2. 三门县水产技术推广站 浙江 三门 317199;
3. 浙江省淡水水产研究所 浙江 湖州 313098
2. Sanmen County Aquatic Technology Extension Station, Sanmen 317199, China;
3. Zhejiang Institute of Freshwater Fisheries, Huzhou 313098, China
环境盐度的变化会影响贝类的生理活动,使体内渗透调节器官结构、渗透压、离子转运及游离氨基酸(free amino acid, FAA)含量发生一系列变化以适应环境盐度的变化(Ran et al, 2017)。水生动物可以通过FAA调节细胞体积,维持体内渗透压平衡。此机制已在丽文蛤(Meretrix lusoria)(Lin et al, 2016)、太平洋牡蛎(Crassostrea gigas)(Hosoi et al, 2003)、皱纹盘鲍(Haliotis discus hannai)(Gao et al, 2017)、凡纳滨对虾(Litopenaeus vannamei)(张丹等, 2016)、三疣梭子蟹(Portunus trituberculatus)(付萍等, 2017)等水生动物中得到证明。双壳贝类中常见的调节渗透压的FAA主要有Ala、Gly、Pro和Tau等(Pourmozaffar et al, 2020),参与缢蛏(Sinonovacula constricta)体内渗透调节的主要FAA还尚待研究。
在双壳贝类中,丙氨酸(Ala)是激素和酶的组成成分,主要参与代谢途径与渗透压调节等(陆捷等, 2023)。Ala参与许多海洋甲壳类和双壳类软体动物的渗透调节(Lynch et al, 1966; Huo et al, 2014)。在日本河蚬(Corbicula japonica)外套膜中,Ala是构成细胞内FAA池的主体,其浓度随盐度的变化而波动(Matsushima et al, 1992; Koyama et al, 2015)。在盐度20~32暴露24 h后,紫贻贝(Mytilus edulis)鳃和外套膜中3种主要的FAA(Ala、Gly和Tau)含量下降(May, 2017)。在砂海螂(Mya arenaria)中,Ala的含量最高(Haider et al, 2019)。盐度胁迫下,香港牡蛎(C. hongkongensis)中主要是Tau和Ala变化最大(Huo et al, 2014)。但目前对丙氨酸代谢通路研究的较少,具体通路尚待研究。
研究表明,肌肽(carnosine, β-alanyl-L-histidine)在生物体内发挥多种功能,包括增加肌肉收缩力、血管紧张素转化酶抑制剂、Ca2+敏感性调节剂、作为缓冲液维持细胞酸碱平衡和自由基清除活性等(Manhiani et al, 2013; Yanase et al, 2015; Baye et al, 2019)。肌肽是一种由β-Ala和L-His组成的水溶性、内源性二肽(赵丹婷等, 2018)。有研究发现,外源肌肽和乙硫氨酸2种生物调节物质可通过抗氧化系统保持细胞膜的结构完整性,有效提高高羊茅(Festuca arundinacea)的耐盐性(王岩, 2016)。
肌肽合成酶(carnosine synthase, CARNS)是一种ATP偶联酶,为ATP-grasp超家族成员,更确切地说是ATPGD1 (含ATP-grasp结构域的蛋白1) (Drozak et al, 2010),其催化反应为:丙氨酸+组氨酸+ATP→肌肽+ADP+Pi。在几种软体动物的基因组数据中已检测到肌肽合成酶基因,如美洲牡蛎(C. virginica) (XP_022325739.1)、厚壳贻贝(M. coruscus)(QKX_ 08451.1)和虾夷扇贝(Mizuhopecten yessoensis)(XP_ 021350145.1),说明肌肽不仅存在于脊椎动物中,也存在于无脊椎动物中。在盐度胁迫后的太平洋牡蛎转录组结果中,ATPGD1基因的表达上调,该基因与渗透调节相关(Meng et al, 2013)。
缢蛏是我国传统四大养殖贝类之一(林国兰, 2016)。养殖水体中的盐度易受到潮汐、季节性降雨或高温天气等影响,经常发生不同程度的波动,从而影响缢蛏的呼吸、代谢、生长和存活(陈牧霞等, 2021; 曹伟等, 2022; 丁红兵等, 2022)。缢蛏属于广盐性贝类,其耐受范围为3~40 (彭茂潇, 2020; Cao et al, 2022),但其广盐性耐受的原理尚不明确。FAA在缢蛏中是否发挥渗透压调节作用及参与渗透压调节作用的FAA是否与其他贝类类似,及主要FAA的代谢通路值得深入研究。本研究探究盐度胁迫后缢蛏鳃、足和血淋巴中渗透压和FAA的变化情况,同时对肌肽合成酶基因(carnosine synthase, Sc-CARNS)的序列特征、组织表达、盐度胁迫及RNA干扰(RNA interference, RNAi)后的mRNA表达特征,丙氨酸和肌肽含量的变化进行分析,以期为FAA在缢蛏盐度适应中的作用提供理论依据,阐明盐度胁迫后缢蛏体内丙氨酸的分解途径,为缢蛏健康养殖及耐盐新品种的选育提供数据支撑。
1 材料与方法 1.1 实验动物实验所用缢蛏取自宁波市海洋与渔业科技创新基地,将规格均匀、体质健康的缢蛏成贝[壳长为(51.64±3.80) mm]在循环养殖水族箱中暂养3 d,连续充气,避免实验过程中的应激反应,水体盐度为20.00±0.34,pH为8.1±0.2,水温为(20.0±0.5) ℃。每天更换1/2养殖塘海水,并投喂定量的小球藻(Chlorella)。
1.2 实验设计 1.2.1 不同盐度下缢蛏湿重测定为探究盐度对缢蛏湿重的影响,排除湿重变化对其体内FAA总量的干扰,根据其盐度耐受范围(3~40),设置低盐组(盐度5)、对照组(盐度20)、高盐组(盐度35) 3个梯度,每组3个重复,每个重复6只缢蛏,盐度胁迫0、2、4、6、8、12、24、48、72、96 h后,称其湿重。
1.2.2 不同盐度下缢蛏鳃、足、血淋巴渗透压测定为探究盐度对缢蛏渗透压的影响,确定其渗透调节类型。设置盐度5、20、35三个梯度,每组3个重复,每个重复160只缢蛏,盐度胁迫0、1、4、8、12、24、48、72 h后,分别从每个实验组随机取3只。用1 mL无菌注射器从缢蛏的闭壳肌中快速抽取血淋巴。取鳃、足于液氮速冻后,–80 ℃保存待用。
1.2.3 不同盐度下缢蛏鳃、足、血淋巴FAA含量测定为探究缢蛏组织中FAA含量随盐度的变化,找到变化最明显的FAA。设置盐度5、20、35三个梯度,每组3个重复,每个重复6只缢蛏,盐度胁迫24 h后,分别从每个实验组随机取3只。用1 mL无菌注射器从缢蛏的闭壳肌中快速抽取血淋巴。取鳃、足于液氮速冻,–80 ℃保存待用。
1.2.4 基因表达分析样品采集利用实时荧光定量PCR (RT-qPCR)分析Sc-CARNS的表达量对盐度的响应,设置盐度5、20、35三个梯度,每组3个重复,每个重复50只缢蛏,盐度胁迫4、8、12、24、48、72、96 h后,分别从每个实验组随机取3只。取对照组缢蛏的鳃、唇瓣、肝胰腺、闭壳肌、外套膜、水管和足进行组织表达分析。取每组的足进行mRNA表达分析。样品于液氮速冻后,–80 ℃保存备用。
1.2.5 siRNA对Sc-CARNS的干扰实验利用RNAi验证Sc-CARNS的功能,实验采取注射正常盐度缢蛏(个体大小均匀)闭壳肌的方式,实验组注射浓度为4 μg Sc-CARNS基因的siRNA,空白对照组和阴性对照组(NC, 非特异性siRNA)分别注射等体积的DEPC处理水和NC链。实验组、空白对照组和阴性对照组分别注射100只,1/2放到盐度为20的养殖缸中(1个缸用网分割成3个部分),另外1/2放入盐度为5的养殖缸中,每组实验设置3个平行。将注射后0、24、48、72、96 h作为取样时间点,各组随机抽取3只,取其足于液氮速冻后,–80 ℃保存备用。
1.3 实验方法 1.3.1 渗透压测定液体:将血清或海水移入0.5 mL的测量管中(不少于50 μL),掌心离心机离心10 s,用冰点渗透压仪(Gonotec 3000, 德国)测定渗透压(徐娴等, 2020)。
组织:将鳃或足用全自动样品快速研磨仪(JXFSTPRP-64L,上海净信)研磨3 min,将匀浆液移入0.5 mL测量管中,短暂离心,测其渗透压(李德全等, 1988)。
1.3.2 FAA含量测定组织:称取1 g真空冷冻干燥后的鳃或足于1.5 mL离心管中,加入1 mL 0.01 mol/L盐酸和2颗小钢珠,研磨6 min,再用0.01 mol/L盐酸定容至50 mL,浸提30 min后14 000 r/min 4 ℃离心15 min;吸取上清液2 mL于5 mL离心管中,加入8%磺基水杨酸2 mL (沉淀蛋白),混匀,静置15 min;14 000 r/min 4 ℃离心15 min,取上清液,过0.22 μm滤膜后,用全自动氨基酸分析仪(日立L8900, 日本)测定FAA含量(张苏平等, 2017)。
血淋巴:取1.5 mL血淋巴于2 mL离心管中,3000 r/min 4 ℃离心5 min;吸取上清液1 mL于10 mL离心管中,加入含0.02 mol/L盐酸的4%磺基水杨酸9 mL,混匀,静置15 min;14 000 r/min 4 ℃离心15 min;取上清液,过0.22 μm滤膜后,上机测定FAA含量(付萍等, 2017)。
1.3.3 基因序列分析及结构域预测将缢蛏盐度胁迫转录组获得的Sc-CARNS的CDS序列通过https://www.ncbi.nlm.nih.gov/orffinder/预测ORF及氨基酸序列;通过https://web.expasy.org/compute_pi/预测蛋白质的分子量和等电点;用https://web.expasy.org/protscale/分析蛋白质疏水性;利用Smart在线软件(http://smart.embl-heidelberg.de/)对蛋白进行功能域预测,通过Swiss Model在线软件(https://swissmodel.expasy.org/interactive)预测蛋白质高级结构。
1.3.4 Sc-CARNS mRNA表达RT-qPCR分析用Trizol提取总RNA,再用PrimeScriptTM RT reagent Kit试剂(TaKaRa)反转录成cDNA,产物于–20 ℃冰箱保存,备用。引物设计参照缢蛏基因转录组文库中的CDS序列,用Primer Premier 6.0软件设计RT-qPCR的引物(表 1),由生工生物工程(上海)股份有限公司合成。使用ChamQ Universal SYBR qPCR Master mix试剂进行RT-qPCR (罗氏Light Cycler 480 Ⅱ,瑞士),结果采用2–ΔΔCt法进行比较分析(孔祥辉等, 2022)。
|
|
表 1 实验所用引物及其序列 Tab.1 Primers and sequences used in the experiment |
用贝类丙氨酸ELISA检测试剂盒(上海酶联)测定丙氨酸含量。用邻苯二甲醛(OPA)显色法测定肌肽含量。
1.4 数据处理用Excel软件对数据进行2–ΔΔCt、平均值(Mean)和标准差(SD)分析,结果用平均值±标准差表示。采用SPSS Statistics 25软件对数据进行统计学分析。先对数据进行单因素方差分析(one-way ANOVA),若有显著差异,再作Dancan´s多重比较,P < 0.05为差异显著。用GraphPad Prism 8.0作图。
2 结果 2.1 盐度胁迫后缢蛏湿重的变化不同盐度对缢蛏湿重的影响不同(图 1)。对照组0~96 h内无明显变化。与对照组相比,低盐胁迫时,缢蛏湿重随胁迫时间先增加后降低,胁迫6~12 h有显著差异(P < 0.05),24 h后无显著差异;高盐时则先降低后升高,96 h内与对照组无显著差异。低盐时,缢蛏的双壳张大,细胞吸水膨胀,体重增大;高盐时则双壳闭合,细胞失水皱缩,体重减小。
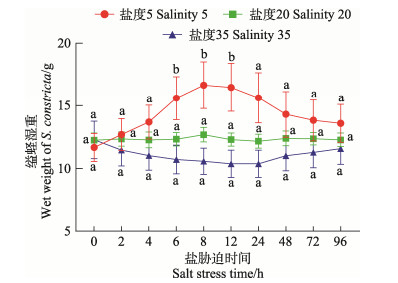
|
图 1 盐度对缢蛏湿重的影响 Fig.1 Effect of salinity on the wet weight of S. constricta 字母不同表示与对照组差异显著(P < 0.05),下同。 Different letters indicate significant differences with the control group (P < 0.05), the same below. |
不同盐度对缢蛏各组织渗透压的影响不同(图 2)。正常盐度下,各组织渗透压在0~72 h内均无显著变化。低盐胁迫下,各组织中渗透压在1~72 h内显著降低(P < 0.05);高盐胁迫时则相反,都在24 h达到稳态。此时,鳃、足、血淋巴中渗透压与对照组相比,低盐组分别降低了66.7%、69.7%和71.6%;高盐组分别增加了68.3%、67.5%和70.2%。同一胁迫时间下,缢蛏各组渗透压大小为盐度35 > 盐度20 > 盐度5。由图 3可以看出,缢蛏各组织渗透压随着外界海水渗透压的升高而升高。结果表明,缢蛏体内渗透压会随外界环境的变化而变化,与外界环境一致时,达到稳定,属于渗透调节类型中的渗透压随变者。
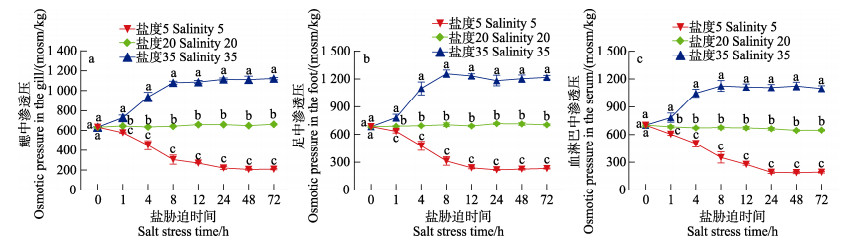
|
图 2 不同盐度胁迫下缢蛏鳃、足和血淋巴中渗透压的变化 Fig.2 Variation of osmotic pressures in gill, foot and hemolymph of S. constricta under different salinity stress |
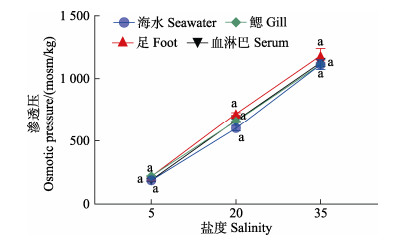
|
图 3 不同盐度下缢蛏鳃、足、血淋巴与海水渗透压之间的关系 Fig.3 Osmotic pressures between gills, foot, hemolymph and seawater of S. constricta under different salinity |
本研究共测了18种FAA,低盐胁迫24 h后缢蛏鳃、足、血淋巴中FAA组成成分见表 2。正常盐度下,鳃中总游离氨基酸(TOFAA)含量为65.05 mg/g,含量最高的为Gly (23.05 mg/g),占TOFAA的35.43%。其次为Ala、Tau、Glu和Thr,分别占TOFAA的25.63%、14.48%、7.26%和3.77%,未检测到Phe和Cys。足中TOFAA含量为96.28 mg/g,Ala含量最高(40.26 mg/g,占41.82%),其次为Arg (17.72%)、Gly (9.14%)、Glu (8.32%)和Asp (6.48%),未检测到Cys和Phe。血淋巴中Ala含量最高(26.69 mg/L),占TOFAA的42.37%,其次为Gly (10.90%)、Thr (8.68%)、Glu (8.16%)和Ser (7.11%),未检测到Cys和Ile。
|
|
表 2 低盐胁迫下缢蛏鳃、足、血淋巴中FAA的含量(Mean±SD) Tab.2 Concentrations of free amino acid in the gill, foot, hemolymph of S. constricta under low salinities (Mean±SD) |
低盐胁迫24 h后,各组织中主要FAA浓度变化如图 4所示,与对照组比,缢蛏鳃中TOFAA的含量降低,Gly含量发生显著下降(P < 0.05),由23.05 mg/g下降到0.75 mg/g;其次为Ala (由16.67 mg/g下降到1.80 mg/g)和Tau (由9.42 mg/g下降到5.56 mg/g)。缢蛏足中TOFAA的含量降低,Ala含量发生显著下降(P < 0.05),由40.26 mg/g下降到9.96 mg/g;其次为Gly (由8.80 mg/g下降到1.04 mg/g)和Asp (由6.24 mg/g下降到2.08 mg/g)。缢蛏血淋巴中TOFAA的含量无显著变化,Ala含量显著降低的含量(P < 0.05),由26.69 mg/L下降到13.48 mg/L;Ser和Thr显著升高(P < 0.05)。
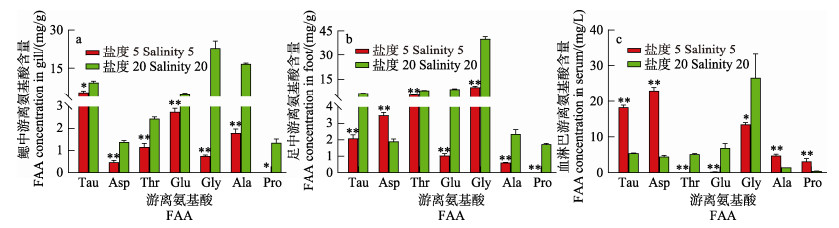
|
图 4 低盐胁迫下缢蛏鳃、足、血淋巴中主要游离氨基酸的浓度变化 Fig.4 Concentrations of the main amino acids in the gill, foot, hemolymph tissue of S. constricta under low salinity stress *代表不同盐度组与对照组差异显著(P < 0.05), **代表差异极显著(P < 0.01),下同。 * represents significant differences between the different salinity stress with control (*P < 0.05), ** represents highly significant differences (P < 0.01). The same below. |
综上所述,低盐胁迫时,缢蛏体内Ala含量降低幅度最大,推测当FAA发挥渗透压调节作用时Ala的贡献率最大。
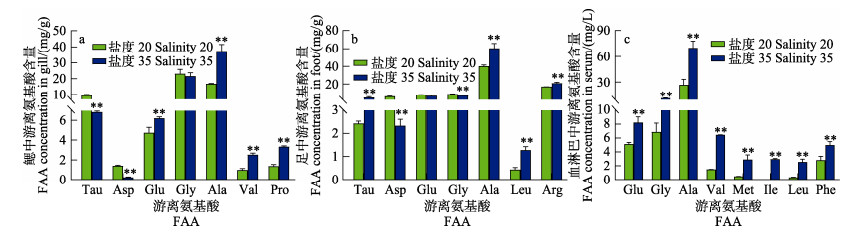
2.4 高盐胁迫后缢蛏鳃、足、血淋巴中FAA组成分析高盐胁迫24 h后,缢蛏鳃、足、血淋巴中FAA组成成分见表 3。缢蛏鳃、足、血淋巴中的TOFAA含量显著升高(P < 0.05)。高盐胁迫24 h后,各组织中主要FAA浓度变化如图 5所示。与对照组相比,高盐组鳃中Ala含量显著升高(P < 0.05),由16.67 mg/g增加到37.29 mg/g,其次为Pro,由1.35 mg/g增加到3.33 mg/g。足中FAA含量升高幅度最大的是Ala,其含量由40.26 mg/g增加到60.18 mg/g,其次为Tau (由2.42 mg/g增加到4.93 mg/g)、Arg (由17.06 mg/g增加到20.95 mg/g)和Pro (由1.72 mg/g增加到2.01 mg/g)。血淋巴中也为Ala含量变化最大,由26.69 mg/L升到69.94 mg/L,其次为Val (由1.46 mg/L增加到6.46 mg/L)和Gly (由6.87 mg/L增加到11.06 mg/L),分别增加了5.00 mg/L和4.19 mg/L。因此,高盐胁迫时,缢蛏体内Ala含量升高幅度最大,是主要的渗透调节氨基酸。
|
|
表 3 高盐胁迫下缢蛏鳃、足、血淋巴中FAA的含量(Mean±SD) Tab.3 Concentrations of free amino acid in the gill, foot, hemolymph of S. constricta under high salinity stress (Mean±SD) |
|
图 5 高盐胁迫下缢蛏鳃、足、血淋巴中主要游离氨基酸的含量变化 Fig.5 Concentrations of the main amino acids in the gill, foot, hemolymph tissue of S. constricta under high salinity stress |
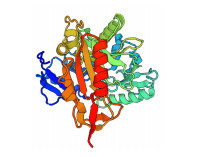
Sc-CARNS基因蛋白质编码区(coding sequence, CDS)长度为1 260 bp。ExPASy软件推导出编码419个氨基酸,包含49个碱性氨基酸(Arg、Lys和His),55个酸性氨基酸(Asp和Glu),182个疏水性氨基酸(Ala、Val、Leu、Pro、Ile、Phe、Trp和Met),133个亲水性氨基酸(Gly、Ser、Thr、Cys、Tyr、Asn和Gln),该蛋白质含有很大比例的疏水性氨基酸,表现为疏水性。该蛋白的分子量为47.15 kDa,等电点pI为5.09。二级结构预测结果显示,缢蛏Sc-CARNS蛋白质序列含有38.66% α-螺旋、6.44% β-折叠和37.23%无规则卷曲。三级结构域预测结果如图 6所示,缢蛏肌肽合成酶含有1个ATP-grasp结构域。
|
图 6 Sc-CARNS蛋白质三级结构预测 Fig.6 Predicted protein tertiary structure of Sc-CARNS 螺旋和箭头分别代表α-螺旋和β-折叠。 Spiral and arrows represent α-helix and β-fold, respectively. |
如图 7所示,Sc-CARNS基因在缢蛏水管、外套膜、鳃、闭壳肌、肝胰腺、唇瓣和足7个组织中均有表达。其中,在闭壳肌、足和水管中显著表达(P < 0.05),说明其主要在肌肉类型的组织中表达,其次为鳃,肝胰腺中的表达量最低。

|
图 7 Sc-CARNS基因在缢蛏不同组织的表达分析 Fig.7 Expression analysis of Sc-CARNS gene in different tissues of S. constricta 不同字母表示存在显著差异(P < 0.05)。下同。 Different letters indicate significant differences (P < 0.05). The same below. |
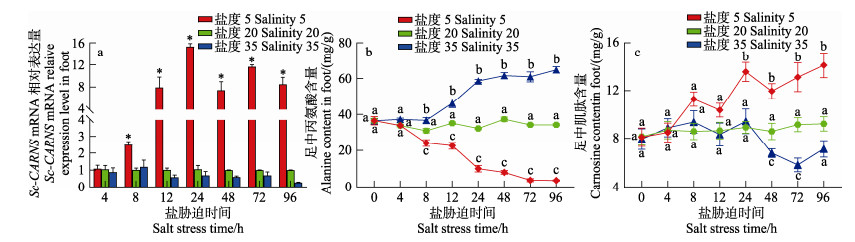
如图 8所示,盐度胁迫96 h内对照组中Sc-CARNS的表达量、丙氨酸和肌肽含量均无显著变化。低盐胁迫后,缢蛏足中Sc-CARNS基因mRNA表达量随胁迫时间先升高后降低,且胁迫4 h后均极显著高于对照组(P < 0.01),并在24 h时达到峰值。丙氨酸的含量随时间显著降低,肌肽含量随时间显著增加(P < 0.05)。高盐胁迫后,Sc-CARNS表达量则呈下降趋势,与对照组相比无显著变化。丙氨酸的含量在8 h后先升高后降低再升高,其含量均不低于对照组。肌肽的含量在24 h后表现为降低趋势。
|
图 8 不同盐度胁迫后缢蛏足中Sc-CARNS mRNA表达量和丙氨酸、肌肽含量变化 Fig.8 Expression of Sc-CARNS mRNA and contents of alanine and carnosine in the foot of S. constricta under different salinity stresses |
上述结果表明,Sc-CARNS主要在低盐胁迫时发挥作用。在低渗条件下,Sc-CARNS高表达,此时丙氨酸含量降低了(30.57±0.51) mg/g,肌肽含量升高了(4.90±1.50) mg/g,变化量比为6∶1。按照生物反应式:丙氨酸+组氨酸+ATP→肌肽+ADP+Pi,丙氨酸与肌肽转化为1∶1。实验结果说明,一部分丙氨酸被转化成肌肽,其余丙氨酸则被其他途径消耗掉了。例如,丙氨酸通过谷丙转氨酶脱氨基作用形成谷氨酸,再通过谷氨酰氨合成酶转化为谷氨酰氨,维持缢蛏体内渗透压平衡(Zhang et al, 2020; Ahmad et al, 2021)。在低盐条件下,双壳贝类需要消耗更多的能量防止生理变化,因此,丙氨酸也可分解成丙酮酸进入三羧酸循环进行能量转换(Maar et al, 2015)。同时,为维持体内肌肽含量的稳定,当肌肽含量过高时,肌肽酶会将其降解,再被转运蛋白运出体外(潘晨, 2021)。
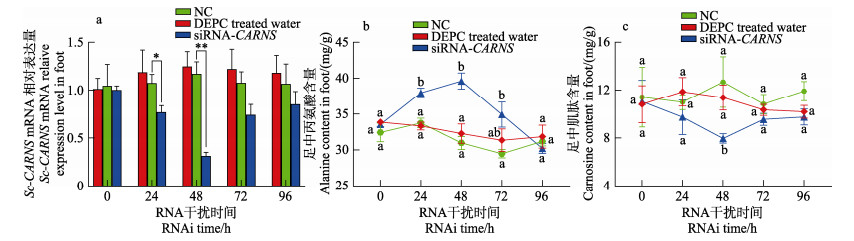
2.8 RNAi对正常盐度下Sc-CARNS表达和调节作用的影响利用RNA干扰技术验证Sc-CARNS在缢蛏中的功能。如图 9所示,正常盐度下,阴性对照组(NC)和DEPC处理水中Sc-CARNS的mRNA表达量、丙氨酸和肌肽的含量无显著变化。干扰组Sc-CARNS mRNA表达水平随干扰时间先降低后升高。在24 h和48 h时,Sc-CARNS mRNA表达水平显著低于NC (P < 0.05),干扰效率分别为24%和69%,48 h的干扰效率最高。此时干扰组中丙氨酸含量随着时间先升高后降低,48 h达到最高值。干扰组肌肽的含量先降低后升高,在48 h达到最低值。
|
图 9 RNA干扰后正常盐度(20)下缢蛏足中Sc-CARNS mRNA表达量和丙氨酸、肌肽含量变化 Fig.9 Expression of Sc-CARNS mRNA and contents of alanine and carnosine in the foot of S. constricta under normal salinity (20) after RNA interference |
RNAi结果显示,Sc-CARNS表达量在48 h干扰效率最高,此时丙氨酸的含量升高了(5.90±0.81) mg/g,肌肽的含量降低了(4.71±1.09) mg/g。且在盐度20时,缢蛏体内无需提供额外的能量,因此,丙氨酸与肌肽符合1∶1的转化关系,证明了肌肽合成酶是丙氨酸转化成肌肽的关键酶。
2.9 RNAi对低盐时Sc-CARNS表达和调节作用的影响利用RNA干扰技术进一步验证Sc-CARNS在缢蛏中的功能。注射干扰链后,低盐胁迫后,缢蛏足中Sc-CARNS mRNA表达量和丙氨酸、肌肽含量的变化情况如图 10所示。低盐胁迫0~24 h,Sc-CARNS mRNA表达量升高,丙氨酸含量降低,肌肽含量升高。注射干扰链24~96 h后,干扰组Sc-CARNS mRNA表达水平随干扰时间先降低后升高,丙氨酸含量随时间先升高后降低,肌肽含量先降低后升高。在干扰48 h时,Sc-CARNS mRNA表达水平显著低于NC (P < 0.05),干扰效率为43%,此时丙氨酸和肌肽的含量都处于极值。
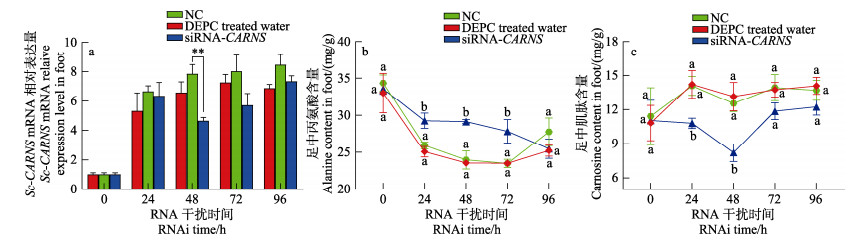
|
图 10 RNA干扰后低盐(5)胁迫下缢蛏足中Sc-CARNS mRNA表达量和丙氨酸、肌肽含量变化 Fig.10 Expression of Sc-CARNS mRNA and contents of alanine and carnosine in the foot of S. constricta under low salt (5) stress after RNA interference |
上述实验结果表明,注射干扰链0~24 h,缢蛏足中Sc-CARNS mRNA表达量、肌肽和丙氨酸含量变化与未干扰时低盐胁迫下的变化一致。在干扰48 h时,Sc-CARNS mRNA表达量达到最低值,此时肌肽含量降低了(4.93±1.34) mg/g,丙氨酸含量升高了(5.17± 1.01) mg/g,与正常盐度下的干扰结果一致,更加印证了他们之间是相互影响的,Sc-CARNS是丙氨酸代谢成肌肽的限速酶,合成肌肽是低盐条件下丙氨酸减少的一条重要途径。
3 讨论 3.1 缢蛏体内渗透压和FAA对盐度的响应按照对水环境盐度变化的渗透调节能力不同,水生生物分为渗透压调节者和渗透压随变者。前者是能主动调节自身渗透压和离子浓度,并维持在一定水平,其调节渗透压的能力较强。而后者则较弱,其血淋巴中渗透压和离子浓度会随外界环境变化而变化,最终与外界环境保持一致(Péqueux, 1995)。通过对缢蛏鳃、足、血淋巴渗透压的测定发现,随着盐度的升高,缢蛏各组织中渗透压随时间先升高后趋于平稳,最终与外界盐度保持基本一致。与魁蚶(Scapharca broughtonii)(蔡星媛等, 2015)和泥蚶(Tegillarca granosa)(李敏等, 2018)的研究结果一致,因此,缢蛏属于渗透压随变者。
水体盐度变化时水生动物可以通过改变胞内可溶物质(FAA)浓度来调节细胞渗透压(Nie et al, 2017)。鳃是水生动物与外界环境进行离子和气体交换的场所,是调节渗透压和血液离子浓度的主要器官(江山等, 2011)。肌肉组织是FAA代谢最适合的场所,是FAA参与渗透压调节最主要的场所(Li et al, 2009; Wang et al, 2012)。血淋巴是应对外界盐度变化的直接场所,血淋巴中的FAA会直接参与渗透压的调节(Cheng et al, 1986; 梁萌青等, 2009; 马金武等, 2016)。因此,鳃、足和血淋巴是研究FAA含量变化最主要的组织。
在正常盐度下,缢蛏体内含量最高的FAA是Ala (张苏平等, 2017)。鳃中含量最高的为Gly,其次为Ala、Tau和Glu。足和血淋巴中含量最高的为Ala,其次分别为Arg、Gly、Glu、Asp和Gly、Thr、Glu,与虾蟹等水生生物中研究结果类似(黄凯等, 2010; Wang et al, 2012; 付萍等, 2016)。说明同一物种的不同组织中含量最高的FAA存在一定的特殊性。缢蛏组织中含量高的Ala、Gly、Glu和Asp都是鲜味氨基酸,会影响肉质和鲜味,赋予它鲜美的口感(陈德慰等, 2012; 张苏平等, 2017)。在高盐度(30~35)水中暂养12~24 h可以提高其鲜度,反之,在低盐度水中暂养会大大降低鲜度。
双壳类动物体内TOFAA的含量与外界环境盐度呈正相关(Pourmozaffar et al, 2020)。Lin等(2016)研究发现,低盐胁迫30 d后,丽文蛤鳃中TOFAA浓度显著下降(P < 0.05)。盐度胁迫后,缢蛏体内TOFAA的含量随盐度的变化而变化。因为低盐胁迫时,细胞吸水膨胀,FAA脱氨分解使细胞与周围海水保持等渗,最终细胞内TOFAA含量显著下降(Haider et al, 2019)。高盐胁迫时,缢蛏则双壳闭合,细胞失水皱缩,部分蛋白分解成FAA,使细胞中TOFAA含量显著升高,以适应环境的变化(Baker et al, 2007; Matoo, 2013; Fuhrmann et al, 2018)。
在水生动物中,Ala、Gly、Pro、Tau和Glu通常是促进细胞内渗透压和渗透形成过程的主要FAA,但不同物种的不同组织中起渗透调节作用的FAA存在一定的差异(Lee et al, 2004; Hosoi et al, 2008)。研究发现,盐度胁迫时,南美蓝对虾(Penaeus stylirostris) (Cobb et al, 19752002)和海蛤(Macoma balthica) (Sokolowski et al, 2003)中主要是Gly调节细胞内的渗透压,而丽文蛤鳃和外套膜中则是Tau (Lin et al, 2021)。在美洲牡蛎肌肉中,FAA占总渗透效应的20.9%,尤其是Tau、Ala和Gly的含量随盐度变化显著(Lynch, 1966)。盐度胁迫时,缢蛏鳃、足和血淋巴中分别为Ala、Gly、Glu、Pro,Ala、Gly、Arg、Tau和Ala、Gly、Pro与外界环境呈正调控,且变化显著(P < 0.05)。Ala是各组织中含量变化最大的氨基酸,与Cao等(2022)在缢蛏鳃中的研究结果一致。Ala从头合成所需的能量要比其他FAA少,体内有多条途径可以合成丙酮酸,再通过谷丙转氨酶合成Ala (Meng et al, 2013)。Ala也可通过肌肽合成酶降解成肌肽(Zhao et al, 2016)。因此,认为Ala在缢蛏渗透压调节过程中发挥重要作用。
3.2 缢蛏体内Sc-CARNS基因对盐度的响应Sc-CARNS在缢蛏肌肉型组织中表达量高,其次是鳃。该结果与其他物种的研究结果类似(Everaert et al, 2013; Pan et al, 2022)。肌肉是调动、存储、代谢FAA最适合的场所,是主要的“FAA库”(Wang et al, 2012)。鳃在调节渗透压和离子转运方面起重要作用,是水环境发生变化后主要的调节器官(金彩霞等, 2008)。在盐度胁迫后的缢蛏转录组结果中,Sc-CARNS基因的表达也上调(团队成果,尚未发表),由此推测,Sc-CARNS基因参与缢蛏渗透压调节过程。
为进一步了解Sc-CARNS基因及其所在的通路在缢蛏渗透压调节过程发挥的作用,本研究通过RT-qPCR分析发现,低盐胁迫后,Sc-CARNS mRNA表达量显著增加(P < 0.05),高盐时则下降。与太平洋牡蛎(Meng et al, 2013)和菲律宾蛤仔(Ruditapes philippinarum) (Nie et al, 2017)中的研究结果一致,表明低渗条件下丙氨酸的代谢速率显著增加。此时,缢蛏足中丙氨酸含量随着盐度的升高而升高。肌肽含量在低盐胁迫下升高,高盐胁迫下降低。与Koyama等(2015)在日本河蚬中研究结果一致,低盐时丙氨酸含量降低,肌肽含量变化则相反。肌肽作为Ca2+和H+耦合调节剂,通过直接或间接机制摄取Ca2+,发挥调节Ca2+水平的作用(Jones et al, 2017)。说明低盐胁迫时,缢蛏可以通过肌肽合成酶的作用降低体内丙氨酸的含量,改变FAA的总含量,进而适应环境盐度的变化。为维持体内肌肽含量的平衡,多余的肌肽会被肌肽酶降解,再由转运蛋白运到细胞外(潘晨, 2021)。
潘晨(2021)研究发现,给厚壳贻贝注射β-丙氨酸后,体内肌肽合成酶的活性出现上调。肌肽含量显著上升,是对照组的2.87倍。同时,部分氨基酸含量发生变化,具体表现为丙氨酸含量上升和半胱氨酸含量下降(王春月, 2021)。本研究通过对正常盐度条件下缢蛏的Sc-CARNS基因进行干扰,0~96 h缢蛏足中Sc-CARNS表达量和肌肽含量均先上升后降低,丙氨酸含量均先下降后上升。表明Sc-CARNS基因的变化会影响丙氨酸和肌肽的含量,肌肽合成酶是丙氨酸降解成肌肽的关键酶,是盐胁迫后影响丙氨酸代谢速率的限速因子。在干扰Sc-CARNS后,缢蛏的收缩功能受损,伴随细胞内Ca2+降低,Ca2+去除能力减慢,调节离子浓度的能力减弱,影响渗透调节过程(Brownlee et al, 1999; Jones et al, 2017; Gonçalves et al, 2021)。
为进一步验证Sc-CARNS基因的功能,通过对低盐胁迫下Sc-CARNS基因进行干扰,24~96 h缢蛏足中Sc-CARNS表达量和肌肽含量均先上升后降低,丙氨酸含量均先下降后上升。干扰48 h时,符合丙氨酸与肌肽1∶1的转化关系,结果再次验证,Sc-CARNS基因会影响缢蛏体内丙氨酸的含量,认为Sc-CARNS基因在低盐胁迫下的渗透压调节过程中发挥重要作用。
4 结论本研究表明,缢蛏属于渗透压随变者,体内渗透压会随着外界环境的变化而变化。缢蛏鳃、足和血淋巴中TOFAA都随着盐度的升高而升高,其中Ala含量变化幅度最大,因此,Ala是盐度变化时缢蛏体内调节渗透压的主要FAA。通过对Sc-CARNS基因功能的研究,初步明确了Sc-CARNS基因在缢蛏盐度适应过程中的作用,表明丙氨酸代谢为肌肽是低盐胁迫时缢蛏体内丙氨酸含量降低的重要途径之一,是缢蛏适应盐度应激的方式之一。后续可以通过补充丙氨酸协助缢蛏适应环境盐度的变化,为其他物种研究FAA在渗透调节中的作用及氨基酸代谢通路提供基础参考,为水生双壳动物在受到盐度胁迫后的环境适应性调控机制提供基础数据。
AHMAD S, JIANG L, ZHENG S, et al. Silencing of a putative alanine aminotransferase (ALT) gene influences free amino acid composition in hemolymph and fecundity of the predatory bug, Cyrtorhinus lividipennis Reuter. Archives of Insect Biochemistry and Physiology, 2021, 108(2): e21836 DOI:10.1002/arch.21836 |
BAKER S, HOOVER E, STURMER L. The role of salinity in hard clam aquaculture. IFAS Extension, University of Florida, 2007, 1-8 |
BAYE E, UKROPEC J, COURTEN M, et al. Carnosine supplementation reduces plasma soluble transferrin receptor in healthy overweight or obese individuals: A pilot randomised trial. Amino Acids, 2019, 51(1): 73-81 DOI:10.1007/s00726-018-2623-6 |
BROWNLEE C, GODDARD H, HETHERINGTON A M, et al. Specificity and integration of responses: Ca2+ as a signal in polarity and osmotic regulation. Journal of Experimental Botany, 1999, 50: 1001-1011 DOI:10.1093/jxb/50.Special_Issue.1001 |
CAI X Y, ZHANG X M, TIAN L, et al. Effect of salinity stress on hemolymph osmolality and gill Na+/K+-ATPase activity of juvenile ark shell (Anadara broughtonii). South China Fisheries Science, 2015, 11(2): 12-19 [蔡星媛, 张秀梅, 田璐, 等. 盐度胁迫对魁蚶稚贝血淋巴渗透压及鳃Na+/K+-ATP酶活力的影响. 南方水产科学, 2015, 11(2): 12-19 DOI:10.3969/j.issn.2095-0780.2015.02.002] |
CAO W, BI S Q, CHI C F, et al. Effects of high salinity stress on the survival, gill tissue, enzyme activity and free amino acid content in razor clam Sinonovacula constricta. Frontiers in Marine Science, 2022, 9: 839614 DOI:10.3389/fmars.2022.839614 |
CAO W, CHI C F, DONG Y H, et al. Effects of high salt stress on survival and enzyme activities of Sinonovacula constricta juvenile. Marine Sciences, 2022, 46(7): 44-51 [曹伟, 迟长凤, 董迎辉, 等. 高盐胁迫对缢蛏幼贝存活和三种酶活性的影响. 海洋科学, 2022, 46(7): 44-51] |
CHEN D W, SU J, LIU X L, et al. Taste evaluation of non-volatile taste compounds in bivalae mollusks from Beibu Gluf, Guangxi. Food Science, 2012, 33(10): 165-168 [陈德慰, 苏键, 刘小玲, 等. 广西北部湾3种贝类中主要呈味物质的测定及呈味作用评价. 食品科学, 2012, 33(10): 165-168] |
CHEN M X, LAI Q F, ZHOU K, et al. Effects of salinity on survival of Razor Clam Sinonovacula constricta under salinity acclimation, feeding and starvation. Chinese Journal of Fisheries, 2021, 34(6): 31-35 [陈牧霞, 来琦芳, 周凯, 等. 盐度驯化、投喂和饥饿条件下盐度对缢蛏存活的影响. 水产学杂志, 2021, 34(6): 31-35 DOI:10.3969/j.issn.1005-3832.2021.06.005] |
CHENG J H, LIAO I C. Effect of salinity on the osmotic and ionic concentrations in the hemolymph of Penaeus monodon and P. penicillatus. Asian Fisheries Forum, Manila (Philippines), 1986, 26-31 |
COBB B F, CONTE F S, EDWARDS M A. Free amino acids and osmoregulation in penaeid shrimp. Journal of Agricultural and Food Chemistry, 1975, 23(6): 1172-1174 DOI:10.1021/jf60202a015 |
DING H B, LI H Y, CHEN Y H, et al. Effects of high salinity on growth and survival, Na+/K+-ATPase activity and energy metabolism related indexes of razor clam Sinonovacula constricta. Journal of Shanghai Ocean University, 2022, 31(4): 831-838 [丁红兵, 李浩宇, 陈义华, 等. 高盐对缢蛏生长存活、Na+/K+-ATPase活性及能量代谢相关指标的影响. 上海海洋大学学报, 2022, 31(4): 831-838] |
DROZAK J, VEIGA-DA-CUNHA M, VERTOMMEN D, et al. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). Journal of Biological Chemistry, 2010, 285(13): 9346-9356 DOI:10.1074/jbc.M109.095505 |
EVERAERT I, DENAEYER H, TAES Y, et al. Gene expression of carnosine-related enzymes and transporters in skeletal muscle. European Journal of Applied Physiology, 2013, 113(5): 1169-1179 DOI:10.1007/s00421-012-2540-4 |
FU P, LÜ J J, LIU P, et al. Effects of different salinities on the free amino acids composition in the gill of Portunus trituberculatus. Progress in Fishery Sciences, 2016, 37(5): 122-126 [付萍, 吕建建, 刘萍, 等. 盐度胁迫对三疣梭子蟹(Portunus trituberculatus)鳃中游离氨基酸含量的影响. 渔业科学进展, 2016, 37(5): 122-126] |
FU P, LÜ J J, LIU P, et al. Effects of different salinity level on free amino acid composition in muscle and hemolymph of the swimming crab Portunus trituberculatus. Journal of Fisheries of China, 2017, 41(3): 374-381 [付萍, 吕建建, 刘萍, 等. 盐度胁迫对三疣梭子蟹肌肉和血淋巴中游离氨基酸含量的影响. 水产学报, 2017, 41(3): 374-381] |
FUHRMANN M, DELISLE L, PETTON B, et al. Metabolism of the Pacific oyster, Crassostrea gigasis influenced by salinity and modulates survival to the Ostreid herpesvirus OsHV-1. Biology Open, 2018, 7: bio028134 DOI:10.1242/bio.028134 |
GAO X L, LI Y, LI X, et al. The response and osmotic pressure regulation mechanism of Haliotis discus hannai (Mollusca, Gastropoda) to sudden salinity changes. Hydrobiologia, 2017, 795(1): 181-198 DOI:10.1007/s10750-017-3129-z |
GONÇALVES L D S, SALES L P, SAITO T R, et al. Histidine dipeptides are key regulators of excitation- contraction coupling in cardiac muscle: Evidence from a novel CARNS1 knockout rat model. Redox Biology, 2021, 44: 102016 DOI:10.1016/j.redox.2021.102016 |
HAIDER F, SOKOLOV E P, TIMM S, et al. Interactive effects of osmotic stress and burrowing activity on protein metabolism and muscle capacity in the soft shell clam Mya arenaria. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2019, 228: 81-93 DOI:10.1016/j.cbpa.2018.10.022 |
HOSOI M, KUBOTA S, TOYOHARA M, et al. Effect of salinity change on free amino acid content in Pacific oyster. Fisheries Science, 2003, 69(2): 395-400 DOI:10.1046/j.1444-2906.2003.00634.x |
HOSOI M, YOSHINAGA Y, TOYOHARA M, et al. Freshwater bivalve Corbicula sandai uses free amino acids as osmolytes under hyperosmotic condition. Fisheries Science, 2008, 74(6): 1339-1341 DOI:10.1111/j.1444-2906.2008.01662.x |
HUANG K, JIANG H C, WU H Y, et al. Salinity responses of free amino acids in the muscle of Litopenaeus vannamei. Marine Fisheries, 2010, 32(4): 422-426 [黄凯, 蒋焕超, 吴宏玉, 等. 盐度对凡纳滨对虾肌肉中游离氨基酸含量的影响. 海洋渔业, 2010, 32(4): 422-426 DOI:10.3969/j.issn.1004-2490.2010.04.012] |
HUO Z M, WANG Z P, LIANG J, et al. Effects of salinity on embryonic development, survival, and growth of Crassostrea hongkongensis. Journal of Ocean University of China, 2014, 13(4): 666-670 DOI:10.1007/s11802-014-2206-4 |
JIANG S, XU Q H. Influence of salinity stress on the activity of gill Na+/K+-ATPase in swimming crab (Portunus trituberculatus). Journal of Fisheries of China, 2011, 35(10): 1475-1480 [江山, 许强华. 盐度胁迫对三疣梭子蟹鳃Na+/K+-ATPase酶活的影响. 水产学报, 2011, 35(10): 1475-1480] |
JIN C X, PAN L Q. Preliminary studies on physiological adaptive mechanism of Procambarus clarkii osmoregulation under different ambient salinities. Acta Hydrobiologica Sinica, 2008, 32(6): 894-899 [金彩霞, 潘鲁青. 盐度变化对克氏原螯虾渗透调节影响机制的初步研究. 水生生物学报, 2008, 32(6): 894-899] |
JONES R, BARNETT C, DAVIDSON J, et al. β-alanine supplementation improves in-vivo fresh and fatigued skeletal muscle relaxation speed. European Journal of Applied Physiology, 2017, 117(5): 867-879 DOI:10.1007/s00421-017-3569-1 |
KONG X H, WANG S S, DONG Y H, et al. Analysis of expression characteristics of related genes in response to acute thermal stress in the razor clam Sinonovacul constricta. Progress in Fishery Sciences, 2022, 43(2): 194-203 [孔祥辉, 王莎莎, 董迎辉, 等. 缢蛏急性高温胁迫应答主要候选基因的表达特征分析. 渔业科学进展, 2022, 43(2): 194-203] |
KOYAMA H, OKAMOTO S, WATANABE, et al. Dynamic changes in the accumulation of metabolites in brackish water clam Corbicula japonica associated with alternation of salinity. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2015, 181: 59-70 DOI:10.1016/j.cbpb.2014.11.007 |
LEE N H, HAN K N, CHOI K S. Effects of salinity and turbidity on the free amino acid composition in gill tissue of the Pacific oyster, Crassostrea gigas. Journal of Shellfish Research, 2004, 23(1): 129-133 |
LI D Q, ZHOU Q, CHENG B S, et al. Determination of osmotic potential of plant cell fluid by freezing point lowering method - Principle and application technology of FM-4 freezing point osmometer. Shandong Agricultural Sciences, 1988(1): 42-45 [李德全, 邹琦, 程炳嵩, 等. 利用冰点降低法测定植物细胞液的渗透势——FM-4型冰点渗透压计的原理及使用技术. 山东农业科学, 1988(1): 42-45] |
LI E C, ARENA L, CHEN L Q, et al. Characterization and tissue-specific expression of the two glutamate dehydrogenase cDNAs in Pacific white shrimp, Litopenaeus vannamei. Journal of Crustacean Biology, 2009, 29(3): 379-386 DOI:10.1651/08-3104.1 |
LI M, ZHENG Y N, XU K L, et al. Effects of salinity stress on survival rate of Tegillarca granosa and enzyme activity. Journal of Zhejiang Agricultural Sciences, 2018, 59(4): 650–653, 657 [李敏, 郑伊诺, 许凯伦, 等. 盐度胁迫对泥蚶存活率及3种酶活力的影响. 浙江农业科学, 2018, 59(4): 650–653, 657] |
LIANG M Q, WANG S W, WANG J L, et al. Effects of different salinities on free amino acid composition in muscle and hemolymph of the shrimp Litopenaeus vannamei. Progress in Fishery Sciences, 2009, 30(2): 34-39 [梁萌青, 王士稳, 王家林, 等. 不同盐度对凡纳滨对虾血淋巴及肌肉游离氨基酸组成的影响. 渔业科学进展, 2009, 30(2): 34-39] |
LIN C H, YEH P L, LEE T H. Ionic and amino acid regulation in hard clam (Meretrix lusoria) in response to salinity challenges. Frontiers in Physiology, 2016, 7, DOI: 10.3389
|
LIN C H, YEH P L, LEE T H. Time-course changes in the regulation of ions and amino acids in the hard clam Meretrix lusoria upon lower salinity challenge. Journal of Experimental Zoology Part A, Ecological and Integrative Physiology, 2021, 335(7): 602-613 |
LIN G L. Sinonovacula constricta pond culture technology. Agricultural Engineering Technology, 2016, 36(23): 60 [林国兰. 缢蛏池塘养殖技术. 农业工程技术, 2016, 36(23): 60] |
LU J, WANG G D, WANG R M, et al. Advances in microbial synthesis of alanine. Food and Fermentation Industries, 2023, 49(5): 299-305 [陆捷, 王国栋, 王瑞明, 等. 丙氨酸制备研究进展. 食品与发酵工业, 2023, 49(5): 299-305] |
LYNCH M P, WOOD L. Effects of environmental salinity of free amino acids of Crassostrea virginica gmelin. Comparative Biochemistry and Physiology, 1966, 19(4): 783-790 |
MA J W, LÜ J J, LIU P, et al. Effects of abrupt salinity stress on serum osmolarity and ion concentration of "Huangxuan No. 1" Portunus trituberculatus. Progress in Fishery Sciences, 2016, 37(1): 58-62 [马金武, 吕建建, 刘萍, 等. 急性盐度胁迫对三疣梭子蟹(Portunus trituberculatus)"黄选1号"血清渗透压及离子含量的影响. 渔业科学进展, 2016, 37(1): 58-62] |
MAAR M, SAUREL C, LANDES A, et al. Growth potential of blue mussels (M. edulis) exposed to different salinities evaluated by a dynamic energy budget model. Journal of Marine Systems, 2015, 148: 48-55 |
MANHIANI P S, NORTHCUTT J K, HAN I, et al. Antioxidant activity of carnosine extracted from various poultry tissues. Poultry Science, 2013, 92(2): 444-453 |
MATOO O B. Interactive effects of ocean acidification and multiple stressors on physiology of marine bivalves. Dissertation of University of North Carolina at Charlotte, 2013
|
MATSUSHIMA O, HAYASHI Y S. Metabolism of D- and L-alanine and regulation of intracellular free amino acid levels during salinity stress in a brackish-water bivalve Corbicula japonica. Comparative Biochemistry and Physiology Part A: Physiology, 1992, 102(3): 465-471 |
MAY M A, BISHOP K D, RAWSON P D. NMR profiling of metabolites in larval and juvenile blue mussels (Mytilus edulis) under ambient and low salinity conditions. Metabolites, 2017, 7(3): 33 |
MENG J, ZHU Q H, ZHANG L L, et al. Genome and transcriptome analyses provide insight into the euryhaline adaptation mechanism of Crassostrea gigas. PLoS One, 2013, 8(3): e58563 |
NIE H T, JIANG L W, CHEN P, et al. High throughput sequencing of RNA transcriptomes in Ruditapes philippinarum identifies genes involved in osmotic stress response. Scientific reports, 2017, 7(1): 4953 |
PAN C, LIAO Z, HE J Y, et al. Carnosine concentration and expression profiles of carnosine related genes in Mytilus after beta-alanine injection. Journal of Oceanology Limnology, 2022, 40(3): 1121-1134 |
PAN C. Transcriptome analysis and expression of genes related to carnosine metabolism in Mytilus coruscus. Master´s Thesis of Zhejiang Ocean University, 2021 [潘晨. 厚壳贻贝肌肽代谢相关基因的表达研究及转录组分析. 浙江海洋大学硕士研究生学位论文, 2021]
|
PENG M X. Study on tolerance of Chinese razor clam (Sinonovacula constricta) to important water environmental factors in inland waters. Doctoral Dissertation of Shanghai Ocean University, 2020 [彭茂潇. 缢蛏对内陆水域重要水环境因子耐受性研究. 上海海洋大学博士研究生学位论文, 2020]
|
PÉQUEUX A. Osmotic regulation in crustaceans. Journal of Crustacean Biology, 1995, 15(1): 1-60 |
POURMOZAFFAR S, JAHROMI S T, Rameshi H, et al. The role of salinity in physiological responses of bivalves. Reviews in Aquaculture, 2020, 12(3): 1548-1566 |
RAN Z S, CHEN H, RAN Y, et al. Fatty acid and sterol changes in razor clam Sinonovacula constricta (Lamarck 1818) reared at different salinities. Aquaculture, 2017, 473: 493-500 |
SOKOLOWSKI A, WOLOWICZ M, HUMMEL H. Free amino acids in the clam Macoma balthica L. (Bivalvia, Mollusca) from brackish waters of the southern Baltic Sea. Comparative Biochemistry and Physiology: Part A Molecular and Integrative Physiology, 2003, 134(3): 579-592 |
WANG C Y. Determination of histidine-containing dipeptides in marine organisms and carnosine-related metabolomes analysis of Mytilus coruscus. Master´s Thesis of Zhejiang Ocean University, 2021 [王春月. 海洋生物组氨酸二肽分析及贻贝肌肽相关代谢组研究. 浙江海洋大学硕士研究生学位论文, 2021]
|
WANG Y R, LI E C, YU N, et al. Characterization and expression of glutamate dehydrogenase in response to acute salinity stress in the Chinese mitten crab, Eriocheir sinensis. PLoS One, 2012, 7(5): e37316 |
WANG Y. Effects of exogenous carnosine and ethionine on tall fescue under salt stress. Master´s Thesis of Nanjing Agricultural University, 2016 [王岩. 外源肌肽和乙硫氨酸对高羊茅耐盐性的影响. 南京农业大学硕士研究生学位论文, 2016]
|
XU X, HE L, LIN Z H, et al. Effects of salinity stress on V-ATPase H expression, enzyme activity and osmotic pression in Sinonovacul constricta. Chinese Journal of Zoology, 2020, 55(5): 606-613 [徐娴, 何琳, 林志华, 等. 盐度胁迫下缢蛏渗透压变化及V-ATPase H基因的表达分析. 动物学杂志, 2020, 55(5): 606-613] |
YANAS K, FUNAGUCHI N, IIHARA H, et al. Prevention of radiation esophagitis by polaprezinc (zinc L-carnosine) in patients with non-small cell lung cancer who received chemoradiotherapy. International Journal of Clinical and Experimental Medicine, 2015, 8(9): 16215-16222 |
ZHANG D, WANG F, DONG S L. Effects of periodic salinity fluctuations on free amino acid contents and transcription patterns of osmo-related genes in Litopenaeus vannamei. Journal of Fishery Sciences of China, 2016, 23(5): 1130-1136 [张丹, 王芳, 董双林. 周期性盐度波动对凡纳滨对虾游离氨基酸含量及渗透调节相关基因表达的影响. 中国水产科学, 2016, 23(5): 1130-1136] |
ZHANG H, SUN G G, LIN Z H, et al. The razor clam Sinonovacula constricta uses the strategy of conversion of toxic ammonia to glutamine in response to high environmental ammonia exposure. Molecular Biology Reports, 2020, 47(12): 9579-9593 |
ZHANG S P, QIU W Q, LU Q, et al. Determination of glutathione and free amino acids in muscles of four shellfish species by automatic amino acid analyzer. Food Science, 2017, 38(4): 170-176 [张苏平, 邱伟强, 卢祺, 等. 全自动氨基酸分析仪法测定4种贝类肌肉中谷胱甘肽和游离氨基酸含量. 食品科学, 2017, 38(4): 170-176] |
ZHAO D T, LIU Y, ZHAO Y, et al. Effect of carnosine on oxidative stress and NF-κB signaling pathway in hippocampus of diabetic rats. Chinese Journal of Clinical Anatomy, 2018, 36(5): 514-519 [赵丹婷, 刘影, 赵艳, 等. 肌肽对糖尿病大鼠海马中氧化应激及NF-κB信号通路的影响. 中国临床解剖学杂志, 2018, 36(5): 514-519] |
ZHAO X L, YU H, KONG L F, et al. High throughput sequencing of small RNAs transcriptomes in two Crassostrea oysters identifies microRNAs involved in osmotic stress response. Scientific Reports, 2016, 6: 22687 |



