2. 中国水产科学研究院黄海水产研究所 农业农村部海水养殖病害防治重点实验室 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Key Laboratory of Marine Aquaculture Disease Control, Ministry of Agriculture and Rural Affairs; Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao 266071, China
虾肝肠胞虫(Enterocytozoon hepatopenaei, EHP)在分类上属于肠微孢子虫科(Enterocytozoonidae)、肠微孢子虫属(Enterocytozoon),是一类主要寄生于对虾肝胰腺上皮细胞的微孢子虫(Texier et al, 2010)。EHP最早发现于泰国生长迟缓的斑节对虾(Penaeus monodon)中(Chayaburakul et al, 2004);Tourtip等(2009)根据其组织学、超微结构等特征,将其正式命名为EHP。成熟的EHP孢子呈卵圆形或梨形,大小为0.7 μm×1.1 μm,其基本结构由孢壁、锚定盘、孢原质、极丝、细胞核、极质体、核糖体和后极泡等组成。该病原靶组织为虾类肝胰腺,感染后引起虾类肝胰腺微孢子虫病(Hepatopancreatic microsporidiosis, HPM) (Salachan et al, 2016; Flegel et al, 2018)。
EHP主要感染斑节对虾、凡纳对虾(P. vannamei)、罗氏沼虾(Macrobrachium rosenbergii)和太平洋蓝对虾(P. stylirostris)(Tangprasittipap et al, 2013; Jang et al, 2022),日本对虾(P. japonicus)也是EHP的疑似易感宿主(Tangprasittipap et al, 2013; Thitamadee et al, 2016)。卤虫(Artemia salina)、桡足类(Copepoda)、多毛类(Polychaetes)均可携带EHP,因此,对虾养殖中使用鲜活或冷冻的卤虫、桡足类和多毛类等存在传播EHP的风险(Tang et al, 2015; 叶键等, 2017; 丁慧昕等, 2018)。EHP也可以通过含有孢子的虾类粪便和养殖水进行传播(Pan et al, 2018; Tang et al, 2016; Salachan et al, 2016; Karthikeyan et al, 2019、2020)。除水平传播方式外,Khac等(2018)通过PCR检测和组织病理学分析发现,EHP或可经由阳性的亲虾垂直传播给子代。目前,EHP在全球流行的范围广泛,泰国、印度、文莱、越南、印度尼西亚、马来西亚、韩国和委内瑞拉等亚洲(Thitamadee et al, 2016; Rajendran et al, 2016)和美洲国家皆存在EHP的广泛流行,并且检出率呈逐年上升趋势(Biju et al, 2016; Moser et al, 2022)。2013年,我国河北、天津等地养殖的凡纳对虾最早检出EHP阳性(刘宝彬等, 2017),2014年,在海南、江苏和山东等地养殖凡纳对虾中也同样检出EHP阳性(刘珍等, 2016);随后,EHP在全国主要对虾养殖区包括山东、河北、江苏、上海、浙江、福建、广东、海南和新疆等地养殖凡纳对虾中流行(农业部渔业渔政管理局等, 2018)。EHP感染对虾后,虽不会引起死亡,但会导致其肝胰腺上皮细胞坏死和破裂,致使肝胰腺消化吸收功能下降和营养储存功能受损,从而引起对虾生长迟缓甚至停滞(Röszer, 2014; Santhoshkumar et al, 2017),由此大大降低对虾产量,严重影响对虾养殖业的绿色高质量发展(刘雅梅等, 2017)。
EHP个体极其微小,且其感染对虾的早期症状不明显,较难通过光学显微镜观察或凭症状现场确诊,因此,常采用实验室的检测方法进行诊断。组织病理切片染色后可对EHP感染中后期的组织病理变化进行观察,但在感染早期难以利用该方法确诊EHP感染,且组织切片制作时间长、流程复杂,不适合作为EHP检测的便捷技术手段。相比之下,分子生物学方法具有快速、简便和灵敏度高等特点,已成为实验室检测EHP的常用手段(李英瑕等, 2022)。
本研究于2021—2022年在我国沿海地区开展养殖虾类EHP流行情况调查,利用TaqMan实时荧光定量PCR (TaqMan qPCR)方法对所采集的虾类样品进行分子生物学检测,并对EHP阳性样品进行组织病理分析,以期阐明2021—2022年EHP在我国沿海省市主要养殖虾类中的流行情况,明确EHP对养殖虾类的危害风险。
1 材料与方法 1.1 样品采集样品采集时,用一次性解剖刀将虾类个体的头胸部纵向一分为二,其中1/2保存在4% PFA固定液中用于后续组织病理学分析,剩余样品部分切碎混匀保存于95%乙醇固定液中,用于TaqMan qPCR的检测。
1.2 DNA提取取30 mg保存在95%乙醇固定液中的样品,利用海洋动物组织基因组DNA试剂盒(天根,北京)按照试剂盒操作手册提取样品的总DNA。然后,使用超微量分光光度计NanoDrop 2000c (ThermoScientific, Waltham, 美国)测量总DNA的浓度和纯度。
1.3 利用TaqMan qPCR方法检测样品中EHP参照中华人民共和国水产行业标准(行SC/T 7232 - 2020)推荐的TaqMan qPCR方法,采用Fast Start Essential DNA Probes Master试剂盒(罗氏,中国)对EHP靶基因片段进行检测。检测中用到的引物序列见表 1。
|
|
表 1 EHP TaqMan荧光定量PCR方法所用引物序列 Tab.1 Primer sequences of EHP TaqMan qPCR |
在冰上配制TaqMan qPCR体系,总体积为20 μL,各组分用量如下:2×Master Mix 10 μL,RNA-free H2O 7.6 μL,引物EHP-157-F (10 μmol/L) 0.6 μL、EHP-157-R (10 μmol/L) 0.6 μL,探针EHP-157-P (10 μmol/L) 0.2 μL,EHP阳性模板1 μL。
利用Bio-Rad实时荧光定量PCR仪进行TaqMan qPCR扩增,反应程序设置:95 ℃预变性10 min;95 ℃变性10 s,60 ℃退火延伸30 s,共40个循环。
1.4 组织病理学观察选择TaqMan qPCR检测呈阳性的样品进行组织切片和病理学观察。将保存在4%-PFA固定液中的样品(已固定12~24 h)转移至70%乙醇中,然后,按照已报道的方法制备病理切片。并进行苏木精–伊红(HE)染色。使用Nikon Eclipse E80光学显微镜(日本)对出现明显病理变化的部位进行观测并拍照记录。
2 结果 2.1 流行病学调查与样品采集情况2021年主要在山东、海南、江苏、辽宁、河北、新疆等省(自治区)的虾类养殖地区开展调查和样品采集。调查发现,北方沿海省份养殖户反映发病对虾中生长迟缓现象较为常见。在上述省份共采集各类虾类样品506份,包括凡纳对虾、斑节对虾、日本对虾、脊尾白虾和罗氏沼虾(表 2和表 3)。
|
|
表 2 样品采集省市及数量信息 Tab.2 The distribution of sampling sites and amount of samples |
|
|
表 3 样品种类及数量信息 Tab.3 The information of sampling species and amount of samples |
2022年主要在山东、江苏、河北、浙江,天津、广西、辽宁、海南等省市(自治区)虾类养殖地区开展调查和样品采集,调查依然发现,北方省市养殖户反映发病对虾生长迟缓现象较多。2022年共采集430份虾类样品,包括凡纳对虾、斑节对虾、脊尾白虾、日本对虾、罗氏沼虾和克氏原螯虾(表 2和表 3)。
2.2 不同虾类中EHP阳性检出率分析利用EHP的TaqMan qPCR方法对所采集的样品进行检测,发现在2021—2022年所采集的7种主要养殖虾类中,仅凡纳对虾和脊尾白虾样品中检出EHP阳性,其余虾类未检出阳性。其中,2021年凡纳对虾中EHP的阳性检出率为14.10% (54/383)、2022年为16.71% (58/347)。脊尾白虾样品量较少,仅在2022年采集到1份样品,样品中有EHP的阳性检出(图 1和表 4)。
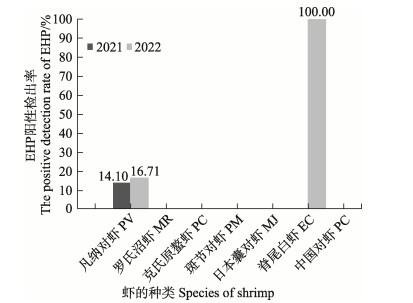
|
图 1 2021—2022年不同虾类EHP阳性检出率 Fig.1 EHP positive detection rates in different shrimp species in 2021–2022 |
|
|
表 4 2021—2022年不同虾类EHP阳性检出数 Tab.4 Number of positive EHP detections for different shrimp species in 2021–2022 |
对所采集虾类样品的TaqMan qPCR检测结果按照采样年份进行分析,结果显示,2021和2022年样品中EHP阳性检出率分别为10.67% (54/506)和13.72% (59/430) (图 2、表 5),总体上,2022年虾类样品中EHP阳性检出率相较于2021年有所上升。

|
图 2 2021—2022年EHP阳性检出率 Fig.2 Annual positive detection rate of EHP in 2021–2022 |
|
|
表 5 2021—2022年EHP阳性检出数 Tab.5 Number of positive EHP detections in 2021–2022 |
TaqMan qPCR检测显示,2021年采集自山东、辽宁和广东三省的样品中存在EHP阳性检出,检出率分别为18.80% (50/266)、10% (2/20)、1.94% (2/103) (图 3和表 6);2022年采集自山东、辽宁、河北和天津四省市的样品中有EHP阳性检出,检出率分别为16.88% (26/154)、14.63% (6/41)、29.17% (7/24)和28.57% (20/70) (图 3和表 6);总体上看,北方省市养殖虾类样品中EHP阳性检出率较高。
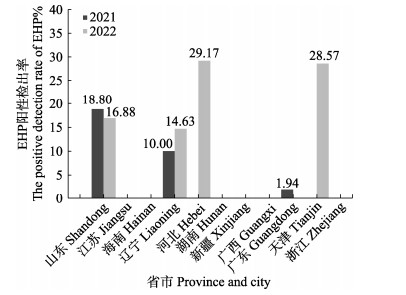
|
图 3 2021—2022年EHP阳性检出省市 Fig.3 Provinces and cities with positive EHP detections in 2021–2022 |
|
|
表 6 2021—2022年EHP阳性检出省市 Tab.6 Provinces and cities with positive EHP detections in 2021–2022 |
在显微镜高放大倍率下,病虾肝胰腺上皮细胞内可见处于不同生活史阶段的EHP,如多核EHP原质团和聚集性孢子,上皮细胞中也可见成熟的EHP孢子和待释放到肝胰腺小管管腔中的孢子(图 4B、C),肝胰腺小管外组织间隙液中可观测到成熟的EHP孢子(图 4D)。
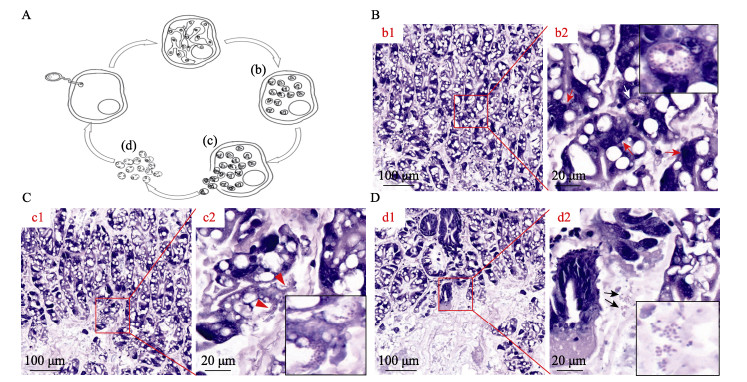
|
图 4 凡纳对虾肝胰腺组织中的虾肝肠胞虫的HE病理切片 Fig.4 HE pathological sections of shrimp EHP in hepatopancreas of P. vannamei A:EHP孢子感染宿主细胞及其胞内生命周期示意图(省略产孢原质团和孢子母细胞2个阶段):孢子萌发,极管刺破细胞膜,将具感染性的孢原质转移到细胞质中;孢原质进行核分裂,而胞质未分裂,产生分枝状的裂殖原质团;孢子母细胞生成孢子;宿主细胞破裂释放成熟的孢子;宿主细胞外的成熟孢子。图A中的b~d分别示意图B~D中EHP生活史的不同周期。B:被感染肝胰腺小管上皮细胞中存在EHP原质团(图b2中红色箭头所示)和聚集性孢子(如图b2中白色箭头所示,并被放大后置于右上角)。其中,图b2为图b1中局部区域的放大图。C:脱落的上皮细胞内含有即将释放到肝胰腺小管腔的EHP孢子(如图c2中红色三角形所示,并被放大后置于左下角)。其中,图c2为图c1中局部区域的放大图。D:游离在肝胰腺小管外血淋巴液中的EHP成熟孢子(如图d2中黑色箭头所示,并被放大后置于左下角)。其中,图d2为图d1中局部区域的放大图。 A: Schematic diagram of EHP spore infected host cells and their intracellular life cycles (The two stages of sporogenous mass and spore mother cell were omitted). Stages of the life cycle of EHP: Spore germination, in which the spore punctures the target host cell membrane with the polar tube and transfers the content into the cytoplasm; The sporoplasm undergoes nuclear division to generate a branched plasmodium; Spore mother cell produce the spore; The host cell ruptures to release mature spores; Mature spores outside the host cell. b~d in Fig. A are schematic of the different periods of EHP life history from Fig. B~D, respectively. B: EHP plasmodia (red arrows in Fig. b2) and aggregated spores (the white arrow in the Fig. b2 image, and is enlarged and placed in the upper right corner) were present in the infected hepatopancreas tubular epithelial cells. Fig. b2 is a magnified view of the framed area in Figure b1. C: Exfoliated epithelial cells contain EHP spores (indicated by the red triangle in Fig. c2 and zoomed in to the bottom right corner) that are about to be released into the lumen of the hepatopancreatic tubules. Fig. c2 is a magnified view of the local area in Fig. c1. D: Mature EHP spores (indicated by the black arrow in the Fig. d2 diagram and also shown in the magnified image at bottom right) free in the haemolymph in the extratubular interstitial fluid of the hepatopancreas. Fig. d2 is a zoomed-in view of the local area in Fig. d1. |
2017年起,农业农村部国家水生动物疫病监测计划开始在全国主要对虾养殖地区开展EHP流行情况的监测,其中,2017年在包括12个省(自治区、直辖市)和新疆生产建设兵团的420个监测点中,EHP平均阳性检出率为22.1% (93/420);2018年在包括15个省(自治区、直辖市)和新疆生产建设兵团采集的样品中,EHP平均阳性检出率为22.45% (288/1283);2019年在包括15个省(自治区、直辖市)和新疆生产建设兵团采集的样品中,EHP平均阳性检出率为14.5% (85/588);2020年在包括9省(直辖市)采集的样品中,EHP平均阳性检出率为14.7% (34/232)。从监测结果来看,EHP的阳性检出率显著高于同时期十足目虹彩病毒(Decapod iridescent virus 1, DIV1: 2018年12.2%,2019年8.5%,2020年8.8%)和致急性肝胰腺坏死弧菌(Vibrio causing acute hepatopancreas necrosis disease, VAHPND, 2019年及之前无监测数据,2020年4.5%)这2种虾类新发病原。2017年以来的国家水生动物疫病监测结果显示,EHP感染引起的HPM在我国主要虾类养殖区域流行分布,包括山东、河北、广东、广西、海南、福建、江苏、浙江和天津等沿海地区以及新疆生产建设兵团所在的内陆地区;EHP阳性检出种类主要包括凡纳对虾、斑节对虾、中国对虾和克氏原螯虾等我国养殖甲壳动物主要经济种类(农业农村部渔业渔政管理局等, 2020),在我国流行危害形势较为严峻,是制约我国虾类养殖业绿色高质量发展的重要疫病病原之一。2021—2022年间,本研究针对沿海省市EHP的流行与感染情况进行了调查和分析,初步揭示了该病原最近2年内在我国主要养殖虾类中的流行趋势。
本研究结果显示,2021年沿海省市虾类样品中EHP的阳性检出率为10.67%,相比于2017—2020年国家水生动物疫病监测计划中EHP的阳性检出率呈下降趋势,但2022年沿海省市虾类样品中EHP的阳性检出率达13.72%,上升到2019—2020年全国监测数据中EHP的阳性检出率水平,即EHP的流行有抬头趋势,值得引起重视。2021年国家水生动物疫病监测计划也在全国部分虾类养殖地区开展了EHP流行情况的监测,共在河北、辽宁、江苏、安徽、江西、山东、湖北和海南8省采集样品92份,测得的EHP阳性检出率为4.5% (5/92),考虑到该年度国家水生动物疫病监测计划所分析样品的数量极为有限(92份),可能难以较为客观地反映我国主要虾类养殖地区的EHP流行情况(农业农村部渔业渔政管理局等, 2022);2022年国家水生动物疫病监测计划中共对在全国采集的70份样品进行EHP监测分析(监测结果尚未发布),样品数量也非常有限。本研究分别对2021年和2022年采集自我国沿海省市的506份和430份虾类样品进行TaqMan qPCR检测分析,分析的样本量较多,检测方法特异性、灵敏性高,因此,检测结果更为可靠,为全面了解我国2021—2022年间沿海地区主要养殖虾类中EHP的流行情况提供了可供参考的重要资料。
本研究对采集自不同省市样品中EHP的检测分析结果显示,2021年采集自辽宁(10%)、山东(18.80%)的样品中EHP阳性检出率较高,2022年采集自辽宁(14.63%)、河北(29.17%)、天津(28.57%)和山东(16.88%) 的样品中EHP阳性检出率较高,该结果说明EHP仍在沿海主要对虾养殖地区流行,且北方沿海省市的流行率高于南方。这与作者在流行病学调查中从养殖户采集到的信息一致,即北方沿海省市养殖场中EHP流行较为严重。2017—2018年国家水生动物疫病监测计划中对EHP的监测结果显示,该病原在广东、福建和上海等地养殖虾类中流行率最高,2019—2021年国家水生动物疫病监测计划中对EHP的监测结果显示,该病原在辽宁、河北、山东等地养殖虾类中流行率最高(农业农村部渔业渔政管理局等, 2019、2020、2021、2022),本研究结果也与国家水生动物疫病监测计划EHP监测数据的变动趋势一致。本研究结果表明,EHP仍然是危害我国沿海地区尤其是北方养殖对虾的主要疫病病原之一。
本研究的结果还显示,EHP主要在凡纳对虾中检出,且阳性检出率高达15.34%。因克氏原螯虾、斑节对虾和脊尾白虾的检测数量较少,不符合流行病学调查的要求,故未对以上虾类的流行情况进行进一步的分析。在对TaqMan qPCR检测呈EHP阳性的样品进行组织病理学分析中,患病对虾肝胰腺上皮细胞中可观测到散在或成簇的EHP孢子以及处于生长阶段的EHP原质团。尽管EHP感染不直接导致虾类死亡,但能引起患病虾生长迟滞而严重影响养殖虾类产量。因此,应继续加强虾类养殖过程中EHP流行情况的监测,并积极开展无EHP种苗推广,最大限度降低其对虾类养殖产业的影响。
综上所述,2021—2022年针对我国沿海省市以及部分内陆城市养殖虾类的流行病学调查和分子流行病学分析表明,EHP流行率较前几年总体呈下降趋势,说明近几年我国虾类养殖产业中对EHP的防控取得了显著成效;但调查分析结果也显示,EHP在我国北方沿海省市养殖虾类中仍广泛流行,应进一步采取包括严格亲体和苗种EHP检疫,推广无EHP种苗等措施,以进一步降低EHP流行范围和危害风险,助力我国虾类养殖产业的绿色高质量发展。
BIJU N, SATHIYARAJ G, RAJ M, et al. High prevalence of Enterocytozoon hepatopenaei in shrimps Penaeus monodon and Litopenaeus vannamei sampled from slow growth ponds in India. Diseases of Aquatic Organisms, 2016, 120(3): 225-230 DOI:10.3354/dao03036 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center. 2019 aquatic animals in China. Beijing: China Agriculture Press, 2019 [农业农村部渔业渔政管理局, 全国水产技术推广总站. 2019中国水生动物卫生状况报告. 北京: 中国农业出版社, 2019]
|
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center. 2020 aquatic animals in China. Beijing: China Agriculture Press, 2020 [农业农村部渔业渔政管理局, 全国水产技术推广总站. 2020中国水生动物卫生状况报告. 北京: 中国农业出版社, 2020]
|
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center. 2021 aquatic animals in China. Beijing: China Agriculture Press, 2021 [农业农村部渔业渔政管理局, 全国水产技术推广总站. 2021中国水生动物卫生状况报告. 北京: 中国农业出版社, 2021]
|
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center. 2022 aquatic animals in China. Beijing: China Agriculture Press, 2022 [农业农村部渔业渔政管理局. 2022中国水生动物卫生状况报告. 北京: 中国农业出版社, 2022]
|
Bureau of Fisheries, Ministry of Agriculture, National Fisheries Technology Extension Center. 2017 aquatic animals in China. Beijing: China Agriculture Press, 2018 [农业部渔业渔政管理局, 全国水产技术推广总站. 2017中国水生动物卫生状况报告. 北京: 中国农业出版社, 2018]
|
CHAYABURAKUL K, NASH G, PRATANPIPAT P, et al. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Diseases of Aquatic Organisms, 2004, 60(2): 89-96 |
DING H X, SHI H, XIE J J, et al. Preliminary study on distribution and transmission ways of Enterocytozoo hepatopenaei in Litopenaeus vannamei's culture environment. Journal of Zhejiang Ocean University: Natural Science, 2018, 37(1): 14-19 [丁慧昕, 施慧, 谢建军, 等. 虾肝肠胞虫(EHP)在凡纳滨对虾养殖水环境中的分布情况及传播途径初步研究. 浙江海洋大学学报(自然科学版), 2018, 37(1): 14-19 DOI:10.3969/j.issn.1008-830X.2018.01.003] |
FLEGEL T W, SRITUNYALUUCKSANA K. Recent research on acute hepatopancreatic necrosis disease (AHPND) and Enterocytozoon hepatopenaei in Thailand. Asian Fisheries Society, 2018, 31(S): 57-269 |
JANG G I, KIM S M, OH Y K, et al. First report of Enterocytozoon hepatopenaei infection in giant freshwater prawn (Macrobrachium rosenbergii de Man) cultured in the Republic of Korea. Animals (Basel), 2022, 12(22): 3149 |
KARTHIKEYAN K, SUDHAKARAN R. Experimental horizontal transmission of Enterocytozoon hepatopenaei in postlarvae of whiteleg shrimp, Litopenaeus vannamei. Journal of Fish Diseases, 2019, 42(3): 397-404 DOI:10.1111/jfd.12945 |
KARTHIKEYAN K, SUDHAKARAN R. Exploring the potentiality of Artemia salina to act as a reservoir for microsporidian Enterocytozoon hepatopenaei of penaeid shrimp. Biocatalysis and Agricultural Biotechnology, 2020, 25: 101607 DOI:10.1016/j.bcab.2020.101607 |
KHAC H V, THANH T N T, THU G N T, et al. Vertical transmission and early diagnosis of the microsporidian Enterocytozoon hepatonaei in whiteleg shrimp Penaeus vannamei. Journal of Pure and Applied Microbiology, 2018, 12(3): 1125-1131 DOI:10.22207/JPAM.12.3.11 |
LI Y X, XU T T, LIU S, et al. Validation of a highly sensitive kit for the rapid detection of Enterocytozoon hepatopenaei in field. Progress in Fishery Sciences, 2022, 43(4): 218-225 [李英瑕, 徐婷婷, 刘爽, 等. 虾肝肠胞虫(EHP)现场快速高灵敏度检测试剂盒的性能评价研究. 渔业科学进展, 2022, 43(4): 218-225] |
LIU B B, YANG B, LÜ X W, et al. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus (IHHNV) and Enterocytozoon hepatopenaei (EHP) of Litopenaeus vannamei by real-time PCR. Progress in Fishery Sciences, 2017, 38(2): 158-166 [刘宝彬, 杨冰, 吕秀旺, 等. 凡纳滨对虾(Litopenaeus vannamei)传染性皮下及造血组织坏死病毒(IHHNV)及虾肝肠胞虫(EHP)的荧光定量PCR检测. 渔业科学进展, 2017, 38(2): 158-166] |
LIU Y M, QIU L, CHENG D Y, et al. The relationship of body length and weight in the litopenaeus vannamei populations detected Enterocytozoon hepatopenaei. Progress in Fishery Sciences, 2017, 38(4): 96-103 [刘雅梅, 邱亮, 程东远, 等. 检出虾肝肠胞虫(Enterocytozoon hepatopenaei)的凡纳滨对虾(Litopenaeus vannamei)群体的体长和体重关系. 渔业科学进展, 2017, 38(4): 96-103] |
LIU Z, ZHANG Q L, WAN X Y, et al. Development of real-time PCR assay for detecting microsporidian Enterocytozoon hepatopenaei and the application in shrimp samples with different growth rates. Progress in Fishery Sciences, 2016, 37(2): 119-126 [刘珍, 张庆利, 万晓媛, 等. 虾肝肠胞虫(Enterocytozoon hepatopenaei)实时荧光定量PCR检测方法的建立及对虾样品的检测. 渔业科学进展, 2016, 37(2): 119-126] |
MOSER R J, FRANZ L, FIRESTONE S M, et al. Enterocytozoon hepatopenaei real-time and shrimp MultiPathTM PCR assay validation for South-East Asian and Latin American strains of Penaeid shrimp. Diseases of Aquatic Organisms, 2022, 149: 11-23 DOI:10.3354/dao03655 |
PAN G Q, BAO J L, MA Z G, et al. Invertebrate host responses to microsporidia infections. Developmental and Comparative Immunology, 2018, 83: 104-113 DOI:10.1016/j.dci.2018.02.004 |
RAJENDRAN K V, SHIVAM S, EZHIL PRAVEENA P, et al. Emergence of Enterocytozoon hepatopenaei (EHP) in farmed Penaeus (Litopenaeus) vannamei in India. Aquaculture, 2016, 454: 272-280 DOI:10.1016/j.aquaculture.2015.12.034 |
RÖSZER T. The invertebrate midintestinal gland ("hepatopancreas") is an evolutionary forerunner in the integration of immunity and metabolism. Cell and Tissue Research, 2014, 358: 685-695 DOI:10.1007/s00441-014-1985-7 |
SALACHAN P V, JAROENLAK P, THITAMADEE S, et al. Laboratory cohabitation challenge model for shrimp hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei (EHP). BMC Veterinary Research, 2016, 13(1): 9 DOI:10.1186/s12917-016-0923-1 |
SANTHOSHKUMAR S, SIVAKUMAR S, VIMAL S, et al. Biochemical changes and tissue distribution of Enterocytozoon hepatopenaei (EHP) in naturally and experimentally EHP‐infected whiteleg shrimp, Litopenaeus vannamei (Boone, 1931), in India. Journal of Fish Diseases, 2017, 40(4): 529-539 DOI:10.1111/jfd.12530 |
TANG K F J, HAN J E, ARANGUREN L F, et al. Dense populations of the microsporidian Enterocytozoon hepatopenaei (EHP) in feces of Penaeus vannamei exhibiting white feces syndrome and pathways of their transmission to healthy shrimp. Journal of Invertebrate Pathology, 2016, 140: 1-7 DOI:10.1016/j.jip.2016.08.004 |
TANG K F J, PANTOJA C R, REDMAN R M, et al. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. Journal of Invertebrate Pathology, 2015, 130: 37-41 DOI:10.1016/j.jip.2015.06.009 |
TANGPRASITTIPAP A, SRISALA J, CHOUWDEE S, et al. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei. BMC Veterinary Research, 2013, 9: 139 DOI:10.1186/1746-6148-9-139 |
TEXIER C, VIDAU C, VIGUÈS B, et al. Microsporidia: A model for minimal parasite-host interactions. Current Opinion in Microbiology, 2010, 13(4): 443-449 DOI:10.1016/j.mib.2010.05.005 |
THITAMADEE S, PRACHUMWAT A, SRISALA J, et al. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture, 2016, 452: 69-87 DOI:10.1016/j.aquaculture.2015.10.028 |
TOURTIP S, WONGTRIPOP S, STENTIFORD G D, et al. Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships. Journal of Invertebrate Pathology, 2009, 102(1): 21-29 DOI:10.1016/j.jip.2009.06.004 |
YE J, SHI L K, WANG L, et al. Beware that Artemia can carry Enterocytozoon hepatopenaei. Scientific Fish Farming, 2017(11): 62 [叶键, 施礼科, 王力, 等. 警惕卤虫可携带虾肝肠孢虫. 科学养鱼, 2017(11): 62] |



