2. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业与可持续发展重点实验室 山东 青岛 266071;
3. 东营市阔海水产科技有限公司 山东 东营 257000
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Marine Fisheries and Sustainable Development, Ministry of Agriculture and Rural Affairs, Qingdao 266071, China;
3. Dongying Kuohai Aquatic Technology Company Ltd, Dongying 257000, China
凡纳对虾(Penaeus vannamei)生长速度快、产量高、肉质鲜美,为我国的主要养殖虾类(Jin et al, 2018)。凡纳对虾分段养殖对提高虾苗的环境适应能力,降低病害和苗种质量的负面影响,提高养殖成功率具有重要的意义,目前已成为对虾养殖的主要模式之一(李俊伟等, 2016)。中间培育(又称“标粗”)是凡纳对虾养殖的重要阶段,经此阶段的虾苗生长状态良好,有利于后续养殖(赵玉超等, 2019)。
目前,凡纳对虾养殖高度依赖饵料投喂,最优的投喂率是获得更大养殖效益的基础(Luo et al, 2021)。投喂率是指饵料量占养殖对象体重的百分数,是影响对虾工厂化养殖的重要因素(褚志鹏等, 2020),也是影响养殖物种存活率和特定生长率的重要因素。投喂率过低会使养殖对象生长缓慢,影响正常发育(Hung et al, 1989);投喂率过高会增加养殖成本,造成残饵过量堆积,引起水质恶化、滋生病菌,导致养殖对象死亡等(刘文逵等, 2007; Silva et al, 2007)。因此,有必要确定适当的投喂率,以提高饵料效率,进而满足可持续水产养殖的需要。有学者从外源和内源因素的角度分别分析了外界环境改变对凡纳对虾生长、生理和水环境的影响机制,如Kaya等(2020)在生物絮团养殖模式中,研究不同投喂率对凡纳对虾生长性能的影响;宫晗等(2023)研究工厂化循环水养殖系统水质指标与微生物群落结构的影响;邢明威等(2022)研究不同饵料投喂对凡纳对虾非特异性免疫的影响。但在中间培育期间,投喂率的确定依赖于养殖经验,缺乏科学依据,鲜有学者对投喂率综合影响进行全面研究。因此,迫切需要阐述不同指标之间的内在联系,确定适宜中间培育的投喂率。
综上,本研究通过分析不同投喂率对凡纳对虾中间培育水质条件、微生物群落结构、非特异性免疫及生长性能的影响,阐明投喂率与中间培育养殖水体及对虾生理、生长指标之间的内在关系,确定凡纳对虾中间培育的适宜投喂率,以期为提高凡纳对虾养殖成功率提供技术指导。
1 材料与方法 1.1 样品获取与实验条件实验在山东省东营市阔海水产有限公司对虾养殖车间进行,随机选取活力好的凡纳对虾仔虾[平均每尾体重为(5.0±0.5) mg]开展实验研究。
1.2 实验设计根据前期养殖经验,中间培育投喂率为对虾体重的6%~8%,因此,本实验设置3个投喂梯度(投喂率分别为体重的5%、7.5%和10%,分别命名为T5、T7.5和T10),每个实验组设置3个重复。实验期间,每日于06:00、10:00、14:00、18:00和22:00进行投喂,每次投喂率分别为体重的1%、1.5%和2%,每隔6 d,随机抽取部分对虾,称量其体重,随之对投喂量进行调整。实验使用容积为200 L的养殖桶(聚乙烯材质,上直径为0.79 m,下直径为0.59 m,高为0.71 m)。养殖过程中投喂浙江恒兴饲料有限公司生产的标粗专用饲料(赖氨酸≥2.8%、粗蛋白≥49.0%、粗脂肪≥7.5%、灰分≤15.0%、总磷1.0%~2.0%、水分≤10.0%)。养殖密度为5 000尾/m³,每个养殖桶虾苗数量为1 000尾。整个实验持续30 d,保证24 h持续充氧,每2 d更换1/3水体,更换水体前清理桶内残饵粪便。
1.3 对虾指标测定实验开始和结束时,在每个养殖桶内随机捞取凡纳对虾10尾,使用游标卡尺和分析天平测定其体长和体重。根据公式计算对虾存活率和特定生长率。
存活率(survival rate, SR, %)=(X2/X1)×100%
特定生长率(specific growth rate, SGR, %/d)= (lnW2‒lnW1)/t×100%
式中,X1代表初始对虾尾数;X2代表终末对虾尾数;W1代表初始对虾体重;W2代表终末对虾体重;t为养殖天数。
1.4 水质指标测定在养殖期间,每隔2 d取水样进行水质测定。利用YSI多参数水质分析仪测定温度、溶解氧、pH和盐度。总氨氮(TAN)和亚硝态氮(NO2–-N)分别采用次溴酸盐氧化分光光度法和萘乙二胺分光光度法进行测定(GB17378.4-2007),化学需氧量(COD)利用高锰酸钾氧化滴定法进行测定。
1.5 非特异性免疫指标测定实验结束时,在每个养殖桶内随机抽取5尾对虾,用无菌海水冲洗干净体表附着物,在无菌条件下解剖对虾,取出肝胰脏,保存于–80 ℃冰箱。超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、酸性磷酸酶(acid phosphatase, ACP)和碱性磷酸酶(alkaline phosphatase, AKP)均采用南京建成生物工程研究所有限公司试剂盒测定。
1.6 微生物群落多样性测定实验结束时,在每个养殖桶内取水样0.5 L,用于养殖水体微生物群落分析。水样均用0.22 μm孔径无菌滤膜进行抽滤。抽滤后的滤膜使用Illumia MiSeq测序平台(Illumina, 美国)进行16S rRNA深度测序。利用PCR对16S rRNA的V3~V4区域进行特异性扩增,上游引物为338F:ACT CCTACGGGAGGCAGCA,下游引物为806R:GGAC TACHVGGGTWTCTAAT。PCR完成后,切胶回收PCR产物,用QuantiFluor-ST蓝色荧光定量系统(Promega)进行定量检测,每个样品的扩增根据DNA浓度以相同的摩尔比混合。最后,在Illumina MiSeq平台上对纯化后的混合物进行测序。
1.7 因子分析目的和过程根据前期所得数据,利用因子分析把数据进行降维,整合为数量较少的公共因子,用具有代表性的公共因子来表达不同变量之间的相互关系。利用IBM SPSS 25.0统计软件对原始数据进行标准化处理,进行KMO检验和Bartlett球形检验,当检验结果表明数据的大部分信息可以被公共因子解释,则可以进行因子分析。通过计算,得到因子载荷矩阵,利用凯撒正态化最大方差法进行旋转,得到旋转后的载荷矩阵。根据所得矩阵,构建因子综合评价模型,由此根据综合得分确定排名。
1.8 数据分析与处理使用Microsoft Excel软件进行数据统计,实验所得数据以平均值±标准差(Mean±SD)表示。使用IBM SPSS 25.0统计软件进行显著性分析、单因素方差分析(one-way ANOVA)、因子分析,以P < 0.05表示差异显著,以P < 0.01表示差异极显著。使用Qiime软件计算α多样性指数,使用“pheatmap”软件包绘制微生物菌群热图,使用“vegan”软件包进行冗余分析(RDA),微生物菌群分析在上海美吉生物医药科技有限公司I-sanger云平台完成。
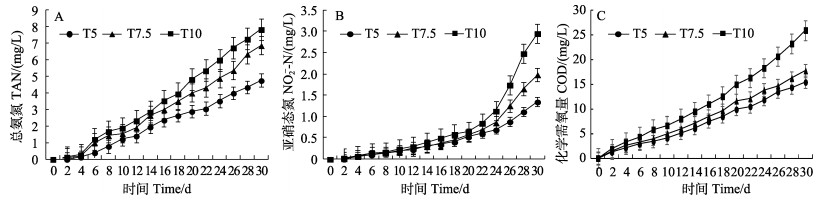
2 结果 2.1 投喂率对养殖水质的影响整个养殖过程中,pH、盐度、温度和溶解氧均保持在适宜对虾生长的范围内,具体数据如表 1所示。不同投喂率养殖水体中TAN、NO2–-N和COD的浓度变化曲线如图 1所示,随着养殖的进行,各组TAN、NO2–-N和COD的浓度均呈逐渐升高的趋势,且投喂率越高,TAN、NO2–-N和COD的浓度越高。当养殖结束时,3个投喂组中TAN浓度分别为4.76、6.87和7.85 mg/L,差异显著(P < 0.05)。NO2–-N浓度在养殖前期积累缓慢,养殖后期的NO2–-N浓度显著升高,实验结束时,其浓度分别为1.35、1.98和2.95 mg/L,差异显著(P < 0.05)。COD浓度随着养殖进行逐渐积累,养殖结束时,其浓度分别为15.43、17.75和25.96 mg/L,差异显著(P < 0.05)。
|
|
表 1 不同投喂率养殖水质指标 Tab.1 Water quality in breeding bucket with different feeding rates |
|
图 1 不同投喂率养殖水体总氨氮(A)、亚硝态氮(B)和化学需氧量(C)浓度变化 Fig.1 Variations of TAN (A), NO2–-N (B), and COD (C) concentrations of aquaculture water in breeding bucket with different feeding rates |
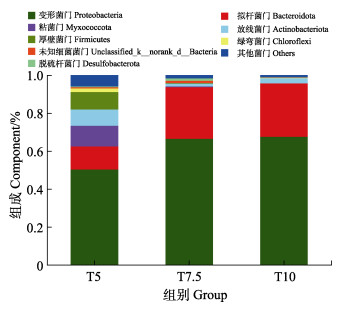
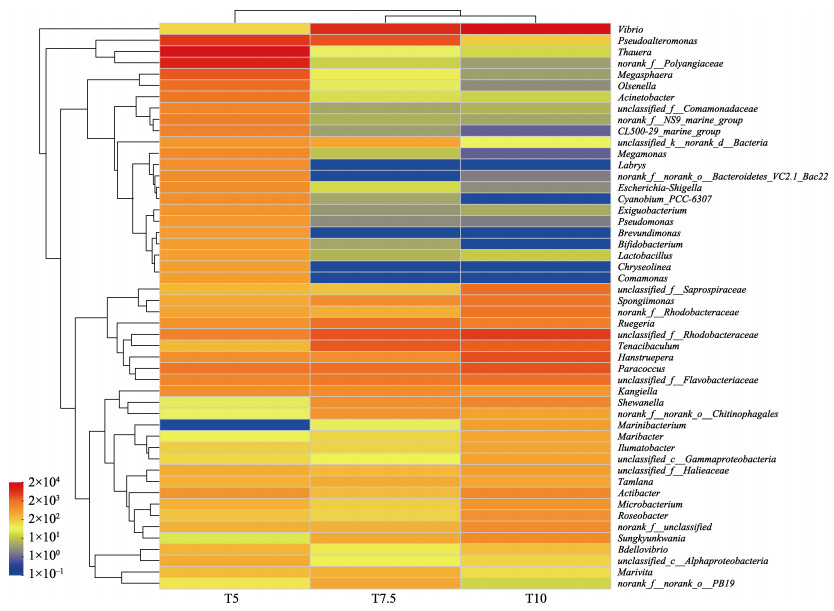
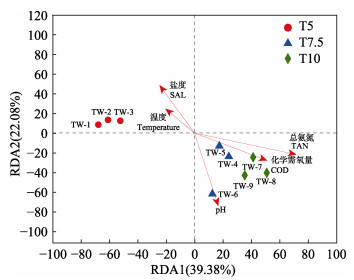
不同投喂率养殖水环境微生物群落多样性指数如表 2所示,Coverage数值均大于99%,这表明测序结果中几乎包含养殖水环境中的所有分类操作单元(operational taxonomic units, OTUs),多样性结果分析具有较高的可靠性。Chao指数和Shannon指数均在T5组最高,分别为447.86和4.14,在T10最低,分别为190.16和2.73,各组间差异显著(P < 0.05)。门水平的养殖水体细菌群落的主要成分如图 2所示,养殖水环境中的优势菌门分别为变形菌门(Proteobacteria, 50.36%~67.53%)、拟杆菌门(Bacteroidetes, 12.09%~ 67.53%)、粘菌门(Myxococcota, 1.14%~10.86%)和放线菌门(Actinobacteria, 1.41%~8.61%)。变形菌门占主导地位,其他菌门的相对丰度存在差别。为深入了解投喂率对水环境微生物群落结构的影响,在属水平建立热图,如图 3所示,热图分析显示了不同投喂率微生物群落结构的差异。在不同投喂率下,弧菌(Vibrio)的相对丰度较高,为主要优势属,此外,副球菌(Paracoccus)、黏着杆菌(Tenacibaculum)均为相对丰度较高的菌属。假交替单胞菌(Pseudoalteromonas)在不同投喂组中均存在,在T7.5组相对丰度最高,为9.78%,在T10组相对丰度最低,仅为0.28%。对养殖水环境的pH、盐度、温度、溶解氧、TAN、NO2–-N和COD的方差膨胀因子(VIF)进行分析。VIF数值越大代表自变量之间的多重共线性关系越强。通常把VIF > 10的因子视为无效因子,最终筛选出pH、盐度、温度、TAN和COD 5个水质因子。利用RDA分析来研究微生物群落与水质因子之间的相关性(图 4)。RDA轴1和2的总方差贡献率为61.46%,箭头长度代表水质因子与不同投喂率养殖水体微生物群落之间的关联程度。结果发现,T5组养殖水体中微生物群落与盐度和温度呈正相关,盐度对微生物群落的影响大于温度;T7.5和T10组养殖水体中微生物群落与TAN、COD和pH呈正相关,TAN对微生物群落的影响大于COD和pH。
|
|
表 2 不同投喂率养殖环境α多样性指数 Tab.2 The α diversity index in breeding bucket with different feeding rates |
|
图 2 不同投喂率养殖水体样品在门水平上的物种丰富度 Fig.2 Community abundance of samples in breeding bucket with different feeding rates on phylum level |
|
图 3 不同投喂率组养殖水体主要细菌菌属热图 Fig.3 Heat map of major bacterial genus in breeding bucket with different feeding rates |
|
图 4 微生物群落与环境参数的冗余分析 Fig.4 Redundancy analysis (RDA) of the microbial communities and environmental parameters TW代表不同投喂组,TW1~TW3、TW4~TW6、TW7~TW9分别为T5、T7.5、T10组3个样本。 TW represents different feeding rates groups, TW1~TW3, TW4~TW6, and TW7~TW9 are three samples of T5, T7.5, and T10 group, respectively. |
使用试剂盒对凡纳对虾肝胰腺的SOD、CAT、ACP和AKP进行测定,结果如图 5所示,凡纳对虾肝胰腺的SOD和CAT在不同投喂组中存在显著差异(P < 0.05),但ACP和AKP在T5和T7.5组中无显著差异(P > 0.05),受投喂率影响较小,但在T10组中,ACP和AKP活性较低,且显著低于T5和T7.5组(P < 0.05)。SOD、CAT、ACP和AKP活性均在T7.5组最高,分别为160.34、70.16、7.08和68.27 U/L;在T10最低,分别为112.88、50.57、5.06和150.64 U/L。
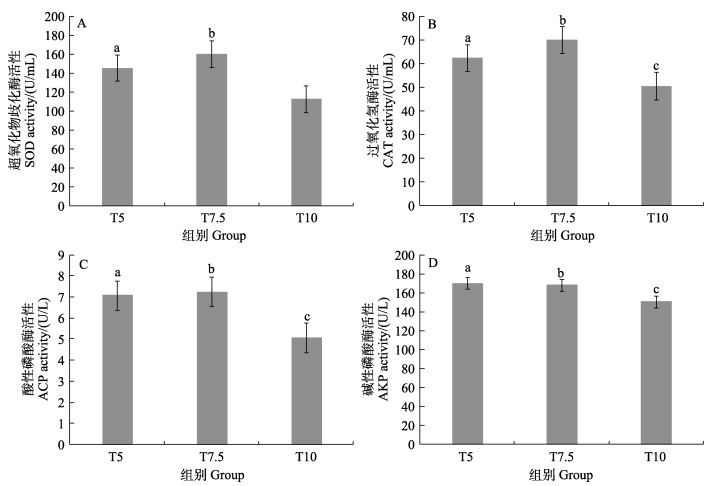
|
图 5 不同投喂率超氧化物歧化酶(A)、过氧化氢酶(B)、酸性磷酸酶(C)和碱性磷酸酶(D)活性变化 Fig.5 Variations of superoxide dismutase (A), catalase (B), acid phosphatase (C) and alkaline phosphatase (D) activity of aquaculture water in breeding bucket with different feeding rates 柱子上标不同字母表示组间存在显著差异(P < 0.05)。 Columns with different superscripts letter are significantly different (P < 0.05). |
采用不同投喂率的凡纳对虾生长指标如表 3所示。实验结束时,T7.5和T10组对虾的体长和体重均著大于T5组(P < 0.05),但T7.5和T10组之间无显著差异(P > 0.05)。存活率与投喂率呈负相关,且3组间存在显著差异(P < 0.05)。T5组的特定生长率最低,为15.14%/d,T7.5和T10组的特定生长率分别为15.63%/d和15.64%/d,无显著差异(P > 0.05)。
|
|
表 3 不同投喂率凡纳对虾生长指标 Tab.3 Growth index of P.vannamei with different feeding rates |
针对本研究所得数据,利用IBM SPSS 25.0统计软件进行因子分析。KMO值为0.635,超过临界值0.6;Bartlett球形度检验的值为128.511,自由度为28,P < 0.001,表明数据相关性极强,适合进行因子分析。使用主成分分析法对数据进行提取,并利用凯撒正态化最大方差法进行旋转,所得结果如表 4所示。前2个公因子包含总方差的95.971%,对所测指标的方差贡献率分别为48.967%和47.004%,因此,提取2个公因子。因子载荷表明生长性能指标、非特异性免疫指标与公因子之间的关联程度。由表 5可知,第1因子对AKP、ACP、SOD和CAT具有高载荷,将该因子命名为免疫因子;第2因子对终末均体长(FL)、终末均体重(FW)、SR和SGR具有高载荷,将该因子命名为生长因子。根据以上分析,得出成分得分系数矩阵(表 6),建立凡纳对虾中间培育的因子评价模型:
| $ \begin{aligned} F_1= & 0.026 \mathrm{FL}+0.12 \mathrm{FW}+0.034 \mathrm{SR}+0.15 \mathrm{SGR}+ \\ & 0.192 \mathrm{AKP}+0.239 \mathrm{ACP}+0.31 \mathrm{SOD}+0.33 \mathrm{CAT} \\ F_2= & 0.231 \mathrm{FL}+0.319 \mathrm{FW}-0.207 \mathrm{SR}+0.336 \mathrm{SGR}- \\ & 0.047 \mathrm{AKP}+0.013 \mathrm{ACP}+0.119 \mathrm{SOD}+0.156 \mathrm{CAT} \end{aligned} $ |
|
|
表 4 提取因子方差解释 Tab.4 Total variance explained of the extract factor |
|
|
表 5 公因子载荷矩阵 Tab.5 Load matrix for factors |
|
|
表 6 成分得分系数矩阵 Tab.6 Component score coefficient matrix |
为准确分析,便于对凡纳对虾中间培育做出评价,采用加权计算的方法进行综合分析,得到综合评价模型:F=0.511F1+0.489F2。计算不同投喂率的综合得分,结果显示,T7.5得分最高,为0.92,T5得分最低,为–0.43。
3 讨论 3.1 投喂率对凡纳对虾中间培育水质及微生物群落结构的影响在中间培育养殖过程中,适宜投喂率有助于养殖水环境的保持。Herawati等(2020)研究表明,水质是影响凡纳对虾生长的重要因素。在本研究中,随着养殖进行,TAN浓度升高,且投喂率越高其升高幅度越大。这可能因为过量投喂使饵料在水体中的密度增加,残饵未被及时清理,引起水体中的TAN浓度上升(陈亨等, 2018; de Melo Filho et al, 2020)。NO2–-N是硝化反应的中间产物,其浓度过高会对养殖物种产生毒害作用(寇红岩等, 2014)。NO2–-N浓度前期较低,但到养殖中后期,其浓度显著升高。该结果与张龙等(2019)的研究结论相似,这可能因为养殖前期TAN浓度较低,硝化反应进行缓慢,但随着TAN的积累,TAN转化为NO2–-N,导致其浓度显著升高(Wang et al, 2019)。COD反映了水体受污染及水质恶化的程度,其浓度过高可能导致养殖物种疾病暴发(马建新等, 2002)。本研究表明,T10组的COD持续升高,且高于T5和T7.5组,这反映出高投喂率可能会增加虾病暴发的风险。基于上述研究结果,过高的投喂率会加剧养殖水质恶化,因此,适当控制中间培育的投喂率对促进对虾健康养殖具有重要意义。
在中间培育养殖过程中,适宜的投喂率有助于维持养殖水体微生物群落结构稳定。外界环境会对养殖水体的微生物群落结构产生影响,微生物群落结构的多样性和丰富度与养殖水体的稳定性密切相关(Yan et al, 2016)。本研究表明,T5组养殖水体微生物群落的Chao指数和Shannon多样性指数显著高于T7.5和T10组。这可能因为T7.5和T10组水体的残饵粪便积累过多,导致水质恶化,使微生物群落的多样性和丰富度下降。袭琼芬等(2013)等研究表明,当养殖水体微生物群落的多样性和丰富度降低时,更易发生养殖病害。因此,投喂率过高会加剧养殖物种的患病风险。本研究发现,变形菌门为养殖水体的优势菌门。在不同养殖条件下,变形菌门分布广泛且通常为最优势菌群(Shin et al, 2015),在中间培育也得到验证。从属水平看,弧菌在T7.5和T10组含量较高,且尤以T10组最高(37.33%),弧菌是水产养殖中的主要致病菌,弧菌浓度超标会导致虾病暴发,从而引起对虾死亡(Quiroz-Guzman et al, 2013)。本研究还发现,假交替单胞菌在不同投喂组中均存在,在T7.5组相对丰度最高,在T10组相对丰度最低。Bowman (2007)研究发现,假交替单胞菌是抑菌、降解纤维素的有益菌,能提高对虾的存活率和特定生长率,这可从侧面反映高投喂率会导致假交替单胞菌丰度下降,对凡纳对虾的生长产生影响。上述菌属相对丰度的差异表明,不同投喂率使养殖水体的群落结构发生改变。RDA分析表明,TAN和COD是影响中间培育养殖水体微生物群落结构的主要因素。这可能是因为不同投喂率使养殖水体有机物含量产生差异,导致水质指标浓度不同,而不同浓度造成养殖水体的微生物群落组成和相对丰度存在差异(张浩, 2015)。张星(2010)对凡纳对虾养殖池的微生物群落和环境因子进行相关性分析,结果表明,TAN和COD等环境因子对养殖池的微生物群落组成起到重要作用,该结论与本研究结果相似。Ruan等(2015)研究表明,养殖水体微生物群落结构受多种因素的共同作用,因此,要分析中间培育微生物群落的多样性及其影响因素还需进行更深入的探究。
3.2 投喂率对凡纳对虾中间培育非特异性免疫及生长性能的影响在中间培育养殖过程中,适宜投喂率有利于对虾提高自身非特异性免疫力。凡纳对虾自身缺乏特异性免疫系统,依靠非特异性免疫来抵抗外界环境的变化(何树青等, 2021)。养殖水体浓度的变化会对凡纳对虾的非特异性免疫系统产生显著影响。葛红星(2014)研究发现,TAN浓度过高会使凡纳对虾的非特异性免疫酶活性降低,同时提高凡纳对虾感染弧菌的几率。本研究中,T10组TAN和COD浓度最高,且非特异性免疫酶活性最低,这表明养殖水体中含氮有机物过高会抑制凡纳对虾的非特异性免疫力(李晨露等, 2022)。Fu等(2018)研究表明,水生动物的应激反应与饵料资源密切相关。因此,研究投喂率与凡纳对虾非特异性免疫之间的关系,将有助于分析外界条件改变对凡纳对虾内在生理的影响,对中间培育对虾的健康养殖具有重要作用。SOD与CAT是机体自由基代谢及反映组织氧化损伤的重要指标,二者活性高低反映机体对外界环境抵抗能力的强弱(Muniswamy et al, 2008)。本研究发现,不同投喂率显著影响SOD和CAT的活性,当改变投喂率时,其活性在T7.5组最高,T10组最低,这表明中间培育凡纳对虾的非特异性免疫力在T7.5组最高,T10组最低,投喂率的增加会使凡纳对虾的非特异性免疫力增加,但过量投喂会对非特异性免疫产生抑制作用,上述结果与赵志刚等(2017)的研究结果一致。ACP和AKP是反映机体免疫和健康的指标,对抵抗外界疾病侵害有重要作用(Muta et al, 1996)。闫戈等(2022)在研究克氏原螯虾(Procambarus clarkii)的非特异性免疫时也发现,ACP和AKP活性下降会导致克氏原螯虾患病风险增加。在本研究中,ACP和AKP在T5和T7.5组差异不大,但在T10组中,其活性显著降低。这表明,高投喂率可能导致中间培育凡纳对虾的非特异免疫力减弱,增加患病风险,影响对虾的正常生长。
因子分析结果表明,T7.5组综合得分最高,是本研究条件下凡纳对虾中间培育的最佳投喂率。投喂率是影响对虾生长发育的重要因素,适宜的投喂率可以减少浪费,降低饵料成本(Amirkolaie, 2011)。投喂率过低会导致对虾摄食不足,生长发育缓慢,投喂率过高会增加对虾肠道负担,并且引起水质恶化,甚至导致对虾死亡(Wang et al, 2021; Jin et al, 2019)。在本研究中,投喂率从5%提高至7.5%,对虾的体长和体重显著升高,但继续提高投喂率,体长和体重无显著变化。Anh等(2021)研究不同养殖密度和投喂率对凡纳对虾的影响,结果发现,当养殖密度和投喂率过高时,凡纳对虾生长发育迟缓。周晖等(2022)研究发现,在生物絮团养殖模式下,适当降低饵料投喂,凡纳对虾能够维持正常的生长速度。这些研究均表明,与高投喂率相比,适当降低饵料投喂能促进凡纳对虾的生长和存活,对虾未从高投喂率中获益,不能利用额外的饵料。投喂率与中间培育不同指标之间存在密切联系,不同投喂率会引起水中残饵粪便存在差异,造成水质恶化,引起养殖水环境中微生物群落结构紊乱,使对虾自身能量代谢消耗过大,以维持胁迫状态下的能量稳态,进而诱导对虾产生氧化应激反应,造成肝胰脏应激和细胞凋亡,对凡纳对虾的生存造成较大威胁(Liang et al, 2016; Sarsangi Aliabad et al, 2022)。因此,在实际生产中,应在生长不同阶段设置合理的投喂率,促进对虾生长,保持对虾健康状态,实现高效养殖。
4 结论通过研究投喂率对凡纳对虾中间培育养殖水环境、免疫及生长的影响,得到如下结论:(1)在中间培育养殖过程中,适宜的投喂率可以提高凡纳对虾的生长性能和非特异性免疫力,但过量投喂会导致水质恶化,引起弧菌暴发,影响对虾的正常生长和存活。(2)在本研究条件下,综合考虑饵料成本、水质条件、非特异性免疫和生长性能等因素,凡纳对虾中间培育的投喂率在7.5%左右为宜。
AMIRKOLAIE A K. Reduction in the environmental impact of waste discharged by fish farms through feed and feeding. Reviews in Aquaculture, 2011, 3(1): 19-26 DOI:10.1111/j.1753-5131.2010.01040.x |
ANH N T N, SHAYO F A, NEVEJAN N, et al. Effects of stocking densities and feeding rates on water quality, feed efficiency, and performance of white leg shrimp Litopenaeus vannamei in an integrated system with sea grape Caulerpa lentillifera. Journal of Applied Phycology, 2021, 33(5): 3331-3345 DOI:10.1007/s10811-021-02501-4 |
BOWMAN J P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Marine Drugs, 2007, 5(4): 220-241 DOI:10.3390/md504220 |
CHEN H, CHEN C, WU J Q, et al. Growth and feed intake of Litopenaeus vannamei under stress-environment interaction. Journal of Shanghai Ocean University, 2018, 27(2): 168-174 [陈亨, 陈琛, 於俊琦, 等. 不同环境胁迫因子耦合对凡纳滨对虾生长与摄食的影响. 上海海洋大学学报, 2018, 27(2): 168-174] |
CHU Z P, JIN J L, CHEN X H, et al. Effects of different feeding rates and frequencies on the growth performance, body composition, and biochemical parameters of juvenile hybrid sturgeon. Journal of Fishery Sciences of China, 2020, 27(2): 177-185 [褚志鹏, 金佳利, 陈细华, 等. 不同投喂率和投喂频率对大杂交鲟幼鱼生长、体成分和生化指标的影响. 中国水产科学, 2020, 27(2): 177-185] |
DE MELO FILHO M E S, OWATARI M S, MOURIÑO J L P, et al. Application of nitrification and denitrification processes in a direct water reuse system for Pacific white shrimp farmed in biofloc system. Aquacultural Engineering, 2020, 88: 102043 DOI:10.1016/j.aquaeng.2020.102043 |
FU S J, PENG J, KILLEN S S. Digestive and locomotor capacity show opposing responses to changing food availability in an ambush predatory fish. Journal of Experimental Biology, 2018, 221(12): jeb173187 |
GE H X. Effects of two main inorganic nitrogen on the immune response of Litopenaeus vannamei and its susceptibility to Vibrio parahemolyticus. Master′s Thesis of Shanghai Ocean University, 2014, 18-22 [葛红星. 两种主要无机氮胁迫下凡纳滨对虾免疫因子变化及其对副溶血弧菌易感性的研究. 上海海洋大学硕士研究生学位论文, 2014, 18-22]
|
GONG H, CHEN P, QIN Z, et al. Analysis of water quality and bacterial community characterization in an industrialized recirculating aquaculture system of Litopenaeus vannamei. Progress in Fishery Sciences, 2023, 44(1): 125-136 [宫晗, 陈萍, 秦桢, 等. 凡纳滨对虾工厂化循环水养殖系统水质指标及微生物菌群结构的分析. 渔业科学进展, 2023, 44(1): 125-136] |
HE S Q, LI R M, YANG Q H, et al. Effects of dietary zinc on growth, serum non-specific immune indexes, disease resistance and intestinal flora structure in juvenile Litopenaeus vannamei. Journal of Fisheries of China, 2021, 45(10): 1726-1739 [何树青, 李日美, 杨奇慧, 等. 锌对凡纳滨对虾生长、非特异性免疫指标、抗病力及肠道菌群结构的影响. 水产学报, 2021, 45(10): 1726-1739 DOI:10.11964/jfc.20200712355] |
HERAWATI V E, PINANDOYO, DARMANTO Y S, et al. Effect of feeding with Phronima sp.on growth, survival rate and nutrient value content of Pacific white shrimp (Litopenaeus vannamei) post-larvae. Aquaculture, 2020, 529: 735674 DOI:10.1016/j.aquaculture.2020.735674 |
HUNG S S O, LUTES P B, CONTE F S. Growth and feed efficiency of white sturgeon (Acipenser transmontanus) sub-yearling at different feeding rates. Aquaculture, 1989, 80(1/2): 147-153 |
JIN M, XIONG J, ZHOU Q C, et al. Dietary yeast hydrolysate and brewer's yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish and Shellfish Immunology, 2018, 82: 121-129 DOI:10.1016/j.fsi.2018.08.020 |
JIN S Y, JACQUIN L, REN Y, et al. Growth performance and muscle composition response to reduced feeding levels in juvenile red swamp crayfish Procambarus clarkii (Girard, 1852). Aquaculture Research, 2019, 50(3): 934-943 DOI:10.1111/are.13968 |
KAYA D, GENC E, GENC M A, et al. Biofloc technology in recirculating aquaculture system as a culture model for green tiger shrimp, Penaeus semisulcatus: Effects of different feeding rates and stocking densities. Aquaculture, 2020, 528: 735526 DOI:10.1016/j.aquaculture.2020.735526 |
KOU H Y, XIAN J A, GUO H, et al. Research progress of toxic effects of nitrite on shrimp. Marine Sciences, 2014, 38(2): 107-115 [寇红岩, 冼健安, 郭慧, 等. 亚硝酸盐对虾类毒性影响的研究进展. 海洋科学, 2014, 38(2): 107-115] |
LI C L, LI J H, CHENG Y X, et al. Effects of different initial diets on water quality, growth, digestive enzymes and non-specific immune enzymes of Procambarus clarkii. Journal of Shanghai Ocean University, 2022, 31(4): 883-892 [李晨露, 李京昊, 成永旭, 等. 不同饵料对克氏原螯虾仔虾养殖水质、生长、消化酶和非特异性免疫酶的影响. 上海海洋大学学报, 2022, 31(4): 883-892] |
LI J W, JIE X Y, GUO Y J, et al. Application of culturing in stages in the shrimp culturing pond. Progress in Fishery Sciences, 2016, 37(6): 74-80 [李俊伟, 颉晓勇, 郭永坚, 等. 分段养殖模式在凡纳滨对虾(Litopenaeus vannamei)养殖过程中的应用. 渔业科学进展, 2016, 37(6): 74-80] |
LIANG Z X, LIU R, ZHAO D P, et al. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Fish and Shellfish Immunology, 2016, 54: 523-528 DOI:10.1016/j.fsi.2016.05.009 |
LIU W K, FAN Q X, ZHU B K, et al. Effects of prey density on the growth and survival of larval hybrid snakehead. Journal of Huazhong Agricultural University, 2007, 26(3): 367-370 [刘文逵, 樊启学, 朱邦科, 等. 饵料密度对杂交鳢仔鱼生长、存活的影响. 华中农业大学学报, 2007, 26(3): 367-370 DOI:10.3321/j.issn:1000-2421.2007.03.022] |
LUO S, LI X Q, ONCHARI M, et al. High feeding level alters physiological status but does not improve feed conversion efficiency and growth performance of juvenile red swamp crayfish Procambarus clarkii (Girard, 1852). Aquaculture, 2021, 537: 736507 DOI:10.1016/j.aquaculture.2021.736507 |
MA J X, LIU A Y, SONG A Q. A study on the relationship between COD and shrimp viral disease. Marine Sciences, 2002, 26(3): 68-71 [马建新, 刘爱英, 宋爱芹. 对虾病毒病与化学需氧量相关关系研究. 海洋科学, 2002, 26(3): 68-71] |
MUNISWAMY D, MUNASWAMY V, HALAPPA R, et al. Impact of sodium cyanide on catalase activity in the freshwater exotic carp, Cyprinus carpio (Linnaeus). Pesticide Biochemistry and Physiology, 2008, 92(1): 15-18 DOI:10.1016/j.pestbp.2008.03.013 |
MUTA T, IWANAGA S. The role of hemolymph coagulation in innate immunity. Current Opinion in Immunology, 1996, 8(1): 41-47 DOI:10.1016/S0952-7915(96)80103-8 |
QUIROZ-GUZMAN E, BALCAZAR J L, VAZQUEZ-JUAREZ R, et al. Proliferation, colonization, and detrimental effects of Vibrio parahaemolyticus and Vibrio harveyi during brine shrimp hatching. Aquaculture, 2013, 406/407: 85-90 DOI:10.1016/j.aquaculture.2013.03.008 |
RUAN Y J, GUO X S, YE Z Y, et al. Bacterial community analysis of different sections of a biofilter in a full-scale marine recirculating aquaculture system. North American Journal of Aquaculture, 2015, 77(3): 318-326 DOI:10.1080/15222055.2015.1017128 |
SARSANGI ALIABAD H, NAII A, MORTEZAEI S R S, et al. Effects of restricted feeding levels and stocking densities on water quality, growth performance, body composition and mucosal innate immunity of Nile tilapia (Oreochromis niloticus) fry in a biofloc system. Aquaculture, 2022, 546: 737320 DOI:10.1016/j.aquaculture.2021.737320 |
SHIN N R, WHON T W, BAE J W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends in Biotechnology, 2015, 33(9): 496-503 DOI:10.1016/j.tibtech.2015.06.011 |
SILVA C R, GOMES L C, BRANDAO F R. Effect of feeding rate and frequency on tambaqui (Colossoma macropomum) growth, production and feeding costs during the first growth phase in cages. Aquaculture, 2007, 264(1/2/3/4): 135-139 |
WANG J J, SHEN M F, XUE H, et al. Effects of feeding frequency on growth performance, antioxidant status and disease resistance of juvenile red swamp crayfish, Procambarus clarkii. Pakistan Journal of Zoology, 2021, 53(2): 467-474 |
WANG X D, SHEN Z H, WANG C L, et al. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish and Shellfish Immunology, 2019, 87: 22-31 DOI:10.1016/j.fsi.2018.12.076 |
XI Q F, ZHANG D M, Ye X S, et al. The bacterial community of coastal sediments influenced by cage culture in Xiangshan Bay, Zhejiang, China. Acta Ecologica Sinica, 2013, 33(2): 483-491 [袭琼芬, 张德民, 叶仙森, 等. 象山港网箱养殖对近海沉积物细菌群落的影响. 生态学报, 2013, 33(2): 483-491] |
XING M W, DENG H G, GAO M R, et al. Effects of Artemia from different origins on growth, muscle composition, and stress resistance of Litopenaeus vannamei postlarvae. Journal of Fishery Sciences of China, 2022, 29(1): 70-78 [邢明威, 邓洪刚, 高美荣, 等. 不同产地卤虫幼体对凡纳滨对虾仔虾生长、肌肉组分和抗胁迫能力的影响. 中国水产科学, 2022, 29(1): 70-78] |
YAN G, GU C, WANG C X, et al. Effect of Euryale ferox extract on growth, antioxidant capacity, and immunity of Procambarus clarkii. Freshwater Fisheries, 2022, 52(1): 83-89 [闫戈, 顾晨, 王承鑫, 等. 芡实提取物对克氏原螯虾生长、抗氧化能力及免疫功能的影响. 淡水渔业, 2022, 52(1): 83-89 DOI:10.3969/j.issn.1000-6907.2022.01.011] |
YAN Q Y, LI J J, YU Y H, et al. Environmental filtering decreases with fish development for the assembly of gut microbiota. Environmental Microbiology, 2016, 18(12): 4739-4754 DOI:10.1111/1462-2920.13365 |
ZHANG H. The dynamic changes of microbial communities in aquaculture environment and interactions with water environment. Master′s Thesis of Soochow University, 2015, 46-48 [张浩. 养殖环境微生物群落的动态变化及与水环境的互作. 苏州大学硕士研究生学位论文, 2015, 46-48]
|
ZHANG L, CHEN Z, WANG L, et al. Effect of stocking density on intermediate cultivation of Litopenaeus vannamei postlarvae. Progress in Fishery Sciences, 2019, 40(1): 76-83 [张龙, 陈钊, 汪鲁, 等. 放养密度对凡纳滨对虾苗种中间培育效果的影响. 渔业科学进展, 2019, 40(1): 76-83] |
ZHANG X. Bacterial community structure analysis in typical aquaculture water by PCR-DGGE technology and its correlation with environmental factors. Master′s Thesis of South China University of Technology, 2010, 60-64 [张星. 典型养殖水体中细菌群落结构的DGGE分析及其与环境因子相关性研究. 华南理工大学硕士研究生学位论文, 2010, 60-64]
|
ZHAO Y C, WANG R J, SHEN M, et al. Effects of high-salt stress on daily weight gain, osmoregulation and immune related enzyme activities in Litopenaeus vannamei postlarvae. Journal of Fisheries of China, 2019, 43(4): 833-840 [赵玉超, 王仁杰, 沈敏, 等. 高盐对凡纳滨对虾仔虾生长、渗透调节及免疫相关酶活性的影响. 水产学报, 2019, 43(4): 833-840] |
ZHAO Z G, LI J N, XU Q Y, et al. Effects of different ration levels in temperature fluctuation on digestive enzyme, metabolic enzyme and antioxidase activities of juvenile amur sturgeon (Acipenser schrenckii Brandt). Chinese Journal of Animal Nutrition, 2017, 29(1): 127-133 [赵志刚, 李晋南, 徐奇友, 等. 变温条件下不同投喂水平对施氏鲟幼鱼消化酶、代谢酶和抗氧化酶活性的影响. 动物营养学报, 2017, 29(1): 127-133 DOI:10.3969/j.issn.1006-267x.2017.01.015] |
ZHOU H, HAN M Q, TANG B G, et al. Indoor biofloc culture of Pacific white shrimp Litopenaeus vannamei in low temperature season. Fisheries Science, 2022, 41(3): 424-430 [周晖, 韩满强, 汤保贵, 等. 低温季节凡纳滨对虾室内生物絮团养殖研究. 水产科学, 2022, 41(3): 424-430] |



