2. 中国水产科学研究院黄海水产研究所 海洋渔业科学与食物产出过程功能实验室 农业农村部海水养殖病害防治重点实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Laboratory for Marine Fisheries Science and Food Production Processes, Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs, Qingdao 266071, China
美洲鲥鱼(Alosa sapidissima)又称美洲鲱鱼,隶属于鲱形总目(Clupeomorpha)、鲱形目(Clupeiformes)、鲱科(Clupeidae)、鲥属(Alosa),是世界上个体最长、生长速度最快的鲥鱼之一(高小强等, 2015)。1998年,美洲鲥鱼率先被上海市水产研究所引入我国进行人工繁殖(施永海等, 2019)。由于美洲鲥鱼肉质鲜美,外形与长江鲥鱼(Tenualosa reevesii)相似,受到人们的广泛喜爱,因此,美洲鲥鱼人工养殖迅速在多省市开展(Jia et al, 2007; 高小强等, 2017)。随着美洲鲥鱼养殖规模的逐步扩大,病害问题也逐渐严峻,然而,国内目前养殖美洲鲥鱼的病害报道较少,仅有水霉病(董亚伦等, 2015)、应激性出血病(肝胆病)、小瓜虫病(白点病)、粘孢子虫病、车轮虫病(潘庭双等, 2008)等,其中以寄生虫病报道最多,细菌性病害研究较少。
嗜水气单胞菌(Aeromonas hydrophila)属于气单胞菌科(Aeromonadaceae)、气单胞菌属(Aeromonas)。作为一种传统水生动物病原,从20世纪80年代起嗜水气单胞菌在我国已广泛流行,引起多种养殖淡水鱼类病害,包括草鱼(Ctenopharyngodon idella) (邓国成等, 2009)、鲢鱼(Hypophthalmichthys molitris) (高汉娇等, 1997)、鲤鱼(Cyprinus carpio) (张玉芬等, 2008)、鲫鱼(Carassius auratus) (刘亚楠等, 2012)、罗非鱼(Oreocheomis niloticus) (董忠典等, 2012)、团头鲂(Megalobrama amblycephala) (田甜等, 2010)、白斑狗鱼(Esox lucius) (秦莉等, 2014)、黄颡鱼(Pelteobagrus fulvidraco) (黄钧等, 2012; 梁正生等, 2012)和翘嘴鳜(Siniperca chuatsi) (朱鑫海等, 2022)等,造成严重的经济损失。嗜水气单胞菌在分类地位上与其他气单胞菌相似,检测和鉴定中容易混淆(DebRoy et al, 2006; Kawada et al, 2008),在菌种鉴定和基因分型方面常用到多位点序列分型(multilocus sequence typing, MLST)或者平均核苷酸同源性(average nucleotide identity, ANI)分析进行鉴定。
2021年,山东省临沂市一养殖场养殖的美洲鲥鱼出现严重发病情况,患病鱼主要症状为体表出血、溃疡,并迅速死亡,高峰期日死亡率为2.5%,超过1 000尾,给养殖企业带来严重的经济损失。本研究从发病的美洲鲥鱼内脏中分离得到形态一致的优势菌,并通过16S rRNA基因测序、生理生化鉴定和人工感染等实验对病原进行确认和鉴定,以期为美洲鲥鱼的养殖和病害防控提供参考数据。
1 材料与方法 1.1 病鱼来源2021年6月,山东省临沂市郯城县一养殖场养殖的美洲鲥鱼大规模发病死亡,鱼体重为200~400 g。初期养殖苗种4万尾,养殖模式为室内水泥池养殖(幼苗)和室外水泥池塘养殖(养成)。水源为地下水,换水1~3次/d,水温为18~20 ℃。投喂加州鲈(Micropterus salmoides)商品化颗粒饲料,日投喂率为2%左右。室内池塘先于外塘发病,累积死亡率为90%左右,患病鱼典型症状为尾部疥疮或溃烂,前期应用恩诺沙星拌料口服,治疗效果不明显。
1.2 细菌分离取发病鱼的鳃丝、体表粘液、尾鳍和内脏分别在显微镜下观察,检测寄生虫感染情况;取发病鱼的脾脏和肾脏组织冻存于液氮中,按照常规方法检测鲤疱疹病毒2型(Cyprinid herpesvirus 2) (罗丹等, 2014)、鲈鱼蛙病毒(largemouth bass ranavirus) (罗晓雯等, 2022)等淡水鱼常见病毒;从患病鱼的肝脏、脾脏和肾脏取样,划线于TSA和LB平板,在28 ℃培养24 h。将获得的优势菌落进行纯化培养,纯化培养后的细菌用甘油保存于–80 ℃冰箱。
1.3 组织病理学观察选取典型发病症状的美洲鲥鱼,分别取肝脏、脾脏和肾脏切成为1 cm3左右组织块,放置于10~15倍体积的Davidson´s AFA固定液室温固定24 h后,利用70%的乙醇保存。对组织块进行脱水、包埋、切片和HE染色,显微镜观察发病美洲鲥鱼的组织病理变化。
1.4 人工感染实验蓝曼龙(Trichogaster trichopterus)是一种经典的水生动物病原感染模型生物,已被广泛用于嗜水气单胞菌(Leung et al, 1997)、杀鱼爱德华氏菌(Edwardsiella piscicida)的研究中(Ling et al, 2001)。考虑到美洲鲥鱼应激较强,不适合实验室养殖、捕捞和感染等操作,本研究选取蓝曼龙作为实验动物用于毒力评价。蓝曼龙购自青岛市南山花鸟虫鱼市场,平均体长为7~8 cm,暂养在60 L水箱中,养殖水温为25 ℃。感染实验前,随机取5尾鱼麻醉解剖,取肝脏和肾脏,匀浆并涂布于TSA和LB培养基,确定实验鱼未携带病原。将实验鱼随机分为4组,每组30尾,养殖于30 L整理箱,水温为25 ℃。用PBS缓冲液将培养好的AS-AH2101菌液浓度分别调整至1×104、1×105、1×106和1×107 CFU/mL。采用背部肌肉注射法,每尾蓝曼龙注射感染AS-AH2101菌液0.1 mL,对照组注射0.1 mL的PBS缓冲液,饲养于相同条件下,并每天记录各组鱼的发病症状和死亡情况。取感染死亡鱼的肝脏和肾脏,进行病原菌的再次分离和16S rRNA基因测序鉴定。感染14 d后,根据改进的寇氏法(杨茂成, 1990)计算菌株的半数致死量(lethal dose 50%, LD50)。
1.5 病原菌的鉴定和药物敏感性检测利用BIOLOG微生物鉴定系统(美国),采用GenⅢ微孔板试剂条根据说明书对细菌进行生理生化特征鉴定。利用引物27F (5′ AGAGTTTGATCCTGGTC AGAACGAACGCT 3′)和1492R (5′ TACGGCTACCT TGTTACGACTTCACCCC 3′)对分离菌株的16S rRNA基因进行PCR扩增和产物测序(Lane, 1991),将得到的序列在GenBank和EzTaxon数据库中进行同源性比对。从GenBank中选取气单胞菌属的代表性菌株,使用MEGA 7.0软件,采用邻位相连法(Neighbor-Joining)构建系统发育进化树(Bootstrap=1 000)。
采用药敏纸片(杭州微生物试剂有限公司)测定分离株对部分抗生素的耐药性。取培养至OD600 nm= 0.5的菌悬液0.1 mL均匀涂布于MH平板,贴上药敏纸片后将平板在28 ℃培养24 h并测量抑菌圈直径。参照美国临床和实验室标准委员会标准,实验重复3次,通过抑菌圈直径大小判定病原菌对药物的耐药性,分别为耐药(R)、中介(I)和敏感(S)。
1.6 病原菌毒力基因检测针对嗜水气单胞菌的主要毒力因子基因:气溶素(aerA)、溶血素(hlyA)、丝氨酸蛋白酶(ahpA)、热稳定细胞肠毒素(ast)、热敏感细胞肠毒素(altA)和密度感应系统调控基因(luxS),根据文献报道的引物(表 1)和反应条件(朱大玲等, 2006; 付乔芳等, 2011)对毒力基因进行扩增、测序和比对,确定分离菌株的毒力基因携带情况。
|
|
表 1 PCR引物 Tab.1 PCR primers used in this study |
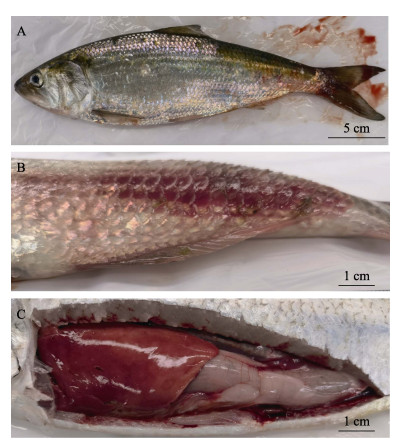
发病美洲鲥鱼的症状包括体表出血,部分鳞片脱落,尾部尤为严重。发病严重时,鱼尾部肿大凸起,内有脓液,呈疥疮病症状,并伴有肠炎和腹水,肝脏暗红坏死,呈败血症症状(图 1);显微镜和肉眼均未观察到寄生虫感染;未检测到鲤疱疹病毒2型、鲈鱼蛙病毒等淡水鱼常见病毒;从10尾发病鱼的肝脏、脾脏、肾脏取样培养,12 h后平板上都得到菌落形态一致的优势菌。16S rRNA基因测序结果显示,所有分离株的序列一致,命名为AS-AH2101。
|
图 1 发病美洲鲥鱼临床症状 Fig.1 The clinical symptoms of diseased A. sapidissima A:尾部渗血;B:尾部肿大凸起,内有脓液,呈疥疮病症状;C:肝脏暗红,呈败血症症状,有肠炎症状。 A: Tail blooding; B: Swollen tail with pus, showing symptoms as furunculosis; C: Dark red liver, showing symptoms of septicemia and enteritis. |
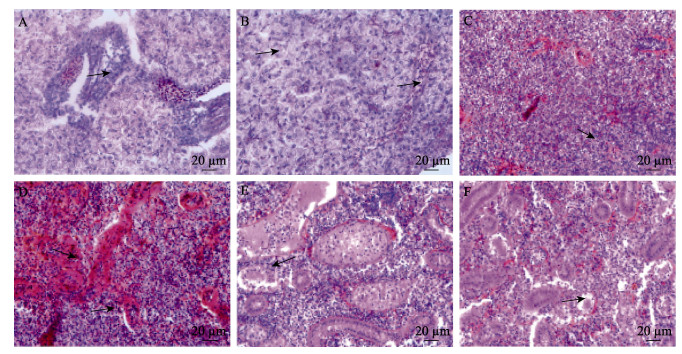
对发病美洲鲥鱼的肝脏、脾脏和肾脏进行切片观察。结果显示,发病鱼肝脏出现弥散性坏死灶、嗜碱性粒细胞增多、细胞肿胀、细胞质空泡变性等症状(图 2A、B);脾脏中同样发现坏死灶,并呈现出血性贫血状态,细胞核破裂、萎缩和消失(图 2C、D);肾脏间质疏松、囊腔变大,淋巴细胞坏死脱落。肾小管轻微变形,肾小体毛细血管球萎缩,近端小管和远端小管内的细胞出现不同程度的细胞结构消失、细胞空泡化和核固缩(图 2E、F)。
|
图 2 发病美洲鲥鱼组织病理症状 Fig.2 Histopathological symptoms of diseased A. sapidissima A:嗜碱性粒细胞增多和弥漫性坏死;B:细胞肿胀,空泡变性;C:嗜碱性粒细胞增多;D:脾脏表现出血性贫血坏死、核破裂和萎缩;E:肾间质疏松,肾小管出现变形;F:肾小球萎缩 A: Basophilia, and diffuse necrosis; B: Cell swelling, vacuolar degeneration; C: Basophilia; D: Spleen showed hemorrhagic anemic necrosis, nuclear rupture and atrophy; E: Loose renal interstitial, and tubular variation; F: Atrophic glomerulus |
由于美洲鲥鱼应激较强,难以进行实验室操作,本实验选择了蓝曼龙作为实验动物,通过肌肉注射感染检测AS-AH2101的致病性。人工感染蓝曼龙在感染后第3天出现死亡,感染鱼肌肉注射部位出现红肿、出血和鳞片脱落,鳍条基部充血或出血,解剖可见与自然发病美洲鲥鱼类似的腹腔内腹水、肝脏坏死等症状。人工感染死亡情况如表 2所示,蓝曼龙注射组感染后死亡率分别为93.3% (1×106 CFU/尾)、60% (1×105 CFU/尾)、40% (1×104 CFU/尾)和13.3% (1× 103 CFU/尾),对照组实验鱼均未发生死亡。经计算,AS-AH2101对蓝曼龙的半数致死量为3.23×104 CFU/尾。从感染死亡的蓝曼龙内脏中可重新分离到菌落形态一致的优势菌,经16S rRNA扩增、测序和比对,结果与AS-AH2101一致,说明AS-AH2101是导致美洲鲥鱼发病并死亡的病原。
|
|
表 2 蓝曼龙人工感染实验 Tab.2 Experimental infection of T. trichopterus |
生理生化鉴定结果如表 3所示,AS-AH2101对龙胆二糖、水苏糖、棉子糖、α-D-乳糖、蜜二糖、3-甲酰葡糖、D-岩藻糖、D-山梨醇、D-阿拉伯醇、肌醇等呈阴性反应;对糊精、D-麦芽糖、D-海藻糖、D-纤维二糖、蔗糖、D-松二糖、β-甲酰-D-葡糖苷等呈阳性反应。经BIOLOG鉴定系统分析,AS-AH2101菌株生理生化特征与嗜水气单胞菌最为接近,可信度为0.999。
|
|
表 3 菌株AS-AH2101生理生化特征 Tab.3 Biochemical analysis of AS-AH2101 strain |
PCR扩增菌株AS-AH2101的16S rRNA基因并进行测序(OP787967),序列比对分析结果显示,菌株AS-AH2101 16S rRNA基因在GenBank中同源性最高的前20位菌株均为嗜水气单胞菌,EzTaxon比对也与嗜水气单胞菌一致。从NCBI数据库中选择气单胞菌属的不同细菌,使用MEGA 7.0软件构建了16S rRNA基因的系统进化树。分析结果显示,AS-AH2101与嗜水气单胞菌聚为一支(图 3),与生理生化鉴定结果吻合。综合以上结果,将AS-AH2101病原菌鉴定为嗜水气单胞菌。

|
图 3 基于16S rRNA基因序列构建的系统发育进化树 Fig.3 Phylogenetic tree constructed by neighbor joining method based on sequences of 16S rRNA |
药敏实验结果显示,AS-AH2101菌株对阿莫西林、氨苄西林、红霉素和水产养殖禁用的头孢拉啶等4种抗生素具有耐药性,对四环素、多西环素和氟苯尼考等11种抗生素敏感(表 4)。
|
|
表 4 AS-AH2101的药敏检测结果 Tab.4 Test results of AS-AH2101 drug sensitivity |
对嗜水气单胞菌的气溶素(aerA)、溶血素(hlyA)、丝氨酸蛋白酶(ahpA)、热稳定细胞肠毒素(ast)、热敏感细胞肠毒素(altA)和密度感应系统调控基因(luxS)等毒力因子基因进行PCR检测。电泳结果显示,6个毒力因子基因在AS-AH2101菌株中均扩增出目标条带,测序结果与目的基因的序列一致,证明AS-AH2101具有这6种毒力基因。
3 讨论嗜水气单胞菌是鱼类、两栖类和爬行类等淡水养殖经济动物的重要病原,在世界各地均有该病原感染淡水养殖鱼类造成出血性败血症的报道(Austin et al, 1996)。嗜水气单胞菌是一种典型的条件致病菌,经常存在于鱼类的体表和肠道中(Allen et al, 1983; Boulanger et al, 1977),当鱼体受到环境压力等胁迫时表现出致病性(Leung et al, 1995)。在本次调查中,我们了解到在4月初水温回升时期,该批次美洲鲥鱼即出现初步症状,并发生少量死亡情况,但养殖企业未引起充分重视。4月中下旬,日死亡数量迅速增加,发病变得难以控制。推测此次发病类似于“草鱼综合征”,在水温回升后,鱼群摄食量上升,消化道压力增大,排泄增多,引起水质恶化和鱼体应激,进一步导致原本在体表或肠道附生的嗜水气单胞菌表现出致病性,造成养殖群体的大规模发病和大量死亡。在人工感染实验中,也通过蓝曼龙进一步验证了病原菌的致病性。本研究发现,蓝曼龙感染3 d迅速出现症状,并发生死亡。感染鱼症状与自然发病的美洲鲥鱼类似。AS-AH2101对蓝曼龙的半数致死量远低于之前报道的其他嗜水气单胞菌(Leung et al, 1997),因此,根据本研究结果,推断嗜水气单胞菌感染是造成此次美洲鲥鱼大规模死亡的原因。
目前,抗生素是很多国家治疗水产动物细菌性感染的主要药物,且其用量随着养殖周期的延长和规模的扩大而迅速增长。1988年,挪威三文鱼养殖共使用抗生素670 kg,而到1992年,抗生素的使用量上升至27.5 t (Grave et al, 1999)。抗生素的大量使用带来了病原菌的耐药性问题。印度一项耐药性调查结果显示,在鱼虾中分离到的319株嗜水气单胞菌对甲氧西林和利福平均具有耐药性,99%以上的菌株对新生霉素和短杆菌肽具有耐药性(Vivekanandhan et al, 2002)。抗生素耐药性已经成为水产养殖中控制嗜水气单胞菌感染的持续性障碍。在本研究中,我们检测了AS-AH2101的耐药性,结果显示,该菌株对头孢拉啶、阿莫西林、氨苄西林和红霉素等4种抗生素具有耐药性。考虑到该养殖企业未使用过这4种抗生素,且这4种抗生素在水产中或者属于禁用药物,或者用量很低,推测AS-AH2101具有这些抗性可能已有较长的时间,或者从其他细菌中通过抗性基因水平转移获得了耐药性(Kim et al, 1993)。同时,根据抗生素耐药性结果,推荐养殖场改用多西环素进行拌料投喂,施用后取得了显著效果。
嗜水气单胞菌的致病机制复杂,具有多种毒力因子,本研究对美洲鲥鱼病原菌AS-AH2101进行了PCR检测,结果显示,AS-AH2101携带6种毒力基因。毒力基因ahpA和aerA分别编码嗜水气单胞菌丝氨酸蛋白酶基因和气溶素基因,是嗜水气单胞菌的主要毒力因子,与致病性存在相关性,强致病性菌株均具有这2个毒力基因(朱大玲等, 2006; 付乔芳等, 2011)。ast编码细胞性肠毒素,hlyA编码溶血素基因,altA编码肠毒素基因,这3种基因也是气单胞菌中常见的毒力因子,具有细胞毒性、溶血毒性和肠毒素毒性,能促进宿主细胞凋亡并发挥致病性功能(吴同垒等, 2011; Buckley et al, 1999; 单晓枫等, 2011; Rosenshie et al, 1993)。luxS编码细菌的密度感应,用以调控毒力基因的表达和分泌,并促进细菌生物膜的形成,增强细菌对宿主免疫杀伤和抗生素药物的抵抗力(Learman et al, 2009; Azakami et al, 2006)。本研究分离得到的病原菌AS-AH2101同时具有6种毒力基因,从基因层面上进一步反映了菌株强致病性,与人工感染实验结果吻合,也解释了此次美洲鲥鱼发病过程中的高死亡率原因。
综上所述,本研究从严重发病的养殖美洲鲥鱼中分离得到了一株嗜水气单胞菌AS-AH2101,通过感染实验和毒力基因分析,证明AS-AH2101具有强致病性,是此次美洲鲥鱼大规模发病死亡的病原,并根据药敏结果使用抗生素取得较好的治疗效果。但从长远来看,抗生素防治嗜水气单胞菌感染会面临耐药性风险,因此,疫苗、噬菌体、益生菌和中草药等抗生素替代品的研发将是未来研究的重点。
AZAKAMI H, TERAMURA I, MATSUNAGA T, et al. Characterization of autoinducer 2 signal in Eikenella corrodens and its role in biofilm formation. Journal of Bioscience and Bioengineering, 2006, 102(2): 110-117 DOI:10.1263/jbb.102.110 |
AUSTIN B, ADAMS C. Fish pathogens, the genus Aeromonas. Chichester, West, Sussex, United Kingdom: John Wiley and Sons, 1996, 197-243 |
ALLEN D A, AUSTIN B, COLWELL R R. Aeromonas media, a new species isolated from river water. International Journal of Systematic and Evolutionary Microbiology, 1983, 33(3): 599-604 |
BOULANGER Y, LALLIER R, COUSINEAU G. Isolation of enterotoxigenic Aeromonas from fish. Canadian Journal of Microbiology, 1977, 23: 1161-1164 DOI:10.1139/m77-174 |
BUCKLEY J T, HOWARD S P. The cytotoxic enterotoxin of Aeromonas hydrophila is aerolysin. Infection and Immunity, 1999, 67(1): 466-467 DOI:10.1128/IAI.67.1.466-467.1999 |
DEBROY S, DAO J, SODERBERG M, et al. Legionella pneumophila type Ⅱ secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(50): 19146-19151 |
DONG Y L, LIANG Z Y. Comparison of effects of several antifungal drugs of Saprolegniasis on American shad eggs. Scientific Fish Farming, 2015(6): 60 [董亚伦, 梁政远. 几种抗霉菌药物对美洲鲥鱼卵水霉病的防治效果比较. 科学养鱼, 2015(6): 60] |
DENG G C, JIANG X Y, YE X, et al. Isolation, identification and characterization of Aeromonas hydrophila from hemorrhagic grass carp. Microbiology China, 2009, 36(8): 1170-1177 [邓国成, 江小燕, 叶星, 等. 草鱼出血病混合感染的嗜水气单胞菌的分离、鉴定与理化特性. 微生物学通报, 2009, 36(8): 1170-1177] |
DONG Z D, ZHOU F N, YUAN Y Y, et al. Oreochromis niloticus pathogenic Aeromonas hydrophila isolation, identification and susceptibility testing. Journal of Northeast Agricultural University, 2012, 43(6): 110-115 [董忠典, 周芬娜, 袁颜颜, 等. 尼罗罗非鱼致病性嗜水气单胞菌的分离鉴定与药敏试验. 东北农业大学学报, 2012, 43(6): 110-115] |
FU Q F, QIU J Q, HU K, et al. The analyse of virulence factors-pathogenicity relationships of Aeromonas hydrophila strains isolated from China. Journal of Biology, 2011, 28(6): 53-57 [付乔芳, 邱军强, 胡鲲, 等. 嗜水气单胞菌国内分离株的毒力因子分布与致病性相关性分析. 生物学杂志, 2011, 28(6): 53-57] |
GAO X Q, HONG L, LIU Z F, et al. The study of allometric growth pattern of American shad larvae and juvenile (Alosa sapidissima). Acta Hydrobiologica Sinica, 2015, 39(3): 638-644 [高小强, 洪磊, 刘志峰, 等. 美洲鲥仔稚鱼异速生长模式研究. 水生生物学报, 2015, 39(3): 638-644] |
GAO X Q, LIU, Z F, HUANG B, et al. Morphological and histological observation of the embryo of American shad (Alosa sapidissima). Progress in Fishery Sciences, 2017, 38(5): 9-18 [高小强, 刘志峰, 黄滨, 等. 美洲鲥(Alosa sapidissima)胚胎发育形态学及组织切片观察. 渔业科学进展, 2017, 38(5): 9-18] |
GRAVE K, LILLEHAUG A, LUNESTAD B T, et al. Surveillance by the use of prescription data. Acta Veterinaria Scandinavica, 1999, 40(3): 185-195 DOI:10.1186/BF03547016 |
GAO H J, CHEN C F, LIN Y T. Studies on fulminant fish disease in reservoir and its prevention and control techniques Ⅴ. Immune response of silver carp to Aeromonas hydrophila. Reservoir Fisheries, 1997(1): 8-10 [高汉娇, 陈昌福, 林永泰. 水库暴发性鱼病及其防治技术研究Ⅴ. 鲢对嗜水气单胞菌的免疫反应. 水利渔业, 1997(1): 8-10] |
HUANG J, WEN H C, SHI J G, et al. Isolation and identification of pathogenic bacteria from Pelteobagrus fulvidraco ulcerative syndrome and its drug sensitive test. Journal of Southern Agriculture, 2012, 43(1): 107-112 [黄钧, 温华成, 施金谷, 等. 黄颡鱼体表溃疡病病原菌分离鉴定及药敏试验. 南方农业学报, 2012, 43(1): 107-112] |
JIA Y J, CHEN Y F, GOUDIE C A, et al. Potential invasion risk of the introduced American shad (Alosa sapidissima) to aquatic ecosystem in China. Acta Zoologica Sinica, 2007, 53(4): 625-629 |
KAWADA M, CHEN C C, ARIHIRO A, et al. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Laboratory Investigation, 2008, 88(8): 883-895 DOI:10.1038/labinvest.2008.47 |
KIM E H, YOSHIDA T, AOKI T. Detection of R plasmid encoded with resistance to florfenicol in Pasteurella piscicida. Fish Pathology, 1993, 28(4): 165-170 DOI:10.3147/jsfp.28.165 |
LIU Y N, LIANG L G, GU W, et al. Isolation, identification and antibiotic susceptibility test of pathogenic bacteria strain from hybridized Prussian carp. Journal of Hydroecology, 2012, 33(5): 108-113 [刘亚楠, 梁利国, 顾伟, 等. 异育银鲫致病性嗜水气单胞菌的分离鉴定与药敏特性研究. 水生态学杂志, 2012, 33(5): 108-113] |
LUO D, LIANG L G, XIE J, et al. Development of duplex PCR for detection of Cyprinid herpesvirus 2. Chinese Journal of Preventive Veterinary Medicine, 2014, 36(5): 379-382 [罗丹, 梁利国, 谢骏, 等. 2型鲤疱疹病毒双重PCR快速检测方法的建立及应用. 中国预防兽医学报, 2014, 36(5): 379-382] |
LUO X W, SHEN J Y, YANG T, et al. Isolation and identification of largemouth bass Ranavirus from Hubei Province. Journal of Fishery Sciences of China, 2022, 29(3): 494-502 [罗晓雯, 沈锦玉, 阳涛, 等. 一株湖北源大口黑鲈蛙病毒的分离鉴定. 中国水产科学, 2022, 29(3): 494-502] |
LEARMAN D R, YI H, BROWN S D, et al. Involvement of Shewanella oneidensis MR-1 LuxS in biofilm development and sulfur metabolism. Applied and Environmental Microbiology, 2009, 75(5): 1301-1307 DOI:10.1128/AEM.01393-08 |
LEUNG K Y, WONG L S, LOW K W, et al. Mini-Tn5 induced growth-and protease-deficient mutants of Aeromonas hydrophila as live vaccines for blue gourami, Trichogaster trichopterus (Pallas). Aquaculture, 1997, 158(1/2): 11-22 |
LING S H, WANG X H, LIM T M, et al. Green fluorescent protein-tagged Edwardsiella tarda reveals portal of entry in fish. FEMS Microbiology Letters, 2001, 194(2): 239-243 DOI:10.1111/j.1574-6968.2001.tb09476.x |
LEUNG K Y, YEAP I V, LAM T J, et al. Serum resistance as a good indicator for virulence in Aeromonas hydrophila strains isolated from diseased fish in South-East Asia. Journal of Fish Diseases, 1995, 18(6): 511-518 DOI:10.1111/j.1365-2761.1995.tb00355.x |
LIANG Z S, HUANG J, SHI J G, et al. Isolation and identification of ascites disease pathogen from Pelteobagrus fulvidraco Richardson and its drug sensitivity test. Journal of Southern Agriculture, 2012, 43(9): 1400-1404 [梁正生, 黄钧, 施金谷, 等. 黄颡鱼腹水病病原菌的分离鉴定及药敏试验. 南方农业学报, 2012, 43(9): 1400-1404] |
LANE D J. 16S/23S rRNA sequencing. In: STACKEBRANDT E, GOODFELLOW M (Eds). Nucleic acid techniques in bacterial systematics. Chichester: John Wiley and Sons, 1991, 115–175
|
PAN T S, WANG L Q, HOU G J. Safe overwintering techniques and common disease control measures of American shad. Reservoir Fisheries, 2008, 28(4): 85-86 [潘庭双, 汪留全, 侯冠军. 美洲鲥鱼安全越冬技术及常见疾病防治措施. 水利渔业, 2008, 28(4): 85-86] |
QIN L, YIN J G, ZHANG W, et al. Isolation and identification of pathogenic Aeromonas hydrophila from Esox lucius around Urumqi in Xinjiang. Progress in Fishery Sciences, 2014, 35(5): 40-45 [秦莉, 殷建国, 张薇, 等. 白斑狗鱼(Esox lucius)致病性嗜水气单胞菌的分离与鉴定. 渔业科学进展, 2014, 35(5): 40-45] |
ROSENSHIE I, FINLAY B B. Exploitation of host signal transduction pathways and cytoskeletal functions by invasive bacteria. BioEssays, 1993, 15(1): 17-24 DOI:10.1002/bies.950150104 |
SHI Y H, XU J B, LIU Y S, et al. Growth regularity and difference of young fish American shad Alosa sapidissima cultured in outdoor and shaded ponds. Journal of Shanghai Ocean University, 2019, 28(2): 161-170 [施永海, 徐嘉波, 刘永士, 等. 敞口池塘和遮荫池塘养殖美洲鲥当年鱼种的生长规律和差异. 上海海洋大学学报, 2019, 28(2): 161-170] |
SHAN X F, GUO W S, WU T L, et al. Cloning and sequence analysis of hemolysin gene fragment from Aeromonas vickerii. Chinese Journal of Veterinary Medicine, 2011, 47(12): 22-24 [单晓枫, 郭伟生, 吴同垒, 等. 框镜鲤维氏气单胞菌溶血素基因片段的克隆与序列分析. 中国兽医杂志, 2011, 47(12): 22-24] |
TIAN T, HU H G, CHEN C F. Identification and pathogenicity of bacterial pathogens isolated in an outbreak of bacterial disease of bluntnose black bream, Megalobrama amblycephala. Journal of Huazhong Agricultural University, 2010, 29(3): 341-345 [田甜, 胡火庚, 陈昌福. 团头鲂细菌性败血症病原菌分离鉴定及致病力研究. 华中农业大学学报, 2010, 29(3): 341-345] |
VIVEKANANDHAN G, SAVITHAMANI K, HATHA A A M, et al. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. International Journal of Food Microbiology, 2002, 76(1/2): 165-168 |
WU T L, SHAN X F, MENG Q F, et al. Bioinformatic analysis and prokaryotic expression of Aerosysin gene of Aeromonas veronii isolated from Cyprinus carpio. Chinese Journal of Preventive Veterinary Medicine, 2011, 33(11): 866-869 [吴同垒, 单晓枫, 孟庆峰, 等. 框镜鲤维氏气单胞菌CY0806株气溶素基因的生物信息学分析及原核表达. 中国预防兽医学报, 2011, 33(11): 866-869] |
YANG M C. Statistics for veterinary science. Beijing: China Prospect Publishing House, 1990: 232-234 [杨茂成. 兽医统计学. 北京: 中国展望出版社, 1990: 232-234]
|
ZHU D L, LI A H, WANG J G, et al. The correlation between the distribution pattern of virulence genes and the virulence of Aeromonas hydrophila strains. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2006, 45(1): 82-85 [朱大玲, 李爱华, 汪建国, 等. 嗜水气单胞菌毒力与毒力基因分布的相关性. 中山大学学报, 2006, 45(1): 82-85] |
ZHANG Y F, ZHANG X J, ZHANG W L, et al. Isolation and identification of Aeromonas hydrophila from carp. Journal of Anhui Agricultural Sciences, 2008, 36(31): 13689-13690, 13709 [张玉芬, 张秀军, 张文丽, 等. 鲤鱼嗜水气单胞菌的分离与鉴定. 安徽农业科学, 2008, 36(31): 13689-13690, 13709] |
ZHU X H, ZHANG Z R, ZHOU L Y, et al. Transcriptomic analysis of the head kidney of Siniperca chuatsi infected with Aeromonas hydrophila. Progress in Fishery Sciences, 2022, 43(4): 208-217 [朱鑫海, 张紫瑞, 周丽颖, 等. 嗜水气单胞菌感染翘嘴鳜头肾转录组分析. 渔业科学进展, 2022, 43(4): 208-217] |



