2. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业与可持续发展重点实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Marine Fisheries and Sustainable Development, Ministry of Agriculture and Rural Affairs, Qingdao 266071, China
大型藻类能够以有机碳的形式释放光合作用产物,其中,能通过0.7 μm孔径滤膜过滤的部分被定义为溶解有机碳(dissolved organic carbon, DOC),滤膜截留部分则称之为颗粒有机碳(particulate organic carbon, POC) (Baines et al, 1991; Pagano et al, 2014)。大型海藻是沿海生态系统的重要组成部分,其固碳能力强、效率高,作为沿海地区的主要初级生产者,其净初级生产力(net primary productivity, NPP)大于浮游植物等其他生产者(Charpy-Roubaud et al, 1990; Krause-Jensen et al, 2016)。海藻生长过程中释放的DOC和POC能在沿海生态系统的碳循环和碳封存中发挥重要作用(Jiao et al, 2010; 张永雨等, 2017; Weigel et al, 2021)。温度、光照强度和光照周期是影响藻类生长、调控有机碳释放的重要环境因素(Wada et al, 2007; Reed et al, 2015; 李科等, 2017)。关于大型海藻释放有机碳的结果差异较大。已有报道显示,苷苔(Ecklonia cava) DOC释放速率范围为0.05~0.24 mg/(g·h) (Wada et al, 2007);坛紫菜(Pyropia haitanensis) DOC释放速率范围为0~1.67 mg/(g·h),POC的释放速率低于0.005 mg/(g·h) (Xu et al, 2022);角叉菜(Chondrus ocellatus)和亨氏马尾藻(Sargassum henslowianum)等11种大型海藻的DOC释放速率为0~7 mg C/(g·h),POC释放速率为0~17 mg C/(g·h) (Chen et al, 2020)。同一种海藻,因产地来源不同,其有机碳的释放速率也有差异,最高可相差约10倍(Chen et al, 2020)。不同种类海藻释放的DOC与NPP之间的比例也不相同,如坛紫菜释放的DOC仅占NPP的6.3%~25.7% (Xu et al, 2022),苷苔则为18%~62% (Wada et al, 2007),鼠尾藻(Sargassum thunbergii)经干燥胁迫,再复水后,DOC的释放速率要高于NPP (Zhao et al, 2022)。
近年在黄海海域暴发的以铜藻(Sargassum horneri)为主要致灾种的马尾藻金潮引起了广泛关注(郑龙啸等, 2022)。金潮暴发越来越频繁,影响范围越来越广,已经成为继浒苔(Enteromorpha)绿潮之后的又一近海大型海藻灾害(Caselle et al, 2018; 王丹等, 2021)。铜藻在生长时能释放大量的DOC和POC,这些DOC和POC可能对沿海生态系统的碳循环产生较大的影响(张永雨等, 2017)。目前,大型海藻有机碳的释放速率、与初级生产力之间的关系及主要调控因素等问题尚不完全清楚,尤其是缺少关于铜藻释放有机碳的研究。因此,本研究以野生铜藻为对象,通过室内正交实验,探究温度、光照和光照周期对铜藻释放有机碳的影响,以期了解不同因素对铜藻释放有机碳的调控效应、有机碳的释放速率、有机碳释放速率与初级生产力之间的关系等问题,为深入了解铜藻的生理生态学特性及其对沿海生态系统碳循环的影响提供科技支撑。
1 材料与方法 1.1 实验材料与预处理实验铜藻于2021年底取自山东省荣成市桑沟湾养殖区。铜藻在取样后冷藏运输至实验室,在循环水养殖系统中暂养3 d后,用于实验,暂养水温设置为20 ℃。用0.8 μm孔径的混合纤维滤膜过滤海水,用于后续实验。实验开始前,采用过滤海水清洗铜藻,去除铜藻表层的附着物。
1.2 实验设计铜藻生长的适宜温度为7.1~20.5 ℃(孙建璋等, 2009)。光照强度小于200 μmol/(m2·s)时,铜藻NPP与光照强度之间线性关系显著,光照强度大于200 μmol/(m2·s)时,线性关系不显著(刘婷, 2019)。基于此,设置温度和光照水平,具体实验设计见表 1。本实验共设温度、光照强度和光照周期3个变量,每个变量设3个水平,分别为低温(5 ℃)、中温(15 ℃)、高温(25 ℃);低光[86 μmol/(m2·s)]、中光[172μmol/(m2·s)]、高光[258 μmol/(m2·s)];光照周期L∶D=6 h∶18 h、L∶D= 12 h∶12 h、L∶D=24 h∶0 h。实验容器为2 L锥形瓶,加入铜藻(3~5 g/L),实验持续24 h,实验中,光照与黑暗的交替顺序为先黑暗后光照。所有实验组均设3个平行,对照组设2个平行。
|
|
表 1 L9(34)正交实验设计表 Tab.1 Orthogonal experimental design of L9(34) |
在实验开始(0 h)和结束(24 h)时,分别取200 mL水样用于DOC和POC浓度分析,取样前,采用Winkler碘量法测量水体的溶解氧(DO)浓度。水样经0.7 μm孔径WhatmanTM滤膜(450 ℃预处理4 h)过滤后,滤液分装在30 mL棕色玻璃瓶内,用于DOC浓度分析;滤膜用锡纸包裹,用于POC浓度分析。所有样品在分析前均于–20 ℃避光冷冻保存。所有实验用玻璃器皿使用前均酸洗、水洗、纯水润洗、烘干。
1.3 样品分析DOC测定:使用日本岛津TOC-LCSH总有机碳分析仪测定样品的DOC含量。POC测定:使用德国ELEMENTAR元素分析仪测定样品的POC含量。
1.4 计算方法与数据处理通过实验前后水体DO浓度的变化来计算铜藻的NPP,计算公式为:
| $ {\text{NPP}} = \frac{{\Delta {\text{O}}2}}{{W \times t}} \times \frac{{12}}{{32}} $ |
式中,NPP为铜藻干质量的净初级生产力[mg C/(g·h)],ΔO2为实验前后水体DO的质量变化,12/32为C与O2的相对分子质量比,W为实验铜藻的干重(g);t为实验时长(h)。
有机碳(DOC、POC)释放速率(rate, R)指单位质量铜藻(干重)在单位时间内引起的水体有机碳(DOC、POC)含量的变化。
| $ R = \frac{{\Delta C \times V}}{{W \times t}} \times \frac{{12}}{{32}} $ |
式中,R为单位质量铜藻(干重)的DOC (POC)释放速率[mg/(g·h)],ΔC为实验前后DOC (POC)浓度变化量(mg/L);V为实验组海水体积(L);W为实验铜藻的干重(g);t为实验时长(h)。
直观分析法是通过对每个因素均值的极差来进行结果分析(滕海英等, 2008)。通过极差可判断影响实验结果的主要因素和次要因素,可通过直观分析表找到各因素水平的最优组合。直观分析表中的
铜藻的NPP的实验结果见表 2。不同条件下,铜藻NPP在2.785~21.190 mg C/(g·h)之间。直观分析结果显示,本研究中,温度是NPP的主要影响因素,光照强度对NPP的影响最小。各因素在不同水平下对NPP影响的顺序为15 ℃>25 ℃>5 ℃,24 h∶0 h>12 h∶12 h>6 h∶18 h,高光>中光>低光。由此得出,铜藻NPP最高的条件组合为15 ℃、L∶D=24 h∶0 h、高光。
|
|
表 2 净初级生产力结果直观分析及最优条件 Tab.2 Visual analysis of NPP results and optimal conditions |
铜藻释放DOC的实验结果见表 3。不同条件下,铜藻DOC的释放速率在0.653~4.785 mg/(g·h)之间。直观分析结果显示,铜藻DOC释放速率最高的条件组合为25 ℃、L∶D=6 h∶18 h、中光。温度、光照强度和光照周期对铜藻DOC释放速率的影响由强到弱依次为温度、光照强度、光照周期。在实验温度范围内,DOC的释放速率随温度的升高而增大;DOC的释放速率随着光照强度的增加表现为先升高再降低的趋势;光照时间越少释放速率就越高。
|
|
表 3 DOC结果直观分析及最优条件 Tab.3 Visual analysis of DOC results and optimal conditions |
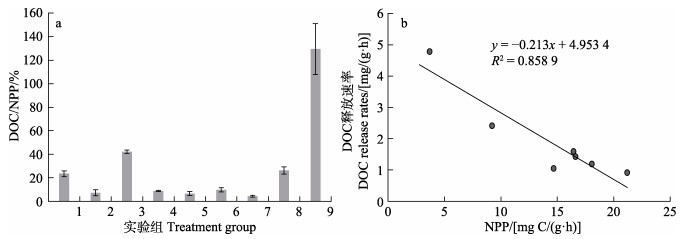
DOC/NPP的值见图 1a。实验结果显示,不同条件下,铜藻释放的DOC占NPP的比例为4%~130%。其中,共计5组占比低于10%,3组占比为20%~42%,1组占比超过100% (129.5%)。图 1b显示,DOC释放速率与NPP之间存在负相关关系(实验组1和3不符合此规律)。
|
图 1 DOC/NPP的值(a)与DOC释放速率随NPP的变化趋势(b) Fig.1 The ratio of DOC/NPP (a) and the trend of DOC release rate with NPP (b) |
铜藻释放POC的实验结果见表 4。不同条件下,铜藻POC的释放速率为0.066~0.322 mg/(g·h)。直观分析结果显示,铜藻POC释放速率最高的条件组合为25℃、L∶D=24 h∶0 h、中光。低温(5 ℃)、高温(25 ℃)、延长光照时长都会促进POC的释放。增加光照强度可以加快POC释放速率,但当光照强度从中光增至高光时,POC释放速率反而有小幅度下降。
|
|
表 4 POC结果直观分析及最优条件 Tab.4 Visual analysis of POC results and optimal conditions |
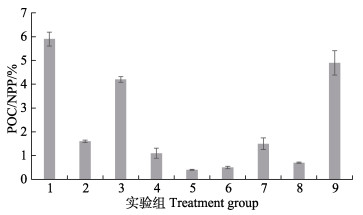
POC/NPP的值见图 2。实验结果显示,不同条件下,铜藻释放的POC占NPP的比例为0.4%~5.9%。其中,共计6组占比低于2%,3组占比为4%~5.9%。
|
图 2 POC占NPP的比例 Fig.2 The ratio of POC to NPP |
本研究发现,室内实验中的DOC和POC释放速率分别在0.653~4.785 mg/(g·h)和0.066~0.322 mg/(g·h)之间。铜藻的DOC释放速率与之前报道的其他马尾藻的DOC释放速率相近(Chen et al, 2020),并高于绿藻、红藻和其他褐藻等大型藻类的DOC释放速率(Paine et al, 2021)。铜藻DOC/NPP在4%~42%之间(实验组9除外),其DOC的释放能力基本符合以往研究中人们对大型藻类的认识(Wada et al, 2007; Watanabe et al, 2020; Xu et al, 2022)。该结果能为精确评估铜藻在不同环境、不同生长时期的有机碳释放通量提供科技支撑。
3.2 温度、光照强度和光照周期对DOC和POC释放速率的影响本研究表明,温度会显著影响铜藻释放DOC和POC的能力,温度与铜藻DOC释放速率之间正相关,与POC释放速率之间表现为胁迫释放。以往对苷苔的研究支持这一结果,即当温度适合苷苔生长时,其DOC的释放量增加、POC释放量减少(Wada et al, 2007)。Barrón等(2014)在对海草甸的研究中也有温度与DOC释放速率正相关相似发现。
以往的研究显示,海带(Saccharina japonica)和坛紫菜等大型海藻的DOC释放速率与光照强度正相关(尼志杰等, 2022; Xu et al, 2022)。然而,本研究中,铜藻释放DOC的速率与光照之间的关系与之前的研究有所差异。对于铜藻来说,光照与DOC释放速率呈正相关可能只存在于某些特定的光照强度区间内,当光照强度超出该区间时,则没有相关性或表现出负相关。因此,可以合理地推断,不同种海藻DOC释放速率对光照强度变化的响应程度不同。由于本研究中光照强度水平设置较少,无法准确判断该推论是否成立,故还需通过开展多光照强度梯度的单因素实验去进一步论证。另外,在针对光照周期的研究中发现,一个光照周期中,黑暗时间越长,DOC释放速率越高。之前的研究表明,环境压力(低pH、低营养盐、缺水、高温和强光等)是海藻释放DOC的驱动因素,海藻在生长过程中面临环境压力时,会出现细胞膜损伤、渗透压升高甚至组织腐烂等情况,细胞膜损伤会引起细胞内容物的大量泄漏(Thornton et al, 2014; Zhao et al, 2022; Xu et al, 2022)。当藻类处于低营养盐胁迫时,光合固碳速率大于其他大分子物质的合成速率,过量的光合产物会被主动释放到细胞外(Fogg, 1983)。在本研究中,光照时间作为铜藻的生长要素之一,光照时间越短,为铜藻带来的生长压力越大。因此,光照时间与DOC的释放速率负相关。在黑暗条件下,铜藻的呼吸作用能够同时降低海水的pH和DO。已有研究证实,pH降低能够促进大型藻类释放DOC (Iñiguez et al, 2016; Diaz-Pulido et al, 2020)。然而,并非所有的大型藻类释放DOC都受pH的影响,如Paine等(2021)对边花昆布(Ecklonia radiata)、边缘勒诺曼藻(Lenormandia marginata)和拉绒石菜(Plocamium cirrhosum) 3种大型藻类的研究发现,pH的降低并不会影响其释放DOC的速率。因此,不能排除DO降低影响DOC释放的可能,故还需单独开展实验分别探究降低pH和降低DO对海藻释放DOC的影响。以上结果表明,铜藻释放有机碳的机制复杂,受温度、光照强度和光照周期等多种因素影响。本研究为后续铜藻释放有机碳的研究奠定了基础,也可为其他海藻释放有机碳的研究提供参考。
3.3 DOC和POC释放速率与NPP之间的关系在DOC释放量与NPP之间的关系的研究中,有人提出“光合产物扩散假说”(Fogg, 1983; Marañón et al, 2005; Weigel et al, 2021),认为DOC释放量与光合作用的固碳量成正比,即DOC释放量占NPP的比值应相对恒定。李科等(2017)研究显示,在1个光照周期内,光照时间越长,铜藻的NPP越高,在L∶D=24 h∶0 h时,NPP最高。为探究铜藻在长光照周期条件下的DOC释放情况,本研究设置了连续光照(L∶D=24 h∶0 h)实验。研究发现,在连续光照条件下,铜藻具有最高的NPP和最低的DOC释放速率,即铜藻释放DOC不符合“光合产物扩散假说”。此外,本研究结果表明,铜藻的DOC释放速率与NPP之间呈负相关关系,但5 ℃实验组中的DOC释放速率与NPP之间的负相关性不显著。这可能是由于铜藻酶活性低、代谢缓慢,细胞膜的通透性并未发生改变或改变幅度较小,从而导致低温条件下,铜藻的DOC释放速率和NPP均处于较低水平,再加上受到其他因素多重胁迫,铜藻的生理状态受到影响,致使细胞膜的通透性发生改变,细胞内容物更容易被释放出来。所以,在低温抑制NPP的条件下,铜藻的DOC释放速率并未得到显著提高。本研究发现,实验组9中DOC/NPP>100%,这一结果可能是由于铜藻面临高温和长时间黑暗双重环境压力时,其生理状态受到严重胁迫,DOC大量释放的同时光合速率远低于适宜条件下的光合速率等因素共同造成的。
本研究发现,铜藻的POC释放速率与NPP之间无显著的相关性,POC/NPP (0.4%~5.9%)与鼠尾藻和坛紫菜相近,低于其他种马尾藻(Chen et al, 2020; Xu et al, 2021)。当NPP相对较高[>9 mg C/(g·h)]时,POC/NPP相对稳定,约为(1±0.6)%;当NPP相对较低[<4 mg C/(g·h)]、处于生长逆境时,POC/NPP则会升高至4%~6%。已有研究发现,POC的释放速率有随生长率增加而增加的趋势,但二者间的相关性较弱(Chen et al, 2020; Xu et al, 2022)。本研究发现,增加光照强度或光照时长都能提高NPP和促进POC的释放,即POC释放速率随光照变化的趋势与NPP随光照变化的趋势基本相同。本研究还发现,铜藻在5 ℃时,NPP最低,25 ℃时,NPP最高,但是铜藻在5 ℃和25 ℃时,POC均有较高的释放速率。低温抑制NPP、促进POC释放的结论已在浮游植物中得到验证(Watanabe, 1980; Verity, 1981)。据此可以合理推测,光照变化是观察到POC释放速率与生长率之间有相关性的主要原因,温度变化则是导致二者间相关性较弱的内在原因。明确DOC/NPP和POC/NPP的范围区间,有助于初步估算金潮发生时,铜藻对沿海生态系统碳循环的贡献。
BAINES S B, PACE M L. The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater systems. Limnology and Oceanography, 1991, 36(6): 1078-1090 DOI:10.4319/lo.1991.36.6.1078 |
BARRÓN C, APOSTOLAKI E T, DUARTE C M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Frontiers in Marine Science, 2014, 1: 42 |
CASELLE J E, DAVIS K, MARKS L M. Marine management affects the invasion success of a non-native species in a temperate reef system in California, USA. Ecology letters, 2018, 21(1): 43-53 DOI:10.1111/ele.12869 |
CHARPY-ROUBAUD C, SOURNIA A. The comparative estimation of phytoplanktonic, microphytobenthic and macrophytobenthic primary production in the oceans. Marine Microbial Food Webs, 1990, 4(1): 31-57 |
CHEN S W, XU K, JI D H, et al. Release of dissolved and particulate organic matter by marine macroalgae and its biogeochemical implications. Algal Research, 2020, 52: 102096 DOI:10.1016/j.algal.2020.102096 |
DIAZ-PULIDO G, BARRÓN C. CO2 enrichment stimulates dissolved organic carbon release in coral reef macroalgae. Journal of Phycology, 2020, 56(4): 1039-1052 DOI:10.1111/jpy.13002 |
FOGG G E. The ecological significance of extracellular products of phytoplankton photosynthesis. Botanica Marina., 1983, 26(1): 3-14 |
IÑIGUEZ C, CARMONA R, LORENZO M R, et al. Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta. Polar Biology, 2016, 39: 1979-1991 DOI:10.1007/s00300-015-1724-x |
JIAO N Z, HERNDL G J, HANSELL D A, et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Reviews Microbiology, 2010, 8(8): 593-599 DOI:10.1038/nrmicro2386 |
KRAUSE-JENSEN D, DUARTE C M. Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience, 2016, 9(10): 737-742 DOI:10.1038/ngeo2790 |
LI K, PAN Y R, WU J P, et al. Effect of LED lightings on growth of Sargassum horneri under different photoperiods. Acta Agriculturae Zhejiangensis, 2017, 29(4): 631-636 [李科, 潘耀茹, 吴嘉平, 等. 不同光照周期下LED光源对铜藻生长的影响. 浙江农业学报, 2017, 29(4): 631-636 DOI:10.3969/j.issn.1004-1524.2017.04.17] |
LIU T. Study on the physiological effects and mechanism of important environmental factors on golden tide causative species Sargassum horneri. Masterxs Thesis of Wenzhou University, 2008 [刘婷. 金潮原因种铜藻(Sargassum horneri)对关键环境因子的生理响应与机理解析. 温州大学硕士研究生学位论文, 2019]
|
MARAÑÓN E, CERMEÑO P, PÉREZ V. Continuity in the photosynthetic production of dissolved organic carbon from eutrophic to oligotrophic waters. Marine Ecology Progress Series, 2005, 299: 7-17 DOI:10.3354/meps299007 |
NI Z J, LI B, SUN Y Q, et al. Effects of light intensity and nutrients on dissolved organic carbon released from Saccharina japonica young seedling. Progress in Fishery Sciences, 2022, 43(5): 8-15 [尼志杰, 李斌, 孙琰晴, 等. 光照强度和营养盐对海带幼苗释放溶解有机碳的影响. 渔业科学进展, 2022, 43(5): 8-15] |
PAGANO T, BIDA M, KENNY J E. Trends in levels of allochthonous dissolved organic carbon in natural water: A review of potential mechanisms under a changing climate. Water, 2014, 6(10): 2862-2897 DOI:10.3390/w6102862 |
PAINE E R, SCHMID M, BOYD P W, et al. Rate and fate of dissolved organic carbon release by seaweeds: A missing link in the coastal ocean carbon cycle. Journal of Phycology, 2021, 57(5): 1375-1391 DOI:10.1111/jpy.13198 |
REED D C, CARLSON C A, HALEWOOD E R, et al. Patterns and controls of reef-scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera. Limnology and Oceanography, 2015, 60(6): 1996-2008 DOI:10.1002/lno.10154 |
SUN J Z, ZHUANG D G, SUN Q H, et al. Aritificial cultivation trials of Sargassum horneri at Nanji islands of China. South China Fisheries Science, 2009, 5(6): 41-46 [孙建璋, 庄定根, 孙庆海, 等. 铜藻人工栽培的初步研究. 南方水产, 2009, 5(6): 41-46 DOI:10.3969/j.issn.1673-2227.2009.06.008] |
TENG H Y, ZHU G Q, HUANG P, et al. Orthogonal experimental design case analysis. Pharmaceutical Care and Research, 2008, 8(1): 75-76 [滕海英, 祝国强, 黄平, 等. 正交试验设计实例分析. 药学服务与研究, 2008, 8(1): 75-76 DOI:10.3969/j.issn.1671-2838.2008.01.025] |
THORNTON D C O. Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. European Journal of Phycology, 2014, 49(1): 20-46 DOI:10.1080/09670262.2013.875596 |
VERITY P G. Effects of temperature, irradiance, and daylength on the marine diatom Leptocylindrus danicus Cleve. I. Photosynthesis and cellular composition. Journal of Experimental Marine Biology and Ecology, 1981, 55(1): 79-91 DOI:10.1016/0022-0981(81)90094-0 |
WADA S, AOKI M N, TSUCHIYA Y, et al. Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. Journal of Experimental Marine Biology and Ecology, 2007, 349(2): 344-358 DOI:10.1016/j.jembe.2007.05.024 |
WANG D, JIANG Y F, WANG X Q, et al. Research progress in ecological dynamics of golden tide dominated by Sargassum. Advances in Earth Science, 2021, 36(7): 753-762 [王丹, 姜亦飞, 王先桥, 等. 我国马尾藻金潮生态动力学研究进展. 地球科学进展, 2021, 36(7): 753-762] |
WATANABE K, YOSHIDA G, HORI M, et al. Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences, 2020, 17(9): 2425-2440 DOI:10.5194/bg-17-2425-2020 |
WATANABE Y. A study of the excretion and extracellular products of natural phytoplankton in Lake Nakanuma, Japan. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie, 1980, 65(6): 809-834 DOI:10.1002/iroh.19800650606 |
WEIGEL B L, PFISTER C A. The dynamics and stoichiometry of dissolved organic carbon release by kelp. Ecology, 2021, 102(2): e03221 DOI:10.1002/ecy.3221 |
XU K, LI M Y, WANG W L, et al. Differences in organic carbon release between conchocelis and thalli of Pyropia haitanensis and responses to changes in light intensity and pH. Algal Research, 2022, 61: 102574 DOI:10.1016/j.algal.2021.102574 |
ZHANG Y Y, ZHANG J H, LIANG Y T, et al. Carbon sequestration processes and mechanisms in coastal mariculture environments in China. Science China Earth Sciences, 2017, 47(12): 1414-1424 [张永雨, 张继红, 梁彦韬, 等. 中国近海养殖环境碳汇形成过程与机制. 中国科学: 地球科学, 2017, 47(12): 1414-1424] |
ZHAO Z F, ZHONG Z H, WANG X, et al. Effects of desiccation and rehydration on carbon fixation and DOC release in Sargassum thunbergii. Aquatic Botany, 2022, 179: 103516 DOI:10.1016/j.aquabot.2022.103516 |
ZHENG L X, WU M Q, ZHAO J, et al. Remote sensing monitoring and temporal and spatial distribution characteristics of gold tide in the South Yellow Sea. Haiyang Xuebao, 2022, 44(5): 12-24 [郑龙啸, 吴孟泉, 赵杰, 等. 南黄海金潮的遥感监测及时空分布特征研究. 海洋学报, 2022, 44(5): 12-24] |



