2. 江西省水产资源与利用重点实验室江西 南昌 330031;
3. 高安市嘉诚特种泥鳅养殖基地 江西 宜春 330800
2. Key Laboratory of Aquatic Resources and Utilization of Jiangxi, Nanchang 330031, China;
3. Jiangxi Jiacheng Loach Breeding Base, Yichun 330800, China
动物的色素沉积在其生存及繁衍中扮演着重要的角色,可以影响动物的体温调节、群体交流、求偶、躲避捕食者以及抵御紫外线等(Kelsh, 2004; Hubbard et al, 2010)。另外,一些体色不同的鱼表现出不同的生长性能(Rodriguez et al, 2019; 傅建军等, 2021)。鱼类体色由色素细胞的类型、分布、发育阶段和虹彩细胞对光线的反射能力决定,并随外界环境和生理状态的变化而适应性变化(Hirata et al, 2003、2005)。在硬骨鱼中存在6种类型的色素细胞,包括黑色素、虹彩色素、黄色素、红色素、白色素和蓝色素细胞(Fujii, 2000; Sugimoto, 2002; Burton, 2002; Burton et al, 2017; Phang et al, 2012)。在红色和金色斗鱼(Betta splendens)的表皮和真皮中主要是红色素细胞和黄色素细胞。在慈鲷(Cichlidae) (Maan et al, 2013)和泥鳅(Misgurnus anguillicaudatus)(Sheng et al, 2022)皮肤中色素细胞主要是黑色素、黄色素和虹彩色素细胞。色素团在色素细胞中被合成并保留其特定的色素和内部结构(Bagnara et al, 2006)。皮肤色素图案反映了色素细胞本身的数量和排列情况。鱼类皮肤具有卵状、斑点、水平条纹和垂直条纹等颜色图案(Santos et al, 2016; Roberts et al, 2009; Kratochwil et al, 2019、2018; Ahi et al, 2017; Hendrick et al, 2019; Sazima et al, 2006)。
鱼类体色及体表图案随发育阶段而变化,不同发育阶段的鱼,其体表色素细胞亦表现出不同的发育特征(Reiss et al, 2012)。体色及体表图案是在胚后发育阶段出现的特征,由于幼鱼生长周期长以及成体体型个体差异大,对它们的研究相对困难。到目前为止,对体色及体表图案形成的分子和发育基础以及进化多样性的研究只集中在斑马鱼(Danio rerio)和青鳉(Oryzias latipes)等少数模式生物中(Meyer et al, 1993、1995; Kelsh et al, 1996; Nagao et al, 2014; Irion et al, 2019; Patterson et al, 2019),对硬骨鱼类体表图案的发育形成和进化仍知之甚少(朱杰等, 2002; 马学坤等, 2006; 于道德等, 2012)。
小眼畸形相关转录因子(microphthalmia-associated transcription factor, MITF)是碱性螺旋–环–螺旋亮氨酸拉链(bHLH-Zip)蛋白家族的成员,作为细胞发育的主要调节因子调节黑色素细胞的存活、增值和分化(Hou et al, 2008)。mitf基因最初因小鼠(Mus musculus)小眼畸形位点的基因发生突变而被发现(Hodgkinson et al, 1993)。在哺乳动物中,单个mitf基因通过使用不同的替代启动子和外显子产生多个亚型(刘文艳等, 2017)。在鱼类中,与小鼠mitf-A和mitf-M相对应的mitfa和mitfb基因已经在包括斑马鱼、剑尾鱼(Xiphophorus hellerii)和红鳍东方鲀(Fugu rubripes)等多个物种中被报道。其中,mitfa基因在皮肤色素细胞发育过程中发挥重要作用(Lister et al, 1999),而mitfb主要在眼内表达,在缺少mitfa活性的情况下为视网膜色素上皮(RPE)细胞的发育发挥必需的功能(Lister et al, 2001)。mitf纯合子斑马鱼突变体在胚胎发育的整个过程中均缺乏黑色素细胞,但虹彩色素细胞的数量则较野生型有所增加(Lister et al, 1999)。
泥鳅是一种小型经济鱼类,广泛分布于亚洲海岸的中国、日本、朝鲜、俄罗斯和印度等地(Wang et al, 2018; Yi et al, 2019)。江西鄱阳湖地区存在3种花斑类型的泥鳅,根据其皮肤黑色斑点的有无及大小分别命名为大花斑泥鳅、小花斑泥鳅和无花斑泥鳅,且在成体泥鳅皮肤中存在3种类型的色素细胞(Sheng et al, 2022)。然而,到目前为止,尚无关于其皮肤色素细胞发育特征的报道,对泥鳅色素沉积过程的分子机制也知之甚少。本文重点研究鄱阳湖泥鳅皮肤色素细胞的发育特征,对3种花斑泥鳅的黑色素细胞发育特征进行比较,并分析mitfa基因的序列特征以及该基因在泥鳅不同发育阶段胚胎及成体不同组织中的转录表达水平,以期为探索泥鳅体表颜色图案的形成、发展和调控机制提供基础数据。
1 材料与方法 1.1 动物样品采集泥鳅人工繁殖实验在南昌大学江西省水产动物资源与利用重点实验室进行,所用亲本取自高安市嘉诚特种泥鳅养殖基地。选取性腺发育成熟、无损伤、无畸形的泥鳅作为人工繁殖的亲鱼,按照大花斑泥鳅(♀)×大花斑泥鳅(♂)、小花斑泥鳅(♀)×小花斑泥鳅(♂)、无花斑泥鳅(♀)×无花斑泥鳅(♂)建立家系。雌雄亲本采用在胸鳍后肌肉注射催产素的方法进行人工催产,每尾雌鳅注射40 IU的催产素(上海生物工程有限公司),雄鳅注射剂量减半。精子和卵子经人工授精结合产生受精卵,随后将受精卵转移至孵化桶中,于(23±1) ℃的微流水中孵化。
2021年5―7月,在从受精卵期到60日龄幼鱼期各个发育阶段的泥鳅胚胎、仔鱼、稚鱼和幼鱼中各随机选取适量发育正常的胚胎或鱼苗样品,用于观察色素细胞类型及黑色素细胞的发育。收集的另一份样品用于检测mitfa基因在泥鳅不同发育阶段胚胎中的mRNA表达水平。体重为(20.0±0.2) g的泥鳅成体在水温为(23±1) ℃的自动循环水养殖系统中暂养3 d,期间每天投喂2次新鲜饵料。之后,从中选取健康的泥鳅3尾,取背部皮肤、腹部皮肤、尾鳍、肠、肝脏、心脏、全脑和肌肉组织用于基因表达分析。
1.2 色素细胞及黑色素细胞的形态采用0.04%三卡因(MS-222, Sigma-Aldrich)对泥鳅进行麻醉处理,观察并拍摄记录了泥鳅从胚胎至孵化后60 d幼鱼的色素细胞形成过程和黑色素细胞形态,并比较了3种类型泥鳅皮肤黑色素细胞的发育特征。
1.3 mitfa基因全长cDNA克隆使用SuperScriptTM Ⅲ逆转录酶试剂盒(Invitrogen),以4 μL泥鳅皮肤总RNA为模板,利用SMART寡核苷酸引物和5′/3′-CDS引物合成第1链cDNA。根据鲤科(Cyprinidae)鱼类mitf基因的保守同源序列设计引物(mitf-F和mitf-R,表 1),通过同源克隆获得泥鳅mitfa基因的中间序列。根据mitfa中间片段序列设计2对特异性引物(3′-GSP和3′-NGSP,5′-GSP和5′-NGSP,表 1),利用SMARTer RACE 5′/3′试剂盒(TaKaRa),通过cDNA末端快速扩增法(rapid amplification of cDNA ends, RACE)经2轮PCR克隆获得mitfa cDNA的3′和5′端序列。实验时,首先使用引物3′-GSP (或5′-GSP)和通用引物混合物(UPM,表 1)进行第1轮PCR。用ddH2O将PCR产物稀释作为模板,使用引物3′-NGSP (或5′-NGSP)和通用引物混合物(UPM)进行第2轮PCR。2轮PCR均采用降落式PCR程序(Golden Star T6 Super PCR Mix Ver.2,擎科,北京):98 ℃ 2 min;98 ℃ 10 s,Tm 15 s,35个循环;72 ℃ 20 s;72 ℃ 5 min。目的片段均使用DNA凝胶回收试剂盒(擎科,北京)进行回收,回收产物用于构建重组质粒,经检验后送至测序公司(擎科,北京)测序获得相应核酸序列。
|
|
表 1 mitfa克隆及基因表达分析引物 Tab.1 Primers for mitfa cloning and gene expression analysis |
使用Trizol试剂(TaKaRa)从收集到的组织样品中提取总RNA。获得的总RNA经微量分光光度计(Genova Nano, 英国)和琼脂糖凝胶电泳检测后置于–80 ℃冰箱备用,或立即使用反转录试剂盒(PrimeScript™ RT reagent Κit with gDNA Eraser, TaKaRa)合成单链cDNA。
通过qRT-PCR技术,使用CFX96 TouchTM实时PCR检测系统(Bio-Rad,美国)检测不同发育阶段泥鳅胚胎和成体不同组织中mitfa mRNA的表达情况。qRT-PCR体系如下:12.5 μL 2×TB Green Premix Ex TaqⅡ,2 μL cDNA模板,上下游引物(mitfa-F和mitfa-R,表 1)各0.5 μL (10 μmol/L),9.5 μL ddH2O,总体积为25 μL。qRT-PCR程序如下:95 ℃ 5min;95 ℃ 5 s,55 ℃ 30 s,72 ℃ 30 s,40个循环。实验设置空白组(不添加模板)作为阴性对照。实验结束后,通过熔解曲线检测扩增的特异性,采用2−ΔΔCt法计算mitfa基因的相对表达量。
2 结果 2.1 泥鳅发育早期体表色素细胞出现的时间顺序3种花斑泥鳅胚胎发育阶段均未见色素细胞形成。以大花斑泥鳅为例,出膜后,1日龄泥鳅体表出现黑色素细胞(图 1a),2日龄仔鱼眼睛中出现虹彩细胞(图 1b),7日龄时体表出现黄色素细胞(图 1c),12日龄时体表出现虹彩细胞(图 1d)。随着泥鳅的进一步发育,其体表黑色素细胞、黄色素细胞和虹彩细胞的数量越来越多。
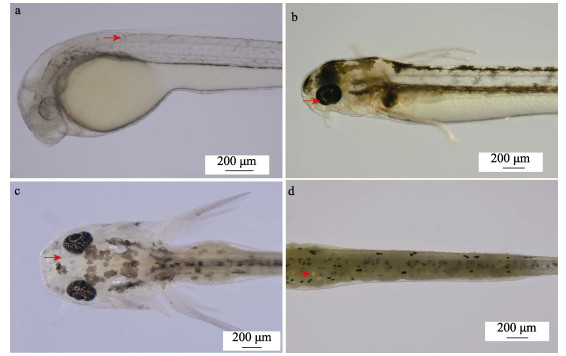
|
图 1 大花斑泥鳅早期发育阶段3种色素细胞发育过程 Fig.1 Development progression of three types of pigment cells in early development stage of large-blackspot loach a:1日龄仔鱼(红色箭头示黑色素细胞);b:2日龄仔鱼(红色箭头示虹彩细胞);c:7日龄稚鱼(红色箭头示黄色素细胞);d:12日龄稚鱼(红色箭头示虹彩细胞)。 a: 1-day-old larva, the red arrow points to the melanocytes; b: 2-day-old larva, the red arrow points to the iridophores; c: 7-day-old juvenile, the red arrow points to xanthophore; d: 12-day-old juvenile, the red arrow points to iridophores. |
从仔鱼期到稚鱼后期(21日龄),泥鳅体表均可见幼体型黑色素细胞;从稚鱼晚期(22日龄)到成体期泥鳅的皮肤上可见成体型黑色素细胞。仔鱼期时,泥鳅体表黑色素细胞由星状发育成雪花状/树突状(图 2a、b);从仔鱼晚期到稚鱼后期(21日龄),黑色素细胞形态由大的浅黑点状向深黑点状发育,最后向细黑点状发育(图 2c、d);从稚鱼后期(22日龄)到成鱼期,细黑点状的幼体型黑色素细胞逐渐发育成树突状或圆点状的成体型黑色素细胞(图 2e、f)。
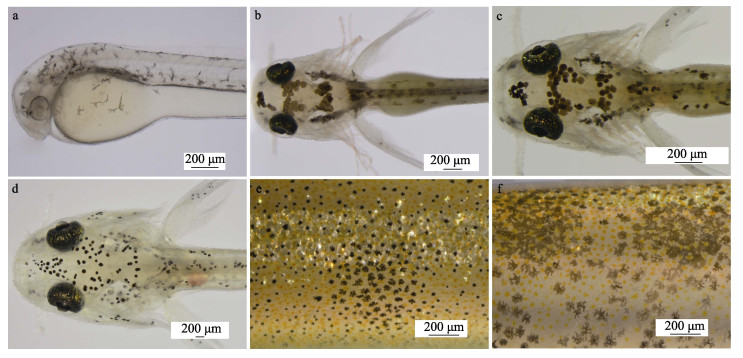
|
图 2 大花斑幼体泥鳅体表黑色素细胞发育 Fig.2 Development of melanocytes on body surface of large-blackspot larvae loach a:1日龄仔鱼;b:5日龄仔鱼;c:6日龄稚鱼;d:21日龄稚鱼;e和f:45日龄泥鳅体表黑色素细胞。 a: 1-day-old larvae; b: 5-day-old larvae; c: 6-day-old juveniles; d: 21-day-old juveniles; e and f: Melanocytes on body surface of 45-old-day loach. |
显微观察并比较3种花斑泥鳅体表黑色素细胞的发育进程。结果显示,孵化后2~21 d的大花斑泥鳅处于仔鱼期和稚鱼期发育阶段,其体表黑色素细胞均为幼体型黑色素细胞(图 3a、b)。孵化后22 d,泥鳅体表开始出现成体型黑色素细胞,多为细黑点状,随后黑色素细胞逐渐聚集形成规则的大黑点(图 3c、d)。孵化后45 d,大花斑泥鳅体表黑色素细胞有规则地聚集,呈黑斑状(图 3e、f)。经局部放大后观察,可见60日龄大花斑泥鳅体表黑色素细胞呈菊花状,许多细长突起从细胞体延伸至外周(图 3g、h)。

|
图 3 大花斑泥鳅体表黑色素细胞的早期发育 Fig.3 Early development of melanocytes on body surface of large-blackspot loach a和b:20日龄泥鳅;c和d:22日龄泥鳅;e和f:45日龄泥鳅;g:60日龄泥鳅侧面皮肤黑色素细胞;h:60日龄泥鳅背部皮肤黑色素细胞。 a and b: 20-day-old loach; c and d: 22-day-old loach; e and f: 45-day-old loach; g: Melanocytes on side skin of 60-day-old loach; h: Melanocytes on the back skin of 60-day-old loach. |
孵化后2~21 d,小花斑泥鳅体表黑色素细胞均为幼体型黑色素细胞(图 4a、b)。从22日龄开始,泥鳅体表皮肤可见成体型黑色素细胞,此时,大多数黑色素细胞呈点状(图 4c、d)。从45日龄开始,泥鳅的形态已与成年泥鳅无异,此时,黑色素细胞呈点状,相当数量的黑色素细胞聚集成小黑点,形成肉眼可见的小花斑(图 4e、f)。继续跟踪观察,可见一部分黑色素细胞从细胞体延伸出短粗的突起而呈树突状,其余则保持点状(图 4g、h)。

|
图 4 小花斑泥鳅体表黑色素细胞的早期发育 Fig.4 Early development of melanocytes on body surface of small-blackspot loach a和b:7日龄泥鳅;c和d:22日龄泥鳅;e和f:45日龄泥鳅;g:60日龄泥鳅侧面皮肤黑色素细胞;h:60日龄泥鳅背部皮肤黑色素细胞。 a and b: 7-day-old loach; c and d: 22-day-old loach; e and f: 45-day-old loach; g: Melanocytes on side skin of 60-day-old loach; h: Melanocytes on the back skin of 60-day-old loach. |
在无花斑泥鳅孵化2~21 d内,其体表黑色素细胞为幼体型黑色素细胞(图 5a、b)。从22日龄开始,体表可见成体型黑色素细胞,此时,大多数黑色素细胞呈树突状(图 5c、d)。孵化后47 d,泥鳅体表的黑色素细胞均为深黑色的成体型黑色素细胞(图 5e、f)。60日龄时,可见黑色素细胞从胞体延伸出3~6个粗大的树突,树突上再延伸出许多小树突,此时的黑色素细胞均匀分布在泥鳅背部(图 5g、h)。

|
图 5 无花斑泥鳅体表黑色素细胞的早期发育 Fig.5 Early development of melanocytes on body surface of non-blackspot loach a和b:7日龄泥鳅;c和d:22日龄泥鳅;e和f:45日龄泥鳅;g:60日龄泥鳅侧面皮肤黑色素细胞;h:60日龄泥鳅背部皮肤黑色素细胞。 a and b: 7-day-old loach; c and d: 22-day-old loach; e and f: 45-day-old loach; g: Melanocytes on side skin of 60-day-old loach; h: Melanocytes on the back skin of 60-day-old loach. |
mitfa基因cDNA序列全长为1 482 bp,包含一个长度为1 227 bp的开放阅读框。推导的蛋白质序列含408个氨基酸,相对分子质量为45.68 kDa,预测等电点为7.16,为非跨膜蛋白。预测的MITF_TFEB_C3_N、bHLH-Zip和DUF 3371结构域分别位于118~396、727~1 038和1 018~1 194位点(图 6)。序列相似性分析显示,mitfa与大西洋鲑(Salmo salar)、虹鳟(Oncorhynchus mykis)、黄颡鱼(Tachysurus fulvidraco)、鲤(Cyprinus carpio)和斑马鱼的同源性较高(58.8%~83.2%),与真海鞘(Halocynthia roretzi) (34.8%)的同源性较低。MITFa的三级结构预测结果显示,其含有MITF_TFEB_C3_N和bHLH-Zip结构域,其中,bHLH-Zip结构域包含下游DNA结合位点和亮氨酸拉链。
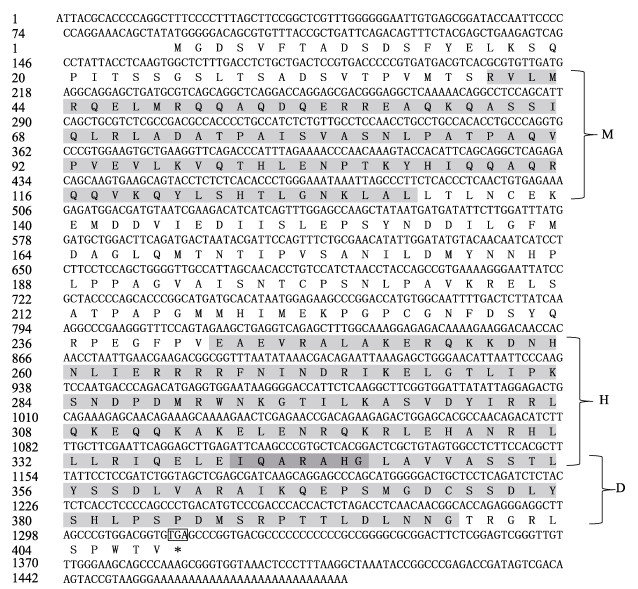
|
图 6 鄱阳湖泥鳅mitfa基因核酸序列和预测氨基酸序列 Fig.6 The nucleotide and predicted amino aquences of mitfa in loach M为MITF_TFEB_C_3_N结构域;H为bHLH-Zip结构域;D为DUF3371结构域;*表示终止密码子;灰色区域为结构域,深灰色区域为bHLH-Zip结构域和DUF3371结构域的重叠区。 M means MITF_TFEB_C_3_N domain, H means bHLH-Zip domain, D means DUF3371 domain. * shows stop codon. The grey region shows domains, dark grey shows overlapping region between bHLH-Zip and DUF3371 domain. |
qRT-PCR结果显示,mitfa基因在泥鳅胚胎发育的各个阶段均有表达。在大花斑泥鳅胚胎中,mitfa的表达水平在受精卵期最高(P < 0.01),其次是多细胞期和原肠胚期(P > 0.05),并在原肠胚期之后趋于稳定(P > 0.05)(图 7a);在小花斑泥鳅胚胎中,mitfa的表达水平在受精卵阶段最高(P < 0.01),其次是多细胞和囊胚阶段,之后,mitfa表达水平在原肠胚期极显著降低(P < 0.01),且与后续几个发育阶段泥鳅胚胎mitfa的表达水平无显著差异(P > 0.05)(图 7b);在无花斑泥鳅胚胎中,mitfa的表达水平在受精卵阶段最高,在多细胞期极显著降低(P < 0.01),并逐步降低至极低表达水平,但多细胞期胚胎与后续胚胎发育各时期胚胎mitfa的表达水平差异并不显著(P > 0.05)(图 7c)。
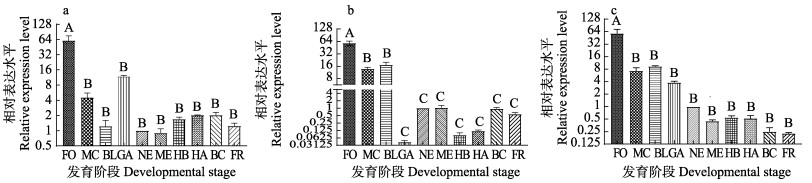
|
图 7 不同发育阶段3种花斑泥鳅mitfa基因的相对表达量 Fig.7 The relative expression level of mitfa in different developmental stages of loach with three types of black spots a:大花斑泥鳅;b:小花斑泥鳅;c:无花斑泥鳅。FO:受精卵期;MC:多细胞期;BL:囊胚期;GA:原肠胚期;NE:神经胚期;ME:肌肉效应期;HB:心跳期;HA:孵化期;BC:血液循环期;FR:鱼苗期。不同大写字母表示存在极显著差异(P < 0.01),下同。 a: Large-blackspot loach; b: Small-blackspot loach; c: Non-blackspot loach. FO: Fertilized ovum stage; MC: Multicellular stage; BL: Blastula stage; GA: Gastrula stage; NE: Neurula stage; ME: Muscle effect stage; HB: Heartbeat stage; HA: Hatching stage; BC: Blood circulation stage; FR: Fry stage. Different capital letters indicate highly significant differences (P < 0.01). The same below. |
qRT-PCR结果显示,在3种泥鳅所有受检组织中都能检测到mitfa mRNA的表达,且在肌肉和背部皮肤中表达水平最高(P < 0.01)(图 8)。在大花斑泥鳅中,mitfa在肠中的表达水平比在腹部皮肤和肝脏中高(P < 0.05),在脑和心脏中的表达水平无显著差异(P > 0.05)(图 8a);在小花斑泥鳅中,肌肉组织中mitfa的表达水平极显著高于背部皮肤(P < 0.01),且二者极显著高于其他组织(P < 0.01),除此之外,mitfa在其他组织中的表达水平均无显著差异(P > 0.05) (图 8b);在无花斑泥鳅中,mitfa在背皮中的表达水平极显著高于肌肉(P < 0.01),在尾鳍、腹皮、脑和内脏器官中的表达水平较低(图 8c)。
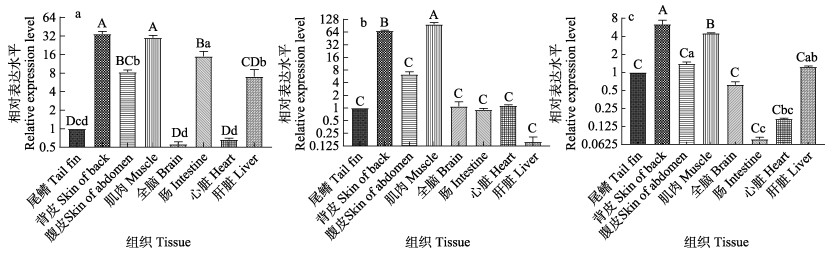
|
图 8 3种花斑泥鳅不同组织中mitfa基因的相对表达量 Fig.8 The relative expression levels of mitfa in different tissues of loach with three types of black spots a:大花斑泥鳅;b:小花斑泥鳅;c:无花斑泥鳅。条形柱上方不同小写字母表示存在显著差异(P < 0.05)。 a: Large-blackspot loach; b: Small-blackspot loach; c: Non-blackspot loach. Different lowercase letters above the bar indicate significant differences (P < 0.05). |
在泥鳅色素细胞的发育时序中,黑色素细胞最先出现,其次是黄色素细胞和虹彩细胞,这与大部分硬骨鱼类如大菱鲆(Scophthamus maximus)(朱杰等, 2002)和黑鲷(Sparus macrocephalus)(于道德等, 2012)中的出现时序一致。在翘嘴鳜(Siniperca chautsi)(刘伟等, 2019)和圆斑星鲽(Verasper variegatus)(严俊丽等, 2017)的胚胎发育阶段观察到黑色素细胞,而橘色双冠丽鱼(Amphilophus citrinellus)(韦敏侠等, 2015)和七彩神仙鱼(Symphysodon spp.)(刘晓东等, 2008)则在胚后阶段才出现黑色素细胞。泥鳅体表黑色素细胞的形态与橘色双冠丽鱼和七彩神仙鱼相似。可见,黑色素细胞的发育时间在硬骨鱼中并不完全一致。
在斑马鱼的胚胎期、仔鱼期和稚鱼期,黑色素细胞均为来源于神经嵴细胞的仔鱼型黑色素细胞。在变态期,成体型色素细胞开始出现并逐渐覆盖鱼体(Patterson et al, 2019)。在成鱼阶段,黑色素细胞均为成体型黑色素细胞,由神经嵴细胞发育、分化形成(Budi et al, 2008)。大菱鲆仔鱼早期发育阶段体表的黑色素细胞为幼体型黑色素细胞,在黑鲷和橘色双冠丽鱼幼鱼阶段,幼体型黑色素细胞发育成为成体型黑色素细胞(朱杰等, 2002; 于道德等, 2012; 韦敏侠等, 2015)。泥鳅黑色素细胞的发育特征与大菱鲆和橘色双冠丽鱼相似。大花斑泥鳅皮肤黑色素细胞的胞体比小花斑和无花斑泥鳅的大;在小花斑和无花斑泥鳅的皮肤中,黑色素细胞的胞体致密,呈树突状(Sheng et al, 2022)。因此,小花斑泥鳅黑色素细胞的发育和形态可能与大花斑泥鳅不同,但与无花斑泥鳅相似。总的来说,黑色素细胞在许多硬骨鱼中发育较早,它们分为幼体型黑色素细胞和成体型黑色素细胞,具有不同的形态变化。
3.2 泥鳅色素图案的形成作为最显著的外部形态特征之一,色素沉着图案在动物的生存和交流中起着重要的作用。据报道,胸斧鱼(Gasteropelecus sternicla)的体表布满黑色斑块和纵向条纹,睛斑鲆(Bothus ocellatus)的体表也布满大小不一的斑块(Ramachandran et al, 1996);翘嘴鳜体表有条状或斑状花纹(刘伟等, 2019);泥鳅体表亦有许多大小不一的黑色斑点。所有这些色素图案有助于鱼的隐蔽,利于其捕食和躲避捕食者。
据报道,50日龄的幼体圆斑星鲽体表会形成黑斑(严俊丽等, 2017)。在斑马鱼的早期发育阶段,3种色素细胞(黑色素细胞、黄色素细胞和虹彩细胞)经特定分布形成其特征性的水平条纹色素图案,这些色素细胞在斑马鱼身体明暗条纹的皮下区域呈现复杂有序的排列,仅单个基因突变就可以显著改变斑马鱼的条纹图案(Parichy, 2006; Ceinos et al, 2015)。本研究发现,黑色斑点在45日龄的泥鳅幼鱼体表形成。进一步观察发现,大花斑泥鳅、小花斑泥鳅和无花斑泥鳅的黑色斑点的形状有所不同。大花斑泥鳅的体表黑色素细胞主要为菊花状,通过细胞聚集形成大黑斑;小花斑泥鳅的体表黑色素细胞聚集形成小黑斑,单个黑色素细胞呈点状和树突状;无花斑泥鳅的体表黑色素细胞分布均匀,其形状主要为点状和树突状。由于色素图案的形成涉及不同类型色素细胞的发育、迁移、聚集和相互作用等一系列过程,因此,泥鳅色素图案形成的细胞和分子机制有待进一步研究。
3.3 mitfa基因序列及其在不同发育时期胚胎和成体组织中的表达在脊索到哺乳动物中都发现mitf基因,mitf基因的长度和某些碱基在不同物种间存在差异。MITF蛋白在不同物种间均含有MITF_TFEB_C_3_N、bHLH-Zip、DUF3371结构域和非跨膜区(吴深基等, 2021)。MITFa蛋白与其他物种MITF蛋白具有许多保守的结构特征,且相对于无脊椎动物,MITFa蛋白显示出与脊椎动物更一致的氨基酸序列(未附氨基酸序列对比图)。作为一种转录因子,MITF可以通过bHLH-zip结构与一系列具有E-box序列的基因结合发挥作用,包括维持黑色素细胞发育分化相关的基因和控制细胞周期相关的基因,进而启动相关的信号通路(Li et al, 2013; Makimura et al, 1993)。对这些保守结构和序列的鉴定表明,MITFa蛋白在其进化过程中具有基本的生化功能,如参与色素沉着。
在青鳉的神经胚阶段,mitf基因的表达在黑色素细胞分化之前就已被检测到(Béjar et al, 2003)。mitfa mRNA在泥鳅胚胎发育的各阶段均有表达,且在受精卵期表达量最高。mitfa在没有黑色素细胞形成的胚胎发育早期表达,原因可能是mitfa基因不仅参与黑色素细胞的发育和分化,而且在与体色形成相关的非黑色素细胞的分化中发挥重要作用(Johnson et al, 2011)。对比来看,mitfa在小花斑泥鳅胚胎发育阶段的表达水平与大花斑泥鳅略有不同,但与无花斑泥鳅相似。
mitf基因与多种细胞功能有关,除了调节黑色素细胞的分化、成熟以及黑色素细胞中黑色素的合成外,mitf基因还可以调节肥大细胞的发育和功能以及骨修复过程中成骨细胞的功能(Jippo et al, 2003; Mansky et al, 2002)。在皮肤中,mitf mRNA的表达水平与体色表型相关(Wu et al, 2020)。在本研究中,mitfa基因在泥鳅的所有受检组织中均有表达,背部皮肤mitfa mRNA表达水平显著高于腹部皮肤。另外,泥鳅背部皮肤包含了躯体最大数量的黑色素细胞(Sheng et al, 2022),皮肤颜色的深浅与mitfa mRNA的表达水平呈正相关。这些积累的数据暗示了mitfa在泥鳅皮肤色素沉着中具有的潜在作用。
AHI E P, SEFC K M. Anterior-posterior gene expression differences in three Lake Malawi cichlid fishes with variation in body stripe orientation. PeerJ, 2017, 5: e4080 DOI:10.7717/peerj.4080 |
BAGNARA J T, MATSUMOTO J. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. The Pigmentary System: Physiology and Pathophysiology, Blackwell Publishing, 2006, 11–59
|
BÉJAR J, HONG Y, SCHARTL M. Mitf expression is sufficient to direct differentiation of medaka blastula derived stem cells to melanocytes. Development, 2003, 130(26): 6545-6553 DOI:10.1242/dev.00872 |
BUDI E H, PATTERSON L B, PARICHY D M. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development, 2008, 135(15): 2603-2614 DOI:10.1242/dev.019299 |
BURTON D, BURTON M. Essential fish biology: Diversity, structure, and function. Oxford University Press, 2017
|
BURTON D. The physiology of flatfish chromatophores. Microscopy Research and Technique, 2002, 58(6): 481-487 DOI:10.1002/jemt.10166 |
CEINOS R M, GUILLOT R, KELSH R N, et al. Pigment patterns in adult fish result from superimposition of two largely independent pigmentation mechanisms. Pigment Cell and Melanoma Research, 2015, 28(2): 196-209 DOI:10.1111/pcmr.12335 |
FU J J, ZHU W B, LUO W T, et al. Comparison of growth, tyrosinase activity, melanin content, and gene expression between common carps with different pigmentations. Journal of Fishery Sciences of China, 2021, 28(8): 939-947 [傅建军, 朱文彬, 罗文韬, 等. 不同体色鲤的生长、酪氨酸酶活性、黑色素含量及基因表达比较. 中国水产科学, 2021, 28(8): 939-947] |
FUJII R. The regulation of motile activity in fish chromatophores. Pigment Cell Research, 2000, 13(5): 300-319 DOI:10.1034/j.1600-0749.2000.130502.x |
HENDRICK L A, CARTER G A, HILBRANDS E H, et al. Bar, stripe and spot development in sand-dwelling cichlids from Lake Malawi. Evodevo, 2019, 10(1): 1-18 DOI:10.1186/s13227-018-0114-1 |
HIRATA M, NAKAMURA K I, KONDO S. Pigment cell distributions in different tissues of the zebrafish, with special reference to the striped pigment pattern. Developmental Dynamics, 2005, 234(2): 293-300 DOI:10.1002/dvdy.20513 |
HIRATA M, NAKAMURA K, KANEMARU T, et al. Pigment cell organization in the hypodermis of zebrafish. Developmental Dynamics, 2003, 227(4): 497-503 DOI:10.1002/dvdy.10334 |
HODGKINSON C A, MOORE K J, NAKAYAMA A, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell, 1993, 74(2): 395-404 DOI:10.1016/0092-8674(93)90429-T |
HOU L, PAVAN W J. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: Do all roads lead to Mitf?. Cell Research, 2008, 18(12): 1163-1176 DOI:10.1038/cr.2008.303 |
HUBBARD J K, UY J A C, HAUBER M E, et al. Vertebrate pigmentation: From underlying genes to adaptive function. Trends in Genetics, 2010, 26(5): 231-239 DOI:10.1016/j.tig.2010.02.002 |
IRION U, NÜSSLEIN-VOLHARD C. The identification of genes involved in the evolution of color patterns in fish. Current Opinion in Genetics and Development, 2019, 57: 31-38 DOI:10.1016/j.gde.2019.07.002 |
JIPPO T, MORⅡ E, ITO A, et al. Effect of anatomical distribution of mast cells on their defense function against bacterial infections: Demonstration using partially mast cell-deficient tg/tg mice. Journal of Experimental Medicine, 2003, 197(11): 1417-1425 DOI:10.1084/jem.20022157 |
JOHNSON S L, NGUYEN A T N, LISTER J A. mitfa is required at multiple stages of melanocyte differentiation but not to establish the melanocyte stem cell. Developmental Biology, 2011, 350(2): 405-413 DOI:10.1016/j.ydbio.2010.12.004 |
KELSH R N, BRAND M, JIANG Y J, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development, 1996, 123(1): 369-389 DOI:10.1242/dev.123.1.369 |
KELSH R N. Genetics and evolution of pigment patterns in fish. Pigment Cell Research, 2004, 17(4): 326-336 DOI:10.1111/j.1600-0749.2004.00174.x |
KRATOCHWIL C F, LIANG Y, GERWIN J, et al. Agouti-related peptide 2 facilitates convergent evolution of stripe patterns across cichlid fish radiations. Science, 2018, 362(6413): 457-460 DOI:10.1126/science.aao6809 |
KRATOCHWIL C F, URBAN S, MEYER A. Genome of the Malawi golden cichlid fish (Melanochromis auratus) reveals exon loss of oca2 in an amelanistic morph. Pigment Cell and Melanoma Research, 2019, 32(5): 719-723 DOI:10.1111/pcmr.12799 |
LI M, ZHU F, HONG Y. Differential evolution of duplicated medakafish mitf genes. International Journal of Biological Sciences, 2013, 9(5): 496-508 DOI:10.7150/ijbs.4668 |
LISTER J A, CLOSE J, RAIBLE D W. Duplicate mitf genes in zebrafish: Complementary expression and conservation of melanogenic potential. Developmental Biology, 2001, 237(2): 333-344 DOI:10.1006/dbio.2001.0379 |
LISTER J A, ROBERTSON C P, LEPAGE T, et al. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development, 1999, 126(17): 3757-3767 DOI:10.1242/dev.126.17.3757 |
LIU W Y, LI Y, CAI Y Q, et al. Research progress of the regulators involved in MITF-related signaling pathways in melanocytes. Animal Husbandry and Veterinary Medicine, 2017, 49(8): 125-128 [刘文艳, 李园, 蔡永强, 等. 黑色素细胞中MITF相关信号通路调控因子的研究进展. 畜牧与兽医, 2017, 49(8): 125-128] |
LIU W, ZHAO J L, WEI L, et al. The early pigmentation and color pattern formation of mandarin fish Siniperca chautsi. Chinese Journal of Zoology, 2019, 54(2): 236-244 [刘伟, 赵金良, 魏磊, 等. 鳜早期色素发育和色彩图案的形成. 动物学杂志, 2019, 54(2): 236-244] |
LIU X D, CHEN Z Z. Study on the chromatophores and the carotenoid components in the skin of discus fish (Symphysodon spp.). Journal of Shanghai Fisheries University, 2008, 17(3): 339-343 [刘晓东, 陈再忠. 七彩神仙鱼皮肤色素细胞观察及类胡萝卜素组分分析. 上海水产大学学报, 2008, 17(3): 339-343] |
MA X K, LIU X Z, WEN H S, et al. Changes of melanophores in the larval skin of Cynoglossus semilaevis Günther. Marine Fisheries Research, 2006, 27(2): 62-68 [马学坤, 柳学周, 温海深, 等. 半滑舌鳎早期发育过程中体表色素变化的研究. 海洋水产研究, 2006, 27(2): 62-68] |
MAAN M E, SEFC K M. Colour variation in cichlid fish: Developmental mechanisms, selective pressures and evolutionary consequences. Seminars in Cell and Developmental Biology. Academic Press, 2013, 24(6/7): 516–528
|
MAKIMURA M, HIRASAWA M, KOBAYASHI K, et al. Inhibitory effect of tea catechins on collagenase activity. Journal of Periodontology, 1993, 64(7): 630-636 DOI:10.1902/jop.1993.64.7.630 |
MANSKY K C, SULZBACHER S, PURDOM G, et al. The microphthalmia transcription factor and the related helix-loop-helix zipper factors TFE-3 and TFE-C collaborate to activate the tartrate-resistant acid phosphatase promoter. Journal of Leukocyte Biology, 2002, 71(2): 304-310 DOI:10.1189/jlb.71.2.304 |
MEYER A, BIERMANN C H, ORTI G. The phylogenetic position of the zebrafish (Danio rerio), a model system in developmental biology: An invitation to the comparative method. Proceedings of the Royal Society of London Series B: Biological Sciences, 1993, 252(1335): 231-236 DOI:10.1098/rspb.1993.0070 |
MEYER A, RITCHIE P A, WITTE K E. Predicting developmental processes from evolutionary patterns: A molecular phylogeny of the zebrafish (Danio rerio) and its relatives. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 1995, 349(1327): 103-111 DOI:10.1098/rstb.1995.0096 |
NAGAO Y, SUZUKI T, SHIMIZU A, et al. Sox5 functions as a fate switch in medaka pigment cell development. PLoS Genetics, 2014, 10(4): e1004246 DOI:10.1371/journal.pgen.1004246 |
PARICHY D M. Evolution of danio pigment pattern development. Heredity, 2006, 97(3): 200-210 DOI:10.1038/sj.hdy.6800867 |
PATTERSON L B, PARICHY D M. Zebrafish pigment pattern formation: insights into the development and evolution of adult form. Annual Review of Genetics, 2019, 53: 505-530 DOI:10.1146/annurev-genet-112618-043741 |
PHANG V P E, LIM T M, KHOO G. Ultrastructure of erythrophores and xanthophores of the siamese fighting fish, Betta splendens. Israeli Journal of Aquaculture-Bamidgeh, 2012, 64: 20620 |
RAMACHANDRAN V S, TYLER C W, GREGORY R L, et al. Rapid adaptive camouflage in tropical flounders. Nature, 1996, 379(6568): 815-818 DOI:10.1038/379815a0 |
REISS P, ABLE K W, NUNES M S, et al. Color pattern variation in Cichla temensis (Perciformes: Cichlidae): Resolution based on morphological, molecular, and reproductive data. Neotropical Ichthyology, 2012, 10: 59-70 DOI:10.1590/S1679-62252012000100006 |
ROBERTS R B, SER J R, KOCHER T D. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science, 2009, 326(5955): 998-1001 DOI:10.1126/science.1174705 |
RODRIGUEZ F H, CACERES G, LHOREN J P, et al. Genetics variation in skin pigmentation patterns and growth in rainbow trout. Animal, 2019, 13(4): 675-682 DOI:10.1017/S175173111800188X |
SANTOS M E, BALDO L, GU L, et al. Comparative transcriptomics of anal fin pigmentation patterns in cichlid fishes. BMC Genomics, 2016, 17(1): 1-16 |
SAZIMA I, CARVALHO L N, MENDONÇA F P, et al. Fallen leaves on the water-bed: diurnal camouflage of three night active fish species in an Amazonian streamlet. Neotropical Ichthyology, 2006, 4: 119-122 DOI:10.1590/S1679-62252006000100013 |
SHENG J, GUAN L, SHENG B, et al. Analysis of pigment cell composition, pigment content, tyrosinase content and activity of three kinds of loaches Misgurnus anguillicaudatus from Poyang Lake. Journal of Fish Biology, 2022, 100(2): 366-377 DOI:10.1111/jfb.14945 |
SUGIMOTO M. Morphological color changes in fish: Regulation of pigment cell density and morphology. Microscopy Research and Technique, 2002, 58(6): 496-503 DOI:10.1002/jemt.10168 |
WANG J, PEI X, LIU H, et al. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). International Journal of Biological Macromolecules, 2018, 106: 544-550 DOI:10.1016/j.ijbiomac.2017.08.046 |
WEI M X, SONG H M, QI B L, et al. Pigment cells development and body color variation of postembryonic development in Amphilophus citrinellus (Günther 1864). Journal of Shanghai Ocean University, 2015, 24(1): 28-35 [韦敏侠, 宋红梅, 祁宝伦, 等. 橘色双冠丽鱼胚后色素细胞发育与体色变化. 上海海洋大学学报, 2015, 24(1): 28-35] |
WU M, CHEN X, CUI K, et al. Pigmentation formation and expression analysis of tyrosinase in Siniperca chuatsi. Fish Physiology and Biochemistry, 2020, 46(4): 1279-1293 DOI:10.1007/s10695-020-00788-7 |
WU S J, HUANG J Q, LI Y J, et al. Cloning and expression analysis of body colour-related gene mitfa in rainbow trout (Oncorhynchus mykiss). Journal of Agricultural Biotechnology, 2021, 29(4): 753-763 [吴深基, 黄进强, 李永娟, 等. 虹鳟体色相关基因mitfa的克隆和表达分析. 农业生物技术学报, 2021, 29(4): 753-763] |
YAN J L, CHEN S Q, CHANG Q, et al. Early ontogeny of chromatophores and skin color changes of spotted halibut (Verasper variegatus). Journal of Fisheries of China, 2017, 41(5): 678-686 [严俊丽, 陈四清, 常青, 等. 圆斑星鲽仔稚鱼色素细胞发育和体色变化. 水产学报, 2017, 41(5): 678-686] |
YI S, WANG W, ZHOU X. Genomic evidence for the population genetic differentiation of Misgurnus anguillicaudatus in the Yangtze River basin of China. Genomics, 2019, 111(3): 367-374 DOI:10.1016/j.ygeno.2018.02.011 |
YU D D, LIU H J, GUAN J, et al. Early ontogeny of chromatophores and body color changes of Acanthopagrus schlegelii. Progress in Fishery Sciences, 2012, 33(5): 1-7 [于道德, 刘洪军, 关健, 等. 黑棘鲷早期色素细胞发育与体色变化. 渔业科学进展, 2012, 33(5): 1-7 DOI:10.3969/j.issn.1000-7075.2012.05.001] |
ZHU J, ZHANG X M, GAO T X, et al. The metamorphosis of turbot Scophthamus maximus and morphological observation on melanophores in larval skin. Journal of Fisheries of China, 2002, 26(3): 193-200 [朱杰, 张秀梅, 高天翔, 等. 大菱鲆早期变态发育和体表黑色素细胞形态学观察. 水产学报, 2002, 26(3): 193-200 DOI:10.3321/j.issn:1000-0615.2002.03.001] |



