2. 中国水产科学研究院黄海水产研究所 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071
2. Yellow Sea Fisheries Research Institute, Ministry of Agriculture and Rural Affairs; Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao 266071, China
rimoc1基因编码的蛋白质Rimoc1是线粒体内膜上的一种蛋白质,参与调节线粒体呼吸链的复合物Ⅰ的活性,进而影响线粒体呼吸功能。线粒体呼吸链是细胞内能量代谢的主要过程之一,它负责将食物中的化学能转化为细胞可以使用的能量(ATP)。如果线粒体呼吸链功能受损,可能导致一系列疾病,包括神经肌肉疾病、代谢疾病和多种癌症等(Youle et al, 2011)。Rimoc1在线粒体内膜上与复合物Ⅰ的NDUFAF1亚基相互作用,增强其对NADH的亲和力,从而增加复合物Ⅰ的催化活性,促进线粒体呼吸链中电子传递的速率(Vaites et al, 2017)。此外,Rimoc1还与复合物Ⅲ和复合物Ⅳ等其他线粒体呼吸链复合物中的蛋白质相互作用,可能在调节线粒体呼吸链整体的稳定性和功能上也具有一定的作用(Yan et al, 2021)。然而,rimoc1在鱼类中的功能尚不明确,在前期的转录组研究中发现,rimoc1基因在半滑舌鳎(Cynoglossus semilaevis)雌、雄鱼发育的早期阶段存在差异表达(Xu et al, 2021)。
半滑舌鳎是我国特有的海水经济鱼类,因其低脂肪、高蛋白质且富含维生素,深受消费者喜爱。半滑舌鳎的生长具有明显的性别二态性,1龄雌鱼的体重及生长速度是雄鱼的2~4倍(Chen et al, 2014),雌鱼为什么比雄鱼生长快?rimoc1基因在雌雄鱼性别分化和生长差异中是否发挥作用?这些问题对于半滑舌鳎遗传改良和养殖业发展具有重要意义和应用价值。然而,有关半滑舌鳎雌、雄鱼rimoc1基因的表达模式及其差异的研究未见报道。
本研究对半滑舌鳎rimoc1基因进行系统发育树、不同组织中的表达模式分析,组织定位、RNA干扰以及启动子活性分析,以期为探讨rimoc1基因在半滑舌鳎性别生长二态性的功能和调控机制奠定基础。
1 材料与方法 1.1 鱼类和组织采集半滑舌鳎来自山东海阳高新技术实验基地。采集成鱼(3龄雄鱼和雌鱼)的组织样本,包括脾脏、肾脏、心脏、肝脏、肠道、肌肉、大脑和性腺,并立即用液氮冷冻,–80 ℃冰箱保存。采集不同发育阶段的半滑舌鳎性腺,包括孵化后40、60和90 d (dph),孵化后6个月(mph),孵化后1.5年(yph)和3 yph。采集后分为两部分,一部分置于液氮中,然后–80 ℃保存以提取RNA,另一部分在4%多聚甲醛固定剂中保存, 用于原位杂交(ISH)。剪取尾鳍并储存在乙醇中,用于DNA提取和遗传性别鉴定。
1.2 序列分析和对比通过ExPASy网站(http://web.expasy.org)对编码序列、分子量和等电点进行预测,通过SMART (http://smart.embl-heidelberg.de/)预测蛋白结构域,使用MEGA 6.0软件构建系统发育树。
1.3 引物设计及合成根据半滑舌鳎基因组数据(GCA_000525025.1)和rimoc1的mRNA序列设计实时定量引物,以β-actin为内参基因,引物序列见表 1,引物由北京睿博兴科生物技术有限公司合成。
|
|
表 1 引物序列 Tab.1 The sequence of primers |
使用TIANamp marine animals DNA试剂盒(TIANGEN)进行基因组DNA的提取,利用超微量核酸检测仪和琼脂糖凝胶电泳检测DNA的浓度和质量。采用共显性性别特异标记引物scaffold68-2-F/R进行PCR后电泳检测,用于半滑舌鳎遗传性别鉴定。从尾鳍中提取基因组DNA并作为PCR模板,用琼脂糖凝胶电泳检查所得的PCR产物。雄性样品只观察到1条带,而雌性样品观察到2条带(刘洋等, 2014)。
1.5 总RNA提取及cDNA合成使用TRIzol试剂(Invitrogen, 美国)分离总RNA,取800 μg用于反转录,使用PrimeScriptTMRT试剂盒(TaKaRa)进行反转录,使用0.8%琼脂糖凝胶电泳和超微量核酸检测仪测定RNA及cDNA的质量和浓度。
1.6 实时荧光定量PCR(qPCR)qPCR用于确定rimoc1在卵巢不同组织和不同发育阶段的表达水平。qPCR使用7500 Fast Real-time PCR仪(AppliedBiosystems, 美国),使用TB Green® Premix Ex Taq™ (TaKaRa)试剂盒进行检测。反应体系体积为20 μL,其中,含有10.0 μL TB GreenPremix Ex Tap、0.4 μL ROX DyeⅡ、0.4 μL正向及反向引物、1.0 μL cDNA和7.8 μL ddH2O。本研究使用的引物见表 1。扩增过程包括95 ℃ 30 s、95 ℃ 5 s和60 ℃ 34 s的40个循环,熔解曲线为默认程序。β-肌动蛋白被用作内参(Li et al, 2010)。基因相对表达量通过2–ΔΔCt方法进行分析(Livak et al, 2001)。使用SPSS软件进行单因素方差分析(one-way ANOVA)并进行t检验,当P<0.05时,定义为显著性差异。
1.7 原位杂交按照Chen等(2014)的方法进行原位杂交(ISH),设计引物以扩增239 bp的rimoc1片段(表 1),连接pBluescript Ⅱ SK(+)构建重组质粒,由此产生的重组质粒用EcoRⅤ和PstⅠ线性化,随后用T3或T7 RNA聚合酶转录,生成地高辛(DIG)标记的正义或反义RNA探针。将1 yph鱼的性腺样本切片进行脱蜡和复水等处理,然后在55 ℃下与探针(终浓度为0.2 μg/mL)孵化过夜。在加入抗DIG抗体孵育过夜之前,在室温下将样品切片封闭4 h (10%山羊血清,150 mmol/L NaCl, 100 mmol/L马来酸,调整pH为7.5)。最后用硝基蓝四氮唑/5-溴-4-氯-3-吲哚基磷酸酯(罗氏公司,德国)显影,用尼康Eclipse 80i显微镜(日本)拍摄照片。
1.8 卵巢细胞系中siRNA介导的rimoc1干扰由睿博公司设计并合成了特异性小干扰RNA rimoc1-siRNA,而GFP-siRNA作为阴性对照。用于RNA干扰的卵巢细胞系来自实验室已有细胞系(Zhang et al, 2011; Sun et al, 2015)。用Lipofectamine 8000试剂将siRNA转染到卵巢细胞系中。比较了干扰后不同时间段(24、48和72 h)的效果,48 h的样本显示出最明显的沉默效果(数据未显示)。rimoc1-siRNA和阴性对照组分别进行了3次重复。转染48 h后,提取总RNA并进行反转录。通过qPCR检测sox9a (XM_008315177.3)、sox9b (XM_017040189.2)、foxl2 (NM_001294199.1)、cyp19a (NM_001294183.1)、igf1 (NM_001294198.1)的表达情况(引物序列见表 1)。
1.9 半滑舌鳎rimoc1启动子活性分析 1.9.1 启动子重组载体的构建根据rimoc1基因组序列,选取基因编码区上游981 bp作为启动子序列,并在两端加上XhoⅠ和HindⅢ两个酶切位点,设计引物rimoc1p F/R (表 1),引物由睿博兴科生物技术有限公司合成。以雌性性腺cDNA为模板进行扩增,随后用1%琼脂糖凝胶电泳验证序列,验证成功后用诺唯赞回收试剂盒进行回收,将得到的片段连接到pGL3-Basic载体上,通过转化后进行阳性克隆检测,随后将样品测序验证,最后提取重组质粒。
1.9.2 转录因子预测通过ALGGEN-PROMO网站预测rimoc1启动子区域存在C/EBPalpha、Sox2、C-Jun的转录因子结合位点,克隆C/EBPalpha、Sox2、C-Jun转录因子的编码区并连接到pcDNA3.1载体上,构建pcDNA3.1-C/EBPalpha、pcDNA3.1-Sox2和pcDNA3.1-C-Jun重组载体。
1.9.3 转录因子克隆及重组载体构建在转录因子序列上设计带有酶切位点HindⅢ的引物克隆转录因子并且回收,将pcDNA3.1用HindⅢ单酶切回收,然后进行无缝克隆连接转化,测序成功后提取质粒。
1.9.4 人类胚胎肾细胞(HEK293T)培养及细胞转染本研究使用来自雌性的人类胚胎肾HEK293T细胞。HEK293T细胞在10%胎牛血清(FBS)(Gibco, 美国)的DME/F-12培养基中培养,不添加生长因子和抗生素。37 ℃恒温培养,并且保持培养箱中5%的CO2浓度。待细胞铺满后,以1传5的方式进行传代。待293T细胞在细胞培养瓶中长到60%~80%密度后铺板,一瓶细胞铺一板(24孔)。最后和pGL3-basic、pGL3-control同时进行转染,每组进行3个孔的平行实验以保证实验的准确性。每孔含500 ng质粒,用lipo8000为转染试剂转染到细胞当中。每孔添加1 μL的转染试剂和40 ng的pRL-TK质粒作为内参。最后将含有10%肽牛血清的DMEM培养基补充到25 μL。
1.9.5 双荧光素酶检测细胞转染36~48 h后,根据细胞状态用双荧光素酶试剂盒(碧云天)进行双荧光素酶检测。将100 μL细胞裂解液加入不透明的细胞检测板中,然后加入100 μL萤火虫荧光素酶,在多功能酶标仪上进行萤火虫荧光素酶的相对发光单位(relative light unit, RLU)检测。待检测完后再加入100 μL海肾荧光素酶检测液,测定海肾荧光素酶的RLU。使用Varioskan Flash spectral scanning multimode reader (Thermo)测定,测定时间为10 s,间隔2 s,波长为450 nm。用IBM SPSS Statistics 23软件处理数据以及Origin 2018软件作图。
2 结果 2.1 rimoc1的克隆及特征本研究获得了1 588 bp的rimoc1全长cDNA,包括64 bp的5´UTR、834 bp的ORF和690 bp的3´UTR片段,ORF编码一个277个氨基酸构成的蛋白质,预测相对分子量为32.22 kDa,等电点为5.05。系统发育分析显示,脊椎动物rimoc1分为两支,其中,半滑舌鳎和其他鱼类的rimoc1聚为一支,其他脊椎动物rimoc1聚为另一支(图 1)。
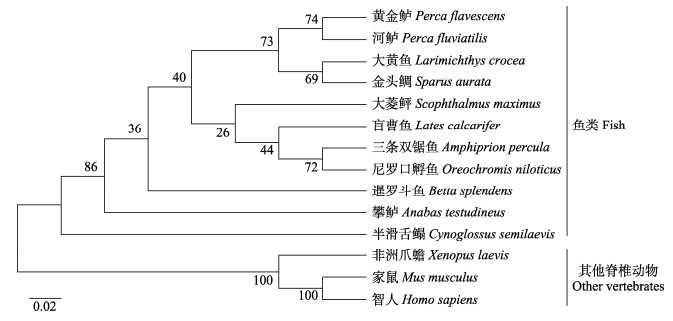
|
图 1 脊椎动物中Rimoc1蛋白系统进化树 Fig.1 Phylogenetic tree of Rimoc1 proteins in vertebrate |
为了分析rimoc1在不同组织间的表达模式,使用来自1.5 yph半滑舌鳎的8个不同组织进行qPCR检测。在雄鱼组织中没有检测到rimoc1表达(数据未显示)。在雌鱼的8个组织中均检测到rimoc1表达,其中,肌肉中的表达量最高,卵巢中的表达量仅次于肌肉,随后是脑。rimoc1在肠、肾脏、脾脏和心脏中的表达量偏低(图 2)。
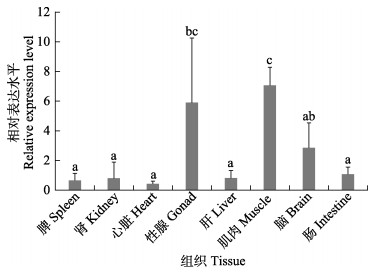
|
图 2 半滑舌鳎rimoc1在不同组织中的表达 Fig.2 Expression of rimoc1 gene in different tissues of C. semilaevis 不同字母表示样本间有显著差异(P<0.05)。图 3和图 7同。 The different letters represent significant differences between samples. The same in Fig.3 and Fig.7. |
通过qPCR进一步检测在性腺发育的不同时间rimoc1的表达情况。如图 3所示,rimoc1在卵巢所有发育阶段中均能检测到表达,自40 d开始表达量逐渐升高,在180 d表达量降低,但在1.5 y时表达量达到峰值,随后表达量开始下降,并在3 y时趋于稳定。
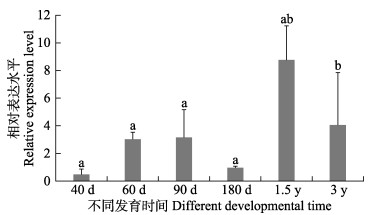
|
图 3 半滑舌鳎rimoc1不同时间卵巢中的表达模式 Fig.3 Gonadal expression pattern of rimoc1 in C. semilaevis ovary at different time |
如图 4A~C所示,在1 yph的雌鱼卵巢中可以观察到信号主要分布在卵母细胞中。在Ⅰ~Ⅳ期的卵母细胞中均能观察到信号,信号在早期的卵母细胞(Ⅰ~Ⅲ期)相对更强(图 4C)。而作为阴性对照的正义探针,在卵巢中未检测出明显的信号(图 4D~H)。
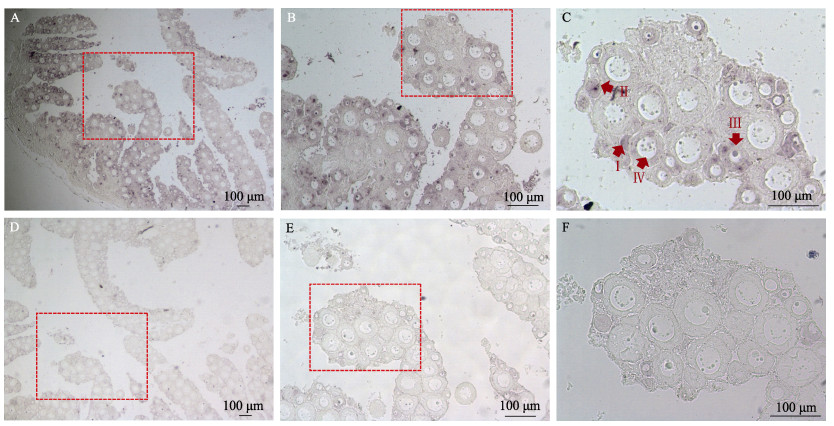
|
图 4 半滑舌鳎rimoc1原位杂交结果 Fig.4 Results of rimoc1 in situ hybridization of C. semilaevis A~C为反义探针,D~F为正义探针。图C中Ⅰ~Ⅳ分别为卵母细胞的各个时期。 A~C: Antisense probe; D~F: Sense probe. Ⅰ~Ⅳ in C are the developing stages of oocyte. |
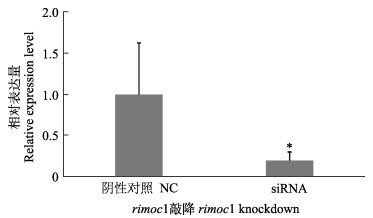
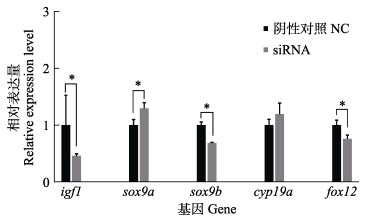
为了确定沉默效果,在siRNA转染48 h后,qPCR检测rimoc1的表达,发现其表达降低至约19%,敲除效果明显(P<0.05)(图 5)。对生长相关基因igf1和性别分化相关基因sox9a、sox9b、cyp19a和fox12的表达水平进行检测,发现igf1表达量降低了54%,sox9b降低了31%,fox12降低了24%,sox9升高了29%,cyp19a有所升高,但差异不显著(图 6)。
|
图 5 半滑舌鳎rimoc1敲降后的表达变化 Fig.5 Expression change in C. semilaevis after rimoc1 knockdown *表示显著差异(P<0.05)。图 6同。 * means significant difference (P < 0.05). The same in Fig.6. |
|
图 6 半滑舌鳎rimoc1敲降后下游基因的表达变化 Fig.6 Change of downstream gene expression after rimoc1 knockdown in C. semilaevis |
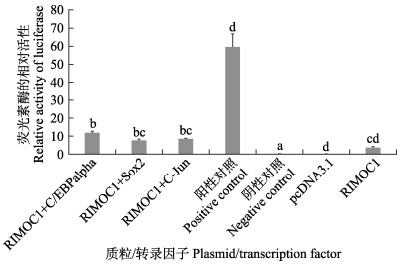
根据双荧光素酶检测结果(图 7),相对于阴性对照组,半滑舌鳎rimoc1启动子具有较强的活性。与对照组只转染rimoc1启动子质粒相比,rimoc1启动子质粒与C/EBPalpha、Sox2、C-Jun转录因子共转染均显示更高的荧光素酶活性,但只与C/EBPalpha结合后的升高有显著差异(P<0.05)。
|
图 7 半滑舌鳎rimoc1启动子转录活性分析 Fig.7 Transcriptional activity analysis of rimoc1 promoter in C. semilaevis |
半滑舌鳎的性别决定发生在50 d左右,而性腺分化则在60 d开始,但细胞层面的分化相对滞后,卵巢细胞分化通常发生在90~120 d,并伴有卵巢腔的出现(Chen et al, 2014)。随后卵母细胞继续分化,并在约2龄时达到性成熟。因此,90 d时的rimoc1高表达与半滑舌鳎自然生长的卵巢细胞分化是一致的。
启动子活性分析显示,rimoc1上游981 bp处具有转录活性,且启动活性较强,与C/EBPalpha、Sox2和C-Jun转录因子共转染之后活性均得到了增强(虽然只有C/EBPalpha组有显著差异),说明这3个转录因子对rimoc1转录起到增强作用。C/EBPalpha主要在角质细胞(Oh et al, 2007)和脂肪细胞(Lefterova et al, 2008)分化中发挥作用,其对半滑舌鳎rimoc1的调控究竟发挥怎样的生物学功能,仍有待进一步研究。
关于rimoc1在性别分化中的功能报道较少,本研究对卵巢细胞系中通过siRNA介导的RNA干扰仅进行了功能初探。通过荧光定量PCR结果可以看出,rimoc1基因干扰后,igf1、sox9a、sox9b和foxl2的表达水平相较于对照组均有不同程度的变化,其中,sox9a表达量上调,而igf1、sox9b和foxl2表达量下调。鱼类igf1与生殖功能和性腺发育密切相关(Lucy et al, 2012; Reinecke, 2010; Vong et al, 2003; Wuertz et al, 2007)。在罗非鱼中,igf1在卵巢发生的前期至卵母细胞成熟过程中均可检测到表达。同时,igf1参与生长激素的调节,从而参与生长调控(Puche et al, 2012)。由于1龄半滑舌鳎开始出现性别生长二态性,同时,不同发育阶段的定量结果表明,rimoc1基因在180 dph的表达量开始上升,直至1.5龄表达量达到峰值。rimoc1基因是否在生长中有着潜在的调控功能,值得深入研究。
sox9是一种关键的转录因子,在调节各种细胞类型的增殖和分化中发挥重要作用(Eftekhary et al, 2019)。sox9在胚胎发育中的作用至关重要,其表达贯穿整个胚胎发育过程,sox9在成人组织中也保持表达,在内皮和外胚层器官的产后损伤修复中也发挥着重要作用。sox9a在性别分化中的作用也屡见报道。由于sox9a和cyp19a共同形成性别分化的调节途径,推测rimoc1通过调节类固醇激素途径参与卵巢发育和卵子发生。sox9b在早期半滑舌鳎的性腺中表现出高表达,而敲除rimoc1基因导致其表达量减低,表明rimoc1和sox9b共同作用来调控半滑舌鳎的性腺分化。foxl2在动物的性别决定和分化、卵巢的发育与维持、胚胎发育和免疫调节等过程中均发挥着作用(He et al, 2020)。研究发现,foxl2在小鼠中能正向调控卵巢的分化(Loffler et al, 2003),foxl2的持续表达可以抑制小鼠生长发育过程中的卵巢细胞向睾丸细胞异常分化,从而维持其表观雌性(Matson et al, 2011),同时,foxl2的缺失也会导致卵巢的发育不良,甚至导致雌性不育(Nicol et al, 2020)。rimoc1基因敲除导致其表达量减低,表明rimoc1可能与半滑舌鳎卵巢发育密切相关。因此,深入了解rimoc1的功能和调控机制,可能是解析性别生长二态性的关键。
4 结论本研究采用qPCR方法、原位杂交、siRNA干扰以及启动子活性分析方法,探究rimoc1基因在半滑舌鳎雌雄鱼中的表达差异及与性别和生长的关系。结果表明,rimoc1在雌鱼中特异表达,在卵巢和肌肉表达量最高。在不同发育阶段的卵巢中,其表达相对稳定,在1.5 yph和3 yph的水平有所增加。启动子活性分析表明,rimoc1启动子可能受到C/EBPdelta、Sox2和c-Jun等转录因子的调控。在半滑舌鳎卵巢细胞系中,通过siRNA干扰rimoc1后发现,生长相关基因igf1、性别分化相关基因sox9b和foxl2的表达水平显著降低,而sox9a的表达升高。推测rimoc1可能在半滑舌鳎的性别分化和生长中发挥重要作用,这为深入研究半滑舌鳎的性别生长二态性提供了基础。
CHEN S L, ZHANG G J, SHAO C W, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics, 2014, 46(3): 253-260 DOI:10.1038/ng.2890 |
EFTEKHARY M, MOHAMMADI-YEGANEH S, BOLANDI Z, et al. A novel natural antisense transcript at human SOX9 locus is down-regulated in cancer and stem cells. Biotechnology Letters, 2019, 42(2): 119-128 |
HE J Q, ZHANG H, SHE Z C, et al. Cloning analysis and expression pattern of Foxl2 gene in the HongKong oyster (Oyster sinensis). Genomics and Applied Biology, 2020, 39(4): 1519-1528 [何金全, 张虹, 佘智彩, 等. 香港牡蛎Foxl2基因克隆分析与表达模式研究. 基因组学与应用生物学, 2020, 39(4): 1519-1528] |
LEFTEROVA M I, ZHANG Y, STEGER D J, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes and Development, 2008, 22(21): 114-135 |
LI Z, YANG L, WANG J, et al. β-actin is a useful internal control for tissue-specific gene expression studies using quantitative real-time PCR in the half-smooth tongue sole Cynoglossus semilaevis challenged with LPS or Vibrio anguillarum. Fish and Shellfish Immunology, 2010, 29(1): 89-93 DOI:10.1016/j.fsi.2010.02.021 |
LIU Y, CHEN S L, GAO F T, et al. SCAR-transformation of sex-specific SSR marker and its application in half-smooth tongue sole (Cynoglossus semiliaevis). Journal of Agricultural Biotechnology, 2014, 22(6): 787-792 [刘洋, 陈松林, 高峰涛, 等. 半滑舌鳎性别特异微卫星标记的SCAR转化及其应用. 农业生物技术学报, 2014, 22(6): 787-792 DOI:10.3969/j.issn.1674-7968.2014.06.015] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001, 25(4): 402-408 DOI:10.1006/meth.2001.1262 |
LOFFLER K A, DAVID Z, PETER K. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology, 2003, 144(7): 3237-3243 DOI:10.1210/en.2002-0095 |
LUCY M C. Growth hormone regulation of follicular growth. Reproduction, Fertility, and Development, 2012, 24(1): 19-28 DOI:10.1071/RD11903 |
MATSON C K, MURPHY M W, SARVER A L, et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature, 2011, 476(7358): 101-104 DOI:10.1038/nature10239 |
NICOL B N, RODRIGUEZ K, YAO H C. Aberrant and constitutive expression of FOXL2 impairs ovarian development and functions in mice. Biology of Reproduction, 2020, 103(5): 966-977 DOI:10.1093/biolre/ioaa146 |
OH W J, RISHI V, OROSZ A, et al. Inhibition of CCAAT/enhancer binding protein family DNA binding in mouse epidermis prevents and regresses papillomas. Cancer Research, 2007, 67(4): 1867-1884 DOI:10.1158/0008-5472.CAN-06-2746 |
PUCHE J E, CASTILLA-CORTÁZAR. Human conditions of insulin-like growth factor-Ⅰ (IGF-Ⅰ) deficiency. Journal of Translational Medicine, 2012, 10(1): 224-239 DOI:10.1186/1479-5876-10-224 |
REINECKE M. Insulin-like growth factors and fish reproduction. Biology of Reproduction, 2010, 82(4): 656-661 DOI:10.1095/biolreprod.109.080093 |
SUN A, WANG T Z, WANG N, et al. Establishment and characterization of an ovarian cell line from half-smooth tongue sole Cynoglossus semilaevis. Journal of Fish Biology, 2015, 86(1): 46-59 DOI:10.1111/jfb.12535 |
VAITES L P, PAULO J A, HUTTLIN E L, et al. Systematic analysis of human cells lacking ATG8 proteins uncovers roles for GABARAPs and the CCZ1/MON1 regulator C18 or f8/RMC1 in macroautophagic and selective autophagic flux. Molecular and Cellular Biology, 2017, 38(1): 1-15 |
VONG Q P, CHAN K M, LEUNG K, et al. Common carp insulin-like growth factor-Ⅰ gene: Complete nucleotide sequence and functional characterization of the 5'-flanking region. Gene, 2003, 322: 145-156 DOI:10.1016/j.gene.2003.08.019 |
WUERTZ S, GESSNER J, KIRSCHBAUM F, et al. Expression of IGF-Ⅰ and IGF-Ⅰ receptor in male and female sterlet, Acipenser ruthenus-evidence for an important role in gonad maturation. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology, 2007, 147(1): 223-230 DOI:10.1016/j.cbpa.2006.12.031 |
XU W T, CUI Z K, WANG N, et al. Transcriptomic analysis revealed gene expression profiles during the sex differentiation of Chinese tongue sole (Cynoglossus semilaevis). Comparative Biochemistry and Physiology Part D Genomics and Proteomics, 2021, 40: 100919 DOI:10.1016/j.cbd.2021.100919 |
YAN B R, LI T, COYAUD E, et al. C5orf51 is a component of the MON1-CCZ1 complex and controls RAB7A localization and stability during mitophagy. Autophagy, 2021, 18(4): 109-121 |
YOULE R J, NARENDRA D P. Mechanisms of mitophagy. Nature Reviews Molecular Cell Biology, 2011, 12(1): 9-14 DOI:10.1038/nrm3028 |
ZHANG B, WANG X L, SHA Z X, et al. Establishment and characterization of a testicular cell line from the half-smooth tongue sole, Cynoglossus semilaevis. International Journal of Biological Sciences, 2011, 7(4): 452-459 DOI:10.7150/ijbs.7.452 |



