2. 广西农产资源化学与生物技术重点实验室 广西 玉林 537000;
3. 中国海洋大学水产学院 山东 青岛 266003
2. Guangxi Key Laboratory of Agricultural Resources Chemistry and Biotechnology, Yulin 537000, China;
3. College of Fisheries, Ocean University of China, Qingdao 266003, China
病害问题是制约对虾养殖业绿色发展的瓶颈问题,每年给对虾养殖业造成巨大经济损失,对相关病原的定期监测是解决该问题的有效途径之一。虾肝肠胞虫(Enterocytozoon hepatopenaei, EHP)和造成对虾急性肝胰腺坏死病(acute hepatopancreatic necrosis disease, AHPND)的副溶血弧菌(Vibrio parahemolvticus, VP)是对虾养殖业中常见的2种致病微生物。其中,EHP是一种高传染性细胞内寄生的微孢子虫(段健诚等, 2022),主要寄生在对虾肝胰腺组织中,导致对虾肝胰腺病变萎缩,生长缓慢(侯子豪, 2022; 段健诚等, 2019; 李英瑕等, 2022)。EHP宿主广泛,包括凡纳对虾(Penaeus vannamei)、斑节对虾(Penaeus monodon)和脊尾白虾(Palaemon carincauda)等;AHPND是由携带毒力基因质粒PirA/B的弧菌导致的病害,此类疫病于2010年首次在我国和越南发现,曾在我国南方地区的对虾养殖场大面积暴发。随后相继出现于马来西亚、泰国、墨西哥和菲律宾等国(孙卫芳等, 2019)。已有报道表明,斑节对虾、凡纳对虾、中国对虾(Penaeus chinensis)、日本对虾(Penaeus japonicus)均为AHPND的易感宿主(陈平亚等, 2022),患病对虾肝胰腺组织中可检测到致病弧菌,并造成肝胰腺萎缩(Han et al, 2015)。除此之外,已知的多数对虾病原,诸如甲壳类虹彩病毒(Decapod iridescent virus 1, DIV1)、白斑综合征病毒(white spot syndrome virus, WSSV)、桃拉病毒(Taura syndrome virus, TSV)、传染性皮下及造血组织坏死病毒(infectious hypodermal and haematopoietic necrosis virus, IHHNV)等对对虾肝胰腺组织均具有亲嗜性。DIV1感染后会造成肝胰腺苍白萎缩(Qiu et al, 2021; 许昊川等, 2021),组织病理学检查显示,感染DIV1的对虾肝胰腺中可见嗜酸性粒细胞和胞浆内的深色嗜酸性包涵体,部分包被或含有微小的嗜碱性染色,血细胞固缩;WSSV和TSV等也可以造成肝胰腺苍白萎缩的症状(绳秀珍等, 2022),IHHNV可以造成凡纳对虾的肝胰腺肿大(任春华等, 2002)。因此,以对虾肝胰腺组织样品制备待检测核酸样本,可广泛应用于已知的大多数对虾病原的检测。
核酸等温扩增技术摆脱了常规核酸变温扩增过程对PCR扩增仪器的依赖,广泛应用于现场快速检测过程中。目前,等温扩增技术涵盖了环介导等温扩增技术(loop-mediated isothermal amplification, LAMP)、置换扩增技术(strand displacement amplification, SDA)以及重组酶聚合酶扩增技术(recombinase polymerase amplification, RPA)等。其中,RPA是近年发展起来的一类利用重组酶促进寡核苷酸引物在DNA双链互补序列中的插入和结合,在Bsu DNA聚合酶的作用下实现对DNA的特定区域进行指数扩增的技术(Deng et al, 2015)。具有反应时间短、特异性强、灵敏度高、可以在37~42 ℃恒温工作等特点,适用于室外的检测与分析(Lobato et al, 2018),结合侧向流层析试纸条技术(lateral flow dipstick, LFD)读取检测结果,操作简单,耗时短,且具有较高的特异性和灵敏度,是一种新兴的适用于现场快速检测的方法。RPA-LFD技术与简化的核酸提取方式相结合,可以真正实现便携式的现场快速核酸检测。
核酸提取过程是核酸检测的限速条件之一。首次报道的核酸提取方法是Meselson等(1957)用密度梯度离心法提取DNA,后续又出现了通过胍盐裂解法(Bowtell, 1987)、碱裂解法(迟原龙等, 2018)、溴化十六烷基三甲基铵(CTAB)裂解法(Murray et al, 1980)、酚抽提法、煮沸法(孙嘉峰等, 2015)、超声波法(麦重伟, 2016)、基于二氧化硅和磁珠的固相提取法、基于纸片的核酸提取方法(Zou et al, 2017)等相关报道。目前的试剂盒核酸提取纯化法,包括裂解、结合、清洗和洗脱等步骤,能提取出高质量、高纯度的核酸,多被科研院所和大型企业实验室所采用,还可结合磁珠法实现全自动提取。然而,其过程繁琐、耗时长,不适用于快速检测的需求。目前,尚未见核酸释放剂与RPA-LFD技术相结合检测对虾组织病原微生物的相关报道。因此,简化对虾病原检测过程中的核酸提取过程,研制一种适用于RPA-LFD技术检测对虾肝胰腺中DNA样品的快速制备试剂,具有广泛的应用前景。本研究旨在针对对虾肝胰腺组织研制一种核酸样品快速制备试剂,进一步实现EHP和AHPND的现场快速检测。
1 材料与方法 1.1 实验材料Tris-HCl、KCl、乙二胺四乙酸二钠(EGTA2Na)、十二烷基硫酸锂(LDS)、聚乙二醇辛基苯基醚(Triton X-100)、牛血清白蛋白(BSA)、明胶、海藻糖和甜菜碱购自北京索莱宝科技有限公司;试纸型核酸扩增试剂盒购自苏州先达基因科技有限公司。实验样本为对虾肝胰腺组织,由中国水产科学研究院黄海水产研究所保存,阳性样本经实验室检测确定为EHP阳性和AHPND阳性,阴性样本经实验室检测确定为EHP阴性和AHPND阴性。
1.2 引物合成及探针制备EHP和AHPND检测引物和探针由生工生物工程(上海)股份有限公司合成。检测EHP引物序列(马超等, 2022)和AHPND的引物序列(董井泉等, 2020)见表 1。
|
|
表 1 虾肝肠孢虫(EHP)和对虾急性肝胰腺坏死病(AHPND)引物序列 Tab.1 Primer sequences of EHP and AHPND |
核酸样品快速制备试剂(核酸释放剂)组分配比使用方法见表 2。按照表 2配制核酸释放剂,分别取30 mg (绿豆大小)经实验室检测确定为EHP阳性和EHP阴性、AHPND阳性和AHPND阴性的对虾肝胰腺组织加入100 μL不同配比的核酸释放剂,100 ℃加热3 min,离心后取上清液,作为检测模板待用;同时分别取EHP阳性和EHP阴性、AHPND阳性和AHPND阴性的对虾肝胰腺组织,用海洋动物组织基因组DNA提取试剂盒提取核酸,作为检测模板待用。
|
|
表 2 核酸释放剂各成分配比 Tab.2 Composition of nucleic acid releaser |
用试纸型核酸扩增试剂盒进行RPA-LFD检测。每个干粉反应管中分别加入试剂盒配套的缓冲液、EHP上下游引物各2 μL (10 μmol/μL),探针1 μL (10 μmol/μL)及经1.3中核酸释放剂处理过的模板2 μL或海洋动物组织基因组DNA提取试剂盒提取核酸模板2 μL。37 ℃反应30 min。反应结束后用无菌水稀释20倍,插入试纸条,读取检测结果。比较用2种方法提取的核酸进行RPA-LFD检测的效果。
1.5 AHPND的RPA-LFD检测的反应体系及反应条件根据1.4的结果,选取最优组合的核酸释放剂处理AHPND阳性和AHPND阴性样本,用试纸型核酸扩增试剂盒进行RPA检测。在每个干粉反应管中加入试剂盒配套的缓冲液、AHPND上下游引物各2 μL (10 μmol/μL),探针1 μL (10 μmol/μL)及经1.4中筛选出的核酸释放剂处理过的模板2 μL或海洋动物组织基因组DNA提取试剂盒提取核酸模板2 μL。37 ℃反应30 min。反应结束后用无菌水稀释20倍,插入试纸条,读取检测结果。比较2种方法提取的核酸进行RPA-LFD检测的效果。
1.6 RPA-LFD检测结果的读取本研究中,下游引物标记有生物素,探针标记有荧光素(FAM),试纸条结合垫有胶体金标记的鼠抗FAM抗体(金标鼠抗FAM抗体),检测线(T)喷涂抗生物素抗体,质控线(C)喷涂羊抗鼠IgG抗体,因此,模板中含有目标基因时,RPA的扩增产物在试纸条层析可以结合金标鼠抗FAM抗体,并被检测线上的抗生物素抗体捕获,使检测线显色。当试纸条的检测线(T)和质控线(C)同时显色时,检测结果为阳性;当试纸条的质控线(C)显色而检测线(T)不显色时,检测结果为阴性;当试纸条的检测线(T)和质控线(C)都不显色时,检测结果无效。
2 结果 2.1 核酸释放剂配比优化按照表 2配制核酸释放剂,处理相同的EHP阳性样品和EHP阴性样品制备待检测DNA模板,进行RPA-LFD反应,检测EHP,结果显示(图 1),方案03、05、08、11、14、17、21、25和28处理后的EHP阳性样品作为检测模板的RPA-LFD反应,均可使试纸条的检测线(T)和质控线(C)同时显色,检测结果呈阳性,以其对应的EHP阴性样品作为检测模板的RPA-LFD反应,均只使试纸条的质控线(C)显色,其检测线(T)不显色,检测结果呈阴性;说明其检测特异性良好。以方案03、05、08和11处理后的EHP阳性样品作检测模板时,RPA-LFD检测的灵敏度相对较低。以方案28处理的EHP阳性样品作为模板,RPA-LFD检测的灵敏度最高,与用试剂盒提取纯化的EHP阳性样品作检测模板时,RPA-LFD检测的灵敏度相近,为最优方案,按此配比配制的核酸释放剂效果最好。其余配比方案不能有效检出条带,未再冗余罗列。
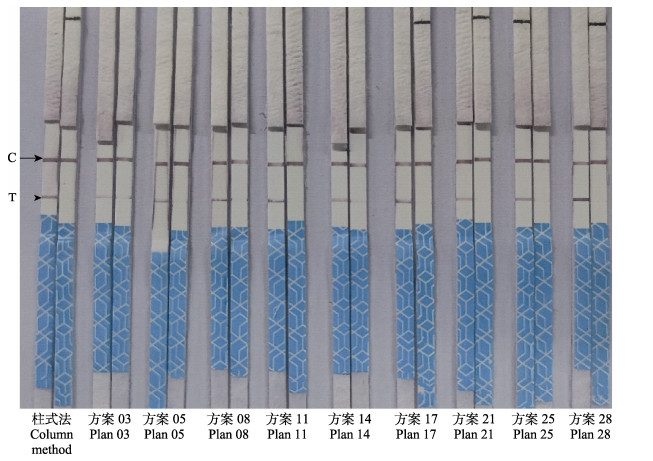
|
图 1 方案03、05、08、11、14、17、21、25和28核酸释放剂提取EHP样本核酸和柱式法提取EHP样本核酸的RPA-LFD检测结果 Fig.1 The RPA-LFD test results of nucleic acid releaser splitting EHP sample by plan 03, 05, 08, 11, 14, 17, 21, 25, 28 and extraction of nucleic acid from EHP sample by column method C:质控线;T:检测线。图 2同。 C: Quality control line; T: Test line. The same in Fig.2. |
分别以方案28处理的AHPND阳性样品和阴性样品以及试剂盒法提取纯化的AHPND阳性样品和阴性样品作为检测模板,用1.4的RPA反应体系检测AHPND,反应结束后用无菌水稀释20倍,插入试纸条,读取检测结果,结果显示(图 2),方案28处理后的AHPND阳性样品作为检测模板的RPA-LFD反应,可使试纸条的检测线(T)和质控线(C)同时显色,检测结果呈阳性,以其对应的EHP阴性样品作为检测模板的RPA-LFD反应,均只使试纸条的质控线(C)显色,其检测线(T)不显色,检测结果呈阴性;说明其检测特异性良好。且与用试剂盒提取纯化的AHPND阳性样品作检测模板时,RPA-LFD检测的灵敏度相近。
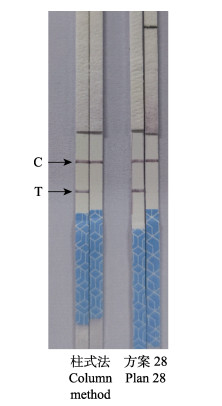
|
图 2 方案28核酸释放剂和柱式法提取AHPND样本核酸的RPA-LFD检测结果 Fig.2 The RPA-LFD test results of nucleic acid releaser splitting AHPND sample by plan 28 and extraction of nucleic acid from AHPND sample by column method |
目前,已有多种核酸提取技术,例如高浓度的胍盐可以解离核蛋白体,同时使细胞内核酸酶失活,保护核酸不被降解,用于分离纯化核酸;Chirgwin等(1979)用异硫氰酸胍和β-巯基乙醇裂解细胞,用氯化铯梯度超速离心或乙醇抽提首次特异性分离出RNA,然而始终无法克服费时费力、危险性大、效率低等缺点;CTAB裂解法适用于产生大量多糖的生物体的核酸提纯,但其实验步骤多,操作较繁琐;酚抽提法提取的DNA可保持天然状态,纯度高,但操作繁琐、费时,且使用毒性有机溶剂苯酚对实验人员有一定的危害(张丽等, 2011);磁珠法安全无毒,但成本较高。这些方法受实验室条件、检测人员操作水平等因素限制较大(黄维等, 2022)。
随着RPA/RAA技术的发展和其在病原检测中的广泛运用,适用于RPA技术的核酸释放剂也随之出现。目前,商品化的核酸释放剂较多,但其是否适用于对虾组织核酸样品提取以进行RPA-LFD检测有待考证。Tris-HCl可以保持细胞裂解缓冲液的pH值稳定,因此,本研究选用Tris-HCl作为裂解缓冲液的主要成分。选用KCl提供盐离子环境,使DNA充分溶解于液相,使蛋白质和DNA分离;LDS是一种阴离子去垢剂,也是一种表面活性剂,可破坏细胞的脂质双分子层膜结构;Triton X-100可发挥同LDS相同的作用,用于细胞裂解和蛋白质提取等(邹晓蕾等, 2013),同时还可以提高核酸保存的稳定性,因此,本研究选用LDS作为裂解液的一种组分;利用EGTA作为螯合剂和抑制剂,结合带有2个正电荷的金属离子(例如Mg2+和Ca2+),降低蛋白酶活性水平;以BSA维持缓冲液的渗透压和pH稳定;海藻糖的扩散系数低,分子运动和分子变性非常微弱,可以保持DNA分子的分散状态,同时,海藻糖对生物体和生物分子还有独特的非特异性保护作用(张玉华等, 2005),因此,本研究以明胶和海藻糖作为反应增稠剂,以甜菜碱作为表面活性剂,促进RPA反应过程。因此,本研究对Tris-HCl、KCl、EGTA2Na、LDS、Triton X-100、BSA、明胶、海藻糖和甜菜碱等常见细胞裂解液组分进行了优化,制备适用于对虾肝胰腺DNA核酸样品提取的核酸释放剂。常宇桐等(2022)基于反转录重组酶聚合酶扩增(RT-RPA)方法研制的微量核酸释放剂,使用异硫氰酸胍、非离子型表面活性剂Tween-20和Triton X-100来裂解细胞,释放细胞中的蛋白质和核酸,使蛋白质变性,促进脂质溶解,用异丙醇保护RNA链中的亲水基团,通过乙醇的洗涤作用溶解部分有机杂质和蛋白质。最终能检测出诺如病毒GⅡ型阳性样本,检测结果与商品化荧光定量试剂盒的结果一致。本研究同样选用Triton X-100来裂解细胞,并使蛋白质变性沉淀,同时用阴离子表面活性剂LDS和甜菜碱辅以裂解细胞,但Triton X-100浓度过高时容易造成污染,增加检测时假阳性的概率,本研究使用2% Triton X-100相较于使用1%和0.5% Triton X-100时检测结果为阳性和阴性的区分度更低,也能说明这一问题;此外,本研究利用Tris-HCl和BSA来维持裂解液的pH值和渗透压稳定,用KCl来提供盐离子环境,使DNA充分溶解于液相;以EGTA螯合带有2个正电荷的金属离子,降低蛋白酶的活性水平;本研究不使用异硫氰酸胍,避免胍盐残留影响后续酶活反应;同时以明胶和海藻糖作为反应增稠剂,保持DNA分子的分散状态;使核酸释放剂的功能更为完善。
综上所述,本项研究优化的核酸释放剂适用于对虾肝胰腺DNA样品制备。成功提取了对虾肝胰腺中的EHP和AHPND的DNA样品,可直接用于RPA-LFD对对虾肝胰腺中这2种病原的检测,有效避免了常规DNA样品繁琐、耗时的制备步骤,极大地提高了核酸水平病原检测效率,与传统试剂盒纯化核酸的方法相比,检测灵敏度相近,具有广泛的应用价值。
BOWTELL D D. Rapid isolation of eukaryotic DNA. Analytical Biochemistry, 1987, 162(2): 463-465 DOI:10.1016/0003-2697(87)90421-0 |
CHANG Y T, LIU L J, ZHANG L P, et al. Development of micro-nucleic acid releasing reagent based on RT-RPA detection method. Chinese Journal of Frontier Health and Quarantine, 2022, 45(3): 172-174 [常宇桐, 刘丽娟, 张丽萍, 等. 基于RT-RPA的微量核酸释放剂的研制. 中国国境卫生检疫杂志, 2022, 45(3): 172-174] |
CHEN P Y, WU S N, HE C H, et al. Establishment of a RAA assay for pathogenic bacteria of AHPND in shrimps. China Animal Health Inspection, 2022, 39(9): 128-133 [陈平亚, 吴山楠, 何翠华, 等. 对虾急性肝胰腺坏死病病原菌RAA检测方法的建立. 中国动物检疫, 2022, 39(9): 128-133] |
CHI Y L, TANG Y, JIN L, et al. Identification of bacteria and yeasts by using an alkaline lysis method for DNA extraction. Food Research and Development, 2018, 39(2): 177-180 [迟原龙, 唐垚, 金璐, 等. 采用碱裂解法提取DNA鉴定细菌和酵母菌. 食品研究与开发, 2018, 39(2): 177-180] |
CHIRGWIN J M, PRZYBYLA A E, MACDONAL R J, et al. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry, 1979, 18(24): 5294-5299 DOI:10.1021/bi00591a005 |
DENG H, GAO Z. Bioanalytical applications of isothermal nucleic acid amplification techniques. Analytica Chimica Acta, 2015, 853: 30-45 DOI:10.1016/j.aca.2014.09.037 |
DONG J Q, YANG X H, ZHAO P P, et al. Establishment and application of RPA-LFS rapid detection method for AHPND in Vibrio parahaemolyticus. Jiangsu: CN111635951A, 2020-09-08 [董井泉, 杨潇含, 赵盼盼, 等. 一种AHPND致病性副溶血弧菌RPA-LFS快速检测方法的建立及应用. 江苏省: CN111635951A, 2020-09-08]
|
DUAN J C, DENG G W, ZHANG Q Q, et al. Advances in shrimp parasitosis of Enterocytozoon hepatopenaei. Journal of Huaihai Institute of Technology (Natural Sciences), 2019, 28(3): 79-83 [段健诚, 邓高威, 张庆起, 等. 虾类寄生虫病——虾肝肠胞虫研究进展. 淮海工学院学报(自然科学版), 2019, 28(3): 79-83] |
DUAN J C, HU J H, SHEN Y H, et al. Effect of Enterocytozoon hepatopenaei infection on the intestinal microflora of Exopalaemon carinicauda. Progress in Fishery Sciences, 2022, 43(3): 75-83 [段健诚, 胡吉卉, 沈宇航, 等. 虾肝肠胞虫感染对脊尾白虾肠道菌群的影响. 渔业科学进展, 2022, 43(3): 75-83] |
HAN J E, TANG K F J, TRAN L H, et al. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Diseases of Aquatic Organisms, 2015, 113(1): 33-40 DOI:10.3354/dao02830 |
HOU Z H. Development of a PCR assay for the detection of Enterocytozoon hepatopenaei and the study of its infection to Litopenaeus vannamei. Master´s of Ludong University, 2022 [侯子豪. 虾肝肠胞虫PCR检测方法的建立及对凡纳滨对虾的感染研究. 鲁东大学硕士研究生学位论文, 2022]
|
HUANG W, ZHAO Z R, HUANG L J, et al. Effect of two conventional nucleic acid extraction methods on the sensitivity of African swine fever qPCR detection. Guangxi Journal of Animal Husbandry & Veterinary Medicine, 2022, 38(4): 162–163, 185 [黄维, 赵正荣, 黄玲娟, 等. 两种常规核酸提取法对非洲猪瘟qPCR检测敏感度的影响. 广西畜牧兽医, 2022, 38(4): 162–163, 185 DOI:10.3969/j.issn.1002-5235.2022.04.005] |
LI Y X, XU T T, LIU S, et al. Validation of a highly sensitive kit for the rapid detection of Enterocytozoon hepatopenaei in field. Progress in Fishery Sciences, 2022, 43(4): 218-225 [李英瑕, 徐婷婷, 刘爽, 等. 虾肝肠胞虫(EHP)现场快速高灵敏度检测试剂盒的性能评价研究. 渔业科学进展, 2022, 43(4): 218-225] |
LOBATO I M, O'SULLIVAN C K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends in Analytical Chemistry, 2018, 98: 19-35 DOI:10.1016/j.trac.2017.10.015 |
MA C, GAO S, WANG W L. Establishment and application of recombinase polymerase amplification and lateral flow strip combined method for the rapid detection of Enterocytozoon hepatonpenae. Journal of Jiangsu Ocean University (Natural Science), 2022, 31(3): 25-30 [马超, 高嵩, 王伟玲. 肝肠胞虫RPA-LFS快速检测方法的建立及应用. 江苏海洋大学学报(自然科学版), 2022, 31(3): 25-30] |
MAI C W. A method of nucleic acid extraction based on ultrasonic lysis. Guangdong. CN105586337A, 2016-05-18 [麦重伟. 一种基于超声波裂解的核酸提取方法. 广东. CN105586337A, 2016-05-18]
|
MESELSON M, STAHL F W, VINOGRAD J. Equilibrium sedimentation of macromolecules in density gradients. Proceedings of the National Academy of Sciences, 1957, 43(7): 581-588 |
MURRAY M G, THOMPSON W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 1980, 8(19): 4321-4325 |
QIU L, CHEN X, GAO W, et al. Molecular epidemiology and histopathological study of a natural infection with Decapod iridescent virus 1 in farmed white leg shrimp, Penaeus vannamei. Aquaculture, 2021, 533: 736105 |
REN C H, SHEN Q, HU C Q. The harmful characteristics and PCR detection of infectious subcutaneous and hematopoietic necrosis virus (IHHNV) in Penaeus vannamei. Conference papers (abstracts) collection of the 20th anniversary of the establishment of the Crustacean Zoology Branch and the 55th anniversary of Academician Liu Ruiyu's engagement in Marine science and education work, 2002, 54 [任春华, 沈琪, 胡超群. 凡纳对虾传染性皮下及造血组织坏死病毒(IHHNV)的危害特点及PCR检测. 甲壳动物学分会成立20周年暨刘瑞玉院士从事海洋科教工作55周年学术研讨会论文(摘要)集, 2002, 54]
|
SHENG X Z, WU M S, YE H B, et al. Research progresses on Decapoda iridescent virus 1. Journal of Fisheries of China, 2022, 46(5): 721-729 [绳秀珍, 吴明顺, 叶海斌, 等. 十足目虹彩病毒1的研究进展. 水产学报, 2022, 46(5): 721-729] |
SUN J F, WANG J W, HUANG Y. Effects of two nucleic acid extraction methods on the quantitative detection of HBV DNA. International Journal of Laboratory Medicine, 2015, 36(18): 2720-2722 [孙嘉峰, 王建伟, 黄毅. 两种核酸提取方法对HBV DNA定量检测结果的影响. 国际检验医学杂志, 2015, 36(18): 2720-2722] |
SUN W F, HUANG X S, HU X J, et al. Detection and analysis of Enterocytozoon hepatopenaei (EHP), Vibrio parahaemolyticus acute hepatopancreatic necrosis disease (VPAHPND) and shrimp hemocyte iridescent virus (SHIV) from Litopenaeus vannamei in coastal areas of Guangdong Province. Journal of Southern Agriculture, 2019, 50(10): 2343-2349 [孙卫芳, 黄小帅, 胡晓娟, 等. 广东沿海地区凡纳滨对虾EHP、VPAHPND和SHIV感染情况调查与分析. 南方农业学报, 2019, 50(10): 2343-2349] |
XU H C, XU S W, WANG W Q, et al. Investigation on major pathogens carried by shrimp seeds of Penaeus vannamei in Ningbo City of Zhejiang Province in 2020. China Animal Health Inspection, 2021, 38(9): 29-32 [许昊川, 徐胜威, 王雯琼, 等. 2020年浙江省宁波市南美白对虾虾苗主要病原携带情况调查. 中国动物检疫, 2021, 38(9): 29-32] |
ZHANG L, YANG L R, WU S Q. Research progress of nucleic acid extraction methods. China Animal Health Inspection, 2011, 28(12): 75-78 [张丽, 杨莲茹, 吴绍强. 核酸提取方法的研究进展. 中国动物检疫, 2011, 28(12): 75-78] |
ZHANG Y H, LING P X, JI B P. Current status of research for trehalose and its prospective applications. Food and Drug, 2005(3): 8-13 [张玉华, 凌沛学, 籍保平. 海藻糖的研究现状及其应用前景. 食品与药品, 2005(3): 8-13] |
ZOU X L, LIU L C, LUO L X. Comparison of different methods for total bacteria RNA extraction. Modern Food Science and Technology, 2013, 29(8): 1948-1954 [邹晓蕾, 刘礼崔, 罗立新. 细菌总RNA提取方法的比较. 现代食品科技, 2013, 29(8): 1948-1954] |
ZOU Y P, MASON M G, WANG Y L, et al. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biology, 2017, 15(11): e2003916 |



