10.1111/are.12406 |
JIA Y D, MENG Z, NIU H, et al. Molecular cloning, characterization, and expression analysis of luteinizing hormone receptor gene in turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 2014, 40(6): 1639-1650 DOI:10.1007/s10695-014-9954-3 |
JIA Y D, NIU H X, MENG Z, et al. Biochemical composition of the ovarian fluid and its effects on the fertilization capacity of turbot Scophthalmus maximus during the spawning season. Journal of Fish Biology, 2015b, 86(5): 1612-1620 DOI:10.1111/jfb.12676 |
JIA Y D, SUN A, MENG Z, et al. Molecular characterization and quantification of the follicle-stimulating hormone receptor in turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 2016, 42(1): 179-191 DOI:10.1007/s10695-015-0128-8 |
LAVENS P, LEBEGUE E, JAUNET H, et al. Effect of dietary essential fatty acids and vitamins on egg quality in turbot broodstocks. Aquaculture International, 1999, 7(4): 225-240 DOI:10.1023/A:1009225028889 |
LAVIOLA L, NATALICCHIO A, GIORGINO F. The IGF-Ⅰ signaling pathway. Current Pharmaceutical Design, 2007, 13(7): 663-669 DOI:10.2174/138161207780249146 |
LI J A, CHU L H, SUN X, et al. IGFs mediate the action of LH on oocyte maturation in zebrafish. Molecular Endocrinology, 2015, 29(3): 373-383 DOI:10.1210/me.2014-1218 |
LI J Z, LIU Z H, WANG D S, et al. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biology of Reproduction, 2011, 84(3): 476-486 DOI:10.1095/biolreprod.110.086363 |
LI J Z, LIU Z Q, KANG T, et al. Igf3: A novel player in fish reproduction. Biology of Reproduction, 2021, 104(6): 1194-1204 DOI:10.1093/biolre/ioab042 |
LI J Z, NIU C Y, CHENG C H K. Igf3 serves as a mediator of luteinizing hormone in zebrafish ovulation. Biology of Reproduction, 2018, 99(6): 1235-1243 DOI:10.1093/biolre/ioy143 |
LI Q H, SUN J, LI Y Z, et al. Effects of nutrition enhancement, light and temperature control on gonad development and ovum quality in Scophthalmus maximus spawner. Journal of Southern Agriculture, 2013, 44(6): 1030-1035 [李庆华, 孙建, 李仰真, 等. 营养强化和控光控温对大菱鲆亲鱼性腺发育及卵子质量的影响. 南方农业学报, 2013, 44(6): 1030-1035] |
LI Z Y, REN X L, GUO Y W, et al. Identification and ovarian developmental regulation of insulin-like growth factor 3 in spotted scat (Scatophagus argus). Aquaculture Reports, 2021, 21: 1-10 |
LIN Q Z, CHEN Y B, HU J, et al. IGF3 gene cloning and expression pattern of Protonibea diacanthus. South China Fisheries Science, 2016, 12(1): 50-58 [林权卓, 陈奕彬, 胡娟, 等. 双棘黄姑鱼IGF3基因克隆及表达模式分析. 南方水产科学, 2016, 12(1): 50-58] |
LINK K, BERISHVILI G, SHVED N, et al. Seawater and freshwater challenges affect the insulin-like growth factors IGF-Ⅰ and IGF-Ⅱ in liver and osmoregulatory organs of the tilapia. Molecular and Cellular Endocrinology, 2010, 327(1/2): 40-46 |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
MARGARET C L, VONG Q P, CHENG C H K, et al. PCR-cloning and gene expression studies in common carp (Cyprinus carpio) insulin-like growth factor-Ⅱ. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression, 2002, 1575(1/2/3): 63-74 |
MENG Z, HU P, LEI J, et al. Expression of insulin-like growth factors at mRNA levels during the metamorphic development of turbot (Scophthalmus maximus). General and Comparative Endocrinology, 2016, 235: 11-17 DOI:10.1016/j.ygcen.2016.05.027 |
National Marine Fish Industry Technology Research and Development Center. Annual report 2021 of China agriculture research system for marine fish culture industry. Qingdao: China Ocean University Press, 2021: 12 [国家海水鱼产业技术研发中心. 国家海水鱼产业技术体系年度报告2021. 青岛: 中国海洋大学出版社, 2021: 12]
|
PATIÑO R, SULLIVAN C V. Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiology and Biochemistry, 2002, 26(1): 57-70 DOI:10.1023/A:1023311613987 |
PERROT V, MOISEEVA E B, GOZES Y, et al. Ontogeny of the insulin-like growth factor system (IGF-Ⅰ, IGF-Ⅱ, and IGF-1R) in gilthead seabream (Sparus aurata): Expression and cellular localization. General and Comparative Endocrinology, 1999, 116(3): 445-460 DOI:10.1006/gcen.1999.7337 |
POLAT H, OZTURK R C, TERZI Y, et al. Effect of photoperiod manipulation on spawning time and performance of turbot (Scophthalmus maximus). Aquaculture Research, 2021, 21(3): 109-115 |
QIN Q, CHEN X H, ZHU X Q, et al. Insulin-like growth factor Ⅰ of yellow catfish (Pelteobagrus fulvidraco): cDNA characterization, tissue distribution, and expressions in response to starvation and refeeding. Fish Physiology and Biochemistry, 2020, 46(1): 177-186 DOI:10.1007/s10695-019-00707-5 |
QUESADA A, ETGEN A M. Functional interactions between estrogen and insulin-like growth factor-Ⅰ in the regulation of α1B-adrenoceptors and female reproductive function. Journal of Neuroscience, 2002, 22(6): 2401-2408 DOI:10.1523/JNEUROSCI.22-06-02401.2002 |
RIZKY D, MAHARDINI A, BYUN J, et al. Molecular cloning of insulin-like growth factor 3 (igf3) and its expression in the tissues of a female damselfish, Chrysiptera cyanea, in relation to seasonal and food-manipulated reproduction. General and Comparative Endocrinology, 2020, 295(1): 1-11 |
ROSE T M, HENIKOFF J G, HENIKOFF S. CODEHOP (COnsensus-DEgenerate Hybrid Oligonucleotide Primer) PCR primer design. Nucleic Acids Research, 2003, 31(13): 3763-3766 |
SACHDEV D, YEE D. The IGF system and breast cancer. Endocrine-Related Cancer, 2001, 8(3): 197-209 |
SERVILI A, CANARIO A V M, MOUCHEL O, et al. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. General and Comparative Endocrinology, 2020, 291: 113439 |
SONG F B, WANG L M, ZHU W B, et al. A novel igf3 gene in common carp (Cyprinus carpio): evidence for its role in regulating gonadal development. PLoS One, 2016, 11(12): 1-17 |
SULLIVAN C V, CHAPMAN R W, READING B J, et al. Transcriptomics of mRNA and egg quality in farmed fish: Some recent developments and future directions. General and Comparative Endocrinology, 2015, 221: 23-30 |
TRIANTAPHYLLOPOULOS K A, CARTAS D, MILIOU H. Factors influencing GH and IGF-Ⅰ gene expression on growth in teleost fish: How can aquaculture industry benefit. Reviews in Aquaculture, 2020, 12(3): 1637-1662 |
VAJDOS F F, ULTSCH M, SCHAFFER M L, et al. Crystal structure of human insulin-like growth factor-1: Detergent binding inhibits binding protein interactions. Biochemistry, 2001, 40(37): 11022-11029 |
VINCENT A M, FELDMAN E L. Control of cell survival by IGF signaling pathways. Growth Hormone and IGF Research, 2002, 12(4): 193-197 |
WANG D S, JIAO B W, HU C J, et al. Discovery of a gonad-specific IGF subtype in teleost. Biochemical and Biophysical Research Communications, 2008, 367(2): 336-341 |
WANG R, QI X F, SCHMIEGE P, et al. Marked structural rearrangement of mannose 6-phosphate/IGF2 receptor at different pH environments. Science Advances, 2020, 6(7): eaaz1466 |
WEN H, QI Q, HU J, et al. Expression analysis of the insulin-like growth factors Ⅰ and Ⅱ during embryonic and early larval development of turbot (Scophthalmus maximus). Journal of Ocean University of China, 2015, 14: 309-316 |
XIE J L, ZHONG Y, ZHAO Y L, et al. Characterization and expression analysis of gonad specific igf3 in the medaka ovary. Aquaculture and Fisheries, 2020, 7(3): 259-268 |
XIE T, GAO Y T, LI M Y, et al. Effect of three different anticoagulants on blood cell morphology, anticoagulation, and hematological parameters in turbot (Scophthalmus maximus). Progress in Fishery Sciences, 2023, 44(2): 68-76 [谢婷, 高云涛, 李明月, 等. 3种抗凝剂对大菱鲆血液抗凝效果、血细胞形态和血液生理生化指标影响. 渔业科学进展, 2023, 44(2): 68-76] |
XU Y B, KONG G K W, MENTING J G, et al. How ligand binds to the type 1 insulin-like growth factor receptor. Nature Communications, 2018, 9(1): 1-13 |
XU Y J, LIU X Z, SHI Y, et al. Physiological role of GH/IGF-Ⅰ axis in ovarian development of Cynoglossus semilaevis. Progress in Fishery Sciences, 2017, 38(1): 73-80 [徐永江, 柳学周, 石莹, 等. GH/IGF-Ⅰ轴对半滑舌鳎(Cynoglossus semilaevis)卵巢发育的调控作用. 渔业科学进展, 2017, 38(1): 73-80] |
YANG G K, LIANG X M, XU S Y, et al. Molecular identification of Igf3 and its roles in grass carp (Ctenopharyngodon idella). Aquaculture, 2022, 548(1): 1-10 |
YANG H R, CHEN H P, ZHAO H H, et al. Molecular cloning of the insulin-like growth factor 3 and difference in the expression of igf genes in orange-spotted grouper (Epinephelus coioides). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2015, 186: 68-75 |
ZHAO S S. Identification, expression and function in gonadal development of igf3 gene in Scophthalmus maximus. Master´s Thesis of Shanghai Ocean University, 2022 [赵珊珊. 大菱鲆igf3基因的鉴定、表达及在性腺发育中的功能研究. 上海海洋大学硕士研究生学位论文, 2022]
|
ZHENG J B, JIA Y Y, CAI L N, et al. Clone and expression analysis of insulin-like growth factor3 (IGF3) gene in Culter alburnus. Acta Hydrobiologica Sinica, 2021, 45(4): 734-740 [郑建波, 贾永义, 蔡李娜, 等. 翘嘴鲌胰岛素样生长因子igf3基因的克隆及表达分析. 水生生物学报, 2021, 45(4): 734-740] |



