2. 海水养殖生物育种与可持续产出全国重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071
2. State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
水温作为水产养殖过程中最重要的生态因素之一,对养殖鱼类的生理生化状态影响巨大(He et al, 2014)。水温变化能直接影响养殖鱼类的生长发育、摄食和代谢等(Chen et al, 2020)。虽然鱼类能适应水温的小幅度波动,但在实际生产过程中,突发的水温变化会引起养殖鱼类产生应激反应,如生理功能障碍、停止摄食和行为异常,甚至会导致鱼类死亡(Liu et al, 2018)。夏季海水升温超过鱼类耐受范围时,会破坏鱼类的消化、免疫和呼吸系统,环境中的致病菌会进一步影响鱼体的正常生理机能(Yanar et al, 2019)。长期高温环境会对鱼体产生不可逆的损伤,破坏鱼体的免疫防御能力,增加鱼体患病的概率,引发鱼类疾病甚至死亡(Xu et al, 2018)。
高温刺激会引起鱼体氧化应激,导致多种毒性作用(Kim et al, 2019)。研究表明,鱼体的抗氧化防御体系包括超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GPx)等抗氧化酶,以清除体内过多的活性氧(Chen et al, 2020)。研究发现,虹鳟(Oncorhynchus mykiss) (夏斌鹏等, 2017)、牙鲆(Paralichthys olivaceus) (徐冬冬等, 2010)、美洲鲥(Alosa sapidissima) (杨明等, 2020)等各种鱼类的抗氧化酶活性变化与高温存在显著相关性。因此,抗氧化酶活性变化可作为鱼体在高温环境中氧化应激状态的重要指示。在抗热应激的过程中,热休克蛋白也会参与(孔祥辉等, 2022)。不同程度热应激下,大口黑鲈(Micropterus salmoides)“优鲈3号”幼鱼肝脏和鳃组织中的hsp70和hsc70的mRNA表达量随着胁迫温度的升高基本呈先上升后下降的趋势(陆健等, 2021)。近期,Wang等(2023)研究发现,斑马鱼(Danio rerio)敲除双特异性磷酸酶基因(dusp1)后,对温度变化极其敏感,主要归因于线粒体功能障碍和鳃中产生过量的活性氧,对DUSP1-MARK-DRP1轴研究表明,dusp1可能对维持线粒体功能完整性和氧化还原稳态起到重要作用,而维持细胞氧化还原稳态可能是应对细胞受到热应激的关键机制。因此,推测dusp1基因也在鱼类抵抗热应激的过程中发挥重要作用。
半滑舌鳎(Cynoglossus semilaevis)是一种暖温型底层鱼类,其肉质细腻、味道鲜美,极受消费者欢迎,市场价值很高。然而,在工厂化养殖过程中,夏季高温对半滑舌鳎的生存和生长产生了极大威胁,使机体发生应激反应,甚至导致死亡(Guo et al, 2016)。肝脏作为鱼类解毒代谢及免疫相关的组织,在响应高温胁迫过程中发挥重要作用(Liu et al, 2016)。随着半滑舌鳎全基因组测序的完成(Chen et al, 2014),分子标记辅助育种(Sharifi et al, 2015)、基因组选择(Lu et al, 2021)、基因编辑等技术(Cui et al, 2017)的应用以及高产抗病新品种“鳎优1号”培育的成功(卢昇等, 2022),使用先进分子育种技术培育耐高温良种已经成为下一阶段半滑舌鳎良种培育的新目标。然而,关于急性高温对半滑舌鳎影响的研究鲜有报道。本研究通过连续升温达到高温胁迫条件(35 ℃)后,分别在0、3、6、12及24 h采集肝脏组织进行苏木精–伊红染色法(HE)和TUNEL染色,观察肝脏组织病理变化及细胞凋亡情况,测定肝脏抗氧化酶活性变化,并检测热应激相关基因heat shock protein family A member 1A (hspa1a)、heat shock protein 90 beta family member 1 (hsp90b1)及dual-specificity phosphatase 1 (dusp1)的表达模式。通过研究半滑舌鳎在急性高温胁迫下的组织细胞、生理生化及分子水平变化规律,初步探究半滑舌鳎对高温胁迫的响应及适应机制,以期为半滑舌鳎耐高温良种培育和健康养殖提供参考依据。
1 材料与方法 1.1 实验用鱼实验用半滑舌鳎幼鱼[(22.6±2.2) cm,(78.7±10.6) g]购于山东省烟台市海阳黄海水产有限公司,个体健壮、无病、活力强。
1.2 实验方案实验用鱼暂养于大型圆形玻璃钢水槽(1 000 L)内,所用海水为砂滤水,水温23 ℃。在该条件下驯化、暂养7 d。
设置对照组和急性高温处理组,其中,对照组(C)在23 ℃下正常养殖,急性高温处理组在以2.5 ℃/h连续升温至水温达到35 ℃后进行急性高温胁迫实验,2个组分别设置3个平行,每个平行27尾鱼。
1.3 样品采集将水温升至实验温度(35 ℃)的时间记为0 h,分别在0、3、6、12和24 h 5个时间点取肝脏组织。对照组和急性高温组的每个平行组分别随机选2尾鱼,MS-222麻醉后,在超净工作台内使用无菌无酶的剪刀、镊子和手术刀采集肝脏组织样品。用于酶活和基因表达检测的样品装入冻存管后立即放进液氮中,然后转移至–80 ℃冰箱保存;用于制作切片的样品使用多聚甲醛保存。动物实验操作遵循中国水产科学研究院黄海水产研究所伦理委员会的要求进行。
1.4 肝脏组织石蜡切片制作及HE染色采集样品时,每条鱼切取一块形状较为完整、约3 mm×3 mm×3 mm肝脏组织,在多聚甲醛中固定24 h,然后转移到70%乙醇中,经过不同浓度乙醇脱水、透明、浸蜡、包埋、切片和展片等步骤制作肝脏石蜡切片。
将制作好的肝脏组织石蜡切片脱蜡至水,然后进行HE染色,最后脱水封片,使用显微镜镜检,采集图像。
1.5 TUNEL检测将制作好的肝脏组织石蜡切片脱蜡至水,通过蛋白酶K修复、破膜、室温平衡、加反应液、细胞核DAPI复染、封片等步骤进行TUNEL染色,最后镜检拍照,采集图像。
1.6 抗氧化酶测定称取适量肝脏组织,根据肝脏重量加入9倍体积的1×PBS缓冲液进行匀浆,4 ℃下3 000 r/min离心8 min,取上清液测定抗氧化酶活力。SOD、CAT、谷GPx酶活性及丙二醛(MDA)含量使用晶美生物的ELISA试剂盒检测。每个样品的总蛋白含量(TP)采用南京建成生物工程研究所的考马斯亮蓝试剂盒测定。吸光度值采用2800MF型多功能酶标仪测定。
1.7 RNA提取及cDNA合成使用TRIzol试剂提取肝脏样品总RNA,测定提取的RNA浓度和OD260/OD280值。配制1%琼脂糖凝胶进行凝胶电泳验证RNA质量,挑选质量和浓度均合格的RNA使用TaKaRa试剂盒进行反转录。
1.8 实时荧光定量PCR检测通过实时荧光定量PCR(qPCR)检测在高温胁迫不同时间点时hspa1a、hsp90b1和dusp1基因在半滑舌鳎肝脏组织中的表达水平。以β-actin作为内参基因,引物根据hspa1a、hsp90b1和dusp1基因核心片段序列设计。反应体系参照SYBR Premix Ex TaqTMⅡ(TaKaRa, 日本)说明书,总体系为20 μL:SYBR Premix Ex TaqTMⅡ(TaKaRa) 10 μL,正反向引物各1.2 μL,cDNA模板4.0 μL,RNase-Free水3.6 μL。使用ABI 7500 Fast Real-Time (Applied Biosystems, 美国)仪器进行定量分析。实验所用引物见表 1。反应程序:95 ℃ 30 s;95 ℃ 3 s,60 ℃ 33 s,40个循环;95 ℃ 15 s;60 ℃ 60 s;95 ℃ 15 s。采用2–ΔΔCt法计算和分析hspa1a、hsp90b1和dusp1的相对表达量。
|
|
表 1 本研究用到的引物信息 Tab.1 Information of primers used in this study |
实验数据使用SPSS 26.0软件中的单因素方差分析(one-way ANOVA),P<0.01为差异极显著水平,P<0.05为差异显著水平。使用Origin 2022软件作图。
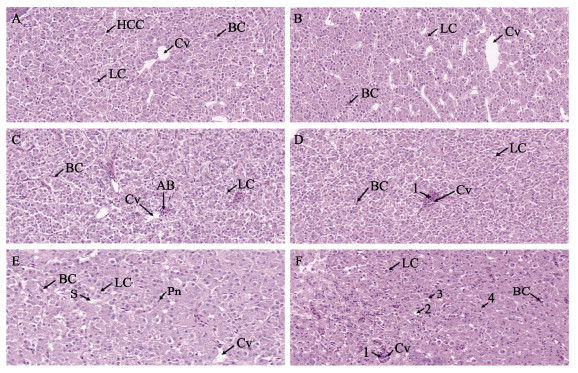
2 结果 2.1 急性高温胁迫对半滑舌鳎肝脏组织病理和细胞凋亡的影响急性高温胁迫后,半滑舌鳎肝脏组织病理变化如图 1所示。对照组肝脏组织中肝细胞形态近圆形,排列整齐,细胞边界清晰,细胞核位于中央,无变形、坏死、淤血、出血等现象(图 1A)。高温0 h后肝脏细胞基本结构并无明显的异常(图 1B);3 h时,出现红细胞沉积和轻微的细胞水肿(图 1C);6 h时,肝小叶整体结构比较完整,而中央静脉区域可见放射状的肝板结构轻微的混乱,肝板之间的血窦间隙增宽、增多,肝脏空泡相对减少,细胞核位于肝细胞的一侧(图 1D);高温胁迫12 h后,肝脏组织损伤较明显,肝细胞排列无序,较多肝细胞均出现空泡化,细胞间界限杂乱模糊,细胞核萎缩,严重的甚至出现溶解,以血窦扩张、空泡持续减少为特点(图 1E);高温胁迫24 h后,肝脏组织原有结构遭到破坏,可以观察到脂质空泡以及肝细胞坏死产生的空洞,肝细胞出现广泛变性坏死,出血严重(图 1F)。
|
图 1 急性高温胁迫对半滑舌鳎肝脏组织结构的影响 Fig.1 Effects of acute heat stress on liver tissue structure of C. semilaevis A:对照组;B~F:高温胁迫组0、3、6、12和24 h。比例尺=20 μm。HCC:肝细胞索;Cv:中央静脉;LC:肝细胞;BC:血细胞;AB:血细胞聚集;S:空泡;Pn:细胞核固缩;1:淤血;2:脂质空泡;3:坏死产生的空洞;4:肝细胞广泛性坏死。 A: Control group; B~F: High temperature stress group at 0, 3, 6, 12, and 24 h. Scale =20 μm. HCC: Hepatocyte cord; Cv: Central vein; LC: Hepatocyte; BC: Hemocyte; AB: Hemocyte aggregation; S: Vacuole; Pn: Nuclear pyresis; 1: Congestion; 2: Lipid vacuole; 3: Necrotic cavity; 4: Hepatocyte extensive necrosis. |
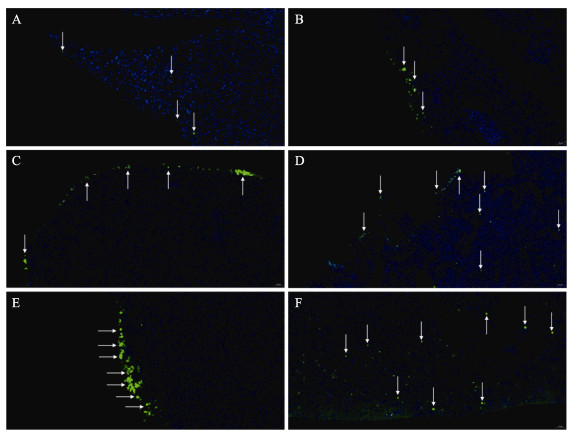
急性高温胁迫后半滑舌鳎肝脏细胞凋亡结果如图 2所示。对照组中肝脏细胞状态良好,偶有凋亡的细胞出现(图 2A)。在高温胁迫0 h即在肝脏边缘发现少量凋亡细胞(图 2B);随着高温胁迫时间的增加,肝脏边缘凋亡细胞数量越来越多(图 2C~E);急性高温胁迫24 h后,肝脏内部出现大量细胞凋亡(图 2F)。
|
图 2 TUNEL染色法检测急性高温胁迫处理后半滑舌鳎肝脏细胞凋亡 Fig.2 Apoptosis of liver cell of C. semilaevis after acute high temperature stress treatment detected by TUNEL staining A:对照组;B~F:高温胁迫组0、3、6、12和24 h。蓝色荧光代表正常细胞的细胞核,绿色荧光代表凋亡细胞的细胞核。比例尺=20 μm。 A: Control group; B~F: High temperature stress group at 0, 3, 6, 12, and 24 h. Blue fluorescence represents the nucleus of normal cells, and green fluorescence represents the nucleus of apoptotic cells. Scale =20 μm. |
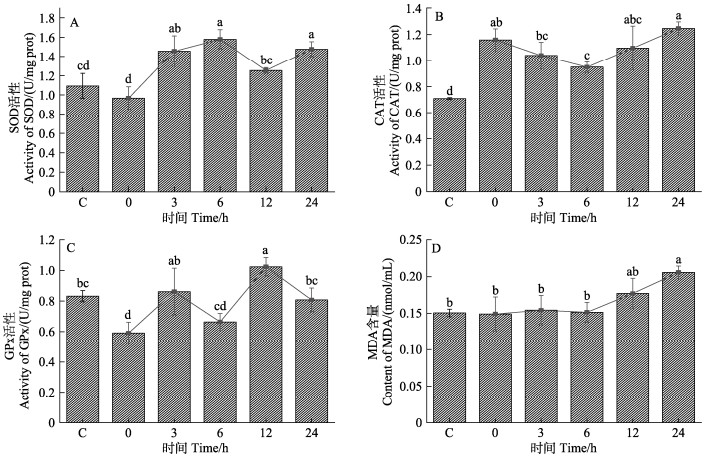
急性高温胁迫后半滑舌鳎肝脏中抗氧化酶SOD、CAT、GPx活性及MDA含量均出现不同程度的升高,具体结果如图 3所示。其中,SOD活性在高温胁迫后3、6和24 h显著高于对照组(P<0.05) (图 3A)。CAT活性在高温胁迫后0~24 h均显著高于对照组(P<0.05) (图 3B)。GPx活性在高温胁迫后0 h显著低于对照组,在12 h显著高于对照组(P<0.05) (图 3C)。MDA含量在急性高温胁迫后0~12 h与对照组无显著差异,24 h显著高于对照组(P<0.05) (图 3D)。
|
图 3 急性高温胁迫对半滑舌鳎抗氧化酶活性的影响 Fig.3 Effects of acute heat stress on antioxidant enzyme activity of C. semilaevis 各时间点不同小写字母表示差异显著(P<0.05)。下同。 Different lowercase letters at each time point indicate significant differences (P<0.05). The same below. |
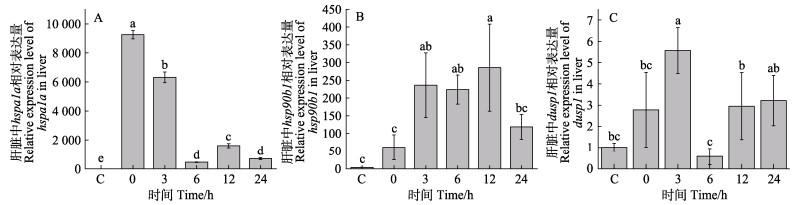
急性高温胁迫后半滑舌鳎肝脏hspa1a、hsp90b1和dusp1基因表达变化如图 4所示。hspa1a基因表达在高温胁迫后显著上调表达,0 h即达到最大值,为对照组的6 000倍,3 h表达量为对照组的4 000倍,6、12和24 h时表达趋势虽然下降,但仍显著高于对照组(图 4A)。hsp90b1表达在高温胁迫后显著上调表达,在12 h达到最大值,为对照组的75倍,3、6和24 h的表达量分别为对照组的60、70和40倍(图 4B)。dusp1基因的表达量呈先升高后降低再升高的趋势,3 h表达量显著高于对照组,其他时间点与对照组差异不显著(图 4C)。
|
图 4 急性高温胁迫下半滑舌鳎肝脏中hspa1a、hsp90b1和dusp1基因的表达量变化 Fig.4 The variation of relative expression level of hspa1a, hsp90b1 and dusp1 in the liver of C. semilaevis under acute high temperature stress |
肝脏是鱼类维持生命活动和物质代谢的重要组织,当肝脏受到损伤后会出现肝细胞空泡化、细胞破损、细胞边界模糊等现象(王晓光, 2015)。在37 ℃急性高温胁迫下,大口黑鲈“优鲈3号”的肝细胞出现空泡化、细胞核溶解、细胞界限杂乱模糊等现象(陆健等, 2021)。在27 ℃急性高温胁迫时,许氏平鲉(Sebastes schlegelii)的肝脏损伤较明显,肝细胞排列杂乱,较多肝细胞出现空泡化、细胞间界限杂乱模糊和细胞核溶解,而在5 ℃急性低温胁迫下,肝脏组织损伤不明显(张思敏等, 2018)。本研究也发现,随着高温胁迫时间延长,肝细胞出现明显损伤。细胞凋亡(apoptosis)是由基因控制、细胞自主的有序性死亡,又称为程序性细胞死亡(programmed cell death),是真核细胞的一种特殊的死亡形式(管强东等, 2022)。研究表明,热应激引起细胞线粒体产生过氧化物损伤机体,从而导致细胞凋亡(Luo et al, 2016)。细胞凋亡的相关研究发现许多对细胞凋亡起到调控作用的基因,如凋亡基因caspasse3 (Chu et al, 2023)、caspasse9 (刘恩光等, 2021)、p38MAPK基因(王艺臻等, 2022)等。本研究发现,高温胁迫后半滑舌鳎肝脏出现明显的细胞凋亡现象,期望后续进一步探讨细胞凋亡基因在半滑舌鳎响应高温胁迫中的调控作用。
高温胁迫会使鱼类产生氧化应激,导致氧化损伤。SOD、CAT和GPx是鱼体应对氧化应激的主要抗氧化酶,MDA作为脂质过氧化的最终产物,可以反映氧自由基对生物体的应激损伤程度(Zang et al, 2012)。其中,SOD对氧化剂和抗氧化剂的平衡起到重要作用,是最基本和最重要的清除剂(徐冬冬等, 2010)。作为一种经典的抗氧化酶,SOD将超氧化物转化为H2O2和O2,再经CAT催化H2O2分解为H2O和O2,从而清除氧自由基,减轻脂质过氧化损伤(王晓煜等, 2020)。GPx能催化还原性谷胱甘肽(GSH)变成氧化性谷胱甘肽(GSSG),促进H2O2分解,使有毒的H2O2还原成无毒的羟基化合物(Ning et al, 2016)。研究人员对虹鳟和硬头鳟(Salmon gairdneri)幼鱼进行急性高温胁迫,发现其肝脏、鳃、肌肉、肾脏、心脏等组织中SOD、CAT和GPx活性均升高,而肝脏组织中的抗氧化酶活性最高(姜旭阳等, 2021)。温度升高也对白梭吻鲈(Sander lucioperca)肝脏抗氧化酶影响显著,升温初期SOD、CAT和GPx活性均升高(王国成等, 2017)。在其他水产物种中也发现类似结果,例如,日本沼虾(Macrobrachium nipponense)在高温胁迫下,肝胰腺中的SOD和CAT活性显著高于对照组,表明高温诱导使日本沼虾的抗氧化力增强(翟书华等, 2022)。对皱纹盘鲍(Haliotis discus hannai)和虾夷扇贝(Mizuhopecten yessoensis)进行高温骤变处理,其MDA含量增加,说明温度急剧改变会激活机体抗氧化系统(姜娓娓, 2017)。同样,本研究中,SOD、CAT、GPx活性和MDA含量在急性高温胁迫24 h内均出现明显的升高。其中,SOD活性在急性高温胁迫0 h显著低于对照组,3 h时显著高于对照组(P<0.05);而GPx活性在高温胁迫0 h和6 h显著低于对照组,12 h后显著高于对照组(P<0.05)。出现这样的变化趋势,可能是在急性高温胁迫期间,活性氧的含量逐渐增加,同时鱼体内产生炎症,出现细胞凋亡现象,需要通过提高抗氧化酶的活性来保护机体不受到损伤。因此,通过以上研究推测,高温胁迫可能致使半滑舌鳎体内活性氧增多,鱼体通过激活肝脏抗氧化系统以清除过多活性氧产生的损害。
热休克蛋白(heat shock proteins, HSPs)是一类高度保守的细胞内蛋白家族,目前在多种生物体中均有发现,能提高机体应对温度胁迫、饥饿胁迫、低氧胁迫等恶劣环境的能力(张晨光等, 2023)。Hsp70和Hsp90是其中重要的2个家族成员,在鱼类中有较广泛的研究。研究表明,温度变化会显著影响鱼类热休克蛋白基因的表达(孙旋辉等, 2023)。对银鲳进行28 ℃、30 ℃和32 ℃的高温胁迫,肝组织hsp70基因的表达量随着胁迫时间增加,在24 h内呈先上升后下降的趋势,并且在不同温度胁迫下的表达量有显著差异(史琛榆等, 2022)。hspa1a即hsp70基因,是热休克蛋白家族中极其重要的基因之一,在高温下能协助蛋白复性及保护生物体免受损伤(Yebra-Pimentel et al, 2019)。本研究中,hspa1a基因在受到高温胁迫后立即呈现极显著上调表达,迅速响应高温胁迫,说明hspa1a基因是半滑舌鳎抵抗热应激的关键基因,在今后可作为耐高温基因标记在相关研究中应用。
研究表明,dusp1基因是通过介导细胞外信号调节蛋白激酶的去磷酸化作用来发挥功能(郭安宁等, 2018)。研究发现,在草鱼(Ctenopharyngodon idellus)受到低温胁迫的大脑组织转录组谱中,dusp1基因主要在冷胁迫下细胞的生长和凋亡调控中起重要作用(Shi et al, 2020)。对斑马鱼胚胎成纤维样细胞进行dusp1敲降,进行10 ℃低温处理,发现低温胁迫下细胞的凋亡比例显著高于对照组,活细胞数显著低于对照组,说明dusp1参与鱼类冷应激过程,在低温胁迫下能保护细胞(Niu et al, 2017)。本研究发现,在急性高温胁迫初期,dusp1基因在肝脏中呈现显著高表达,这可能是在暗示dusp1基因能响应高温胁迫;但在急性高温胁迫6 h时其表达量显著下调,可能是因为此时细胞凋亡的范围开始逐渐扩散,影响到dusp1基因的表达。综上结果表明,半滑舌鳎的dusp1基因在耐高温功能中可能发挥重要作用,这为探究暖温性鱼类高温适应性机制提供了新的证据。
4 结论本研究通过对半滑舌鳎进行35 ℃急性高温胁迫,选取24 h内5个时间点的肝脏组织,分析组织细胞、生理生化、基因表达等水平的应激性反应,结果表明,急性高温胁迫会造成半滑舌鳎肝脏组织病变和细胞凋亡,引起机体抗氧化防御系统发生反应,即SOD、CAT、GPx和MDA等抗氧化相关指标产生变化以减轻机体损伤,并激活hspa1a、hsp90b1和dusp1等热应激相关基因显著表达以尽快适应高温胁迫带来的不利影响。以上结果初步探明了半滑舌鳎在急性高温胁迫下的应激反应,对其高温适应性分子基础的遗传解析和耐高温良种培育具有指导意义。
CHEN S L, ZHANG G J, SHAO C W, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics, 2014, 46(3): 253-260 DOI:10.1038/ng.2890 |
CHEN Y N, LIU E G, LI C J, et al. Effects of heat stress on histopathology, antioxidant enzymes, and transcriptomic profiles in gills of pikeperch Sander lucioperca. Aquaculture, 2020, 534(5): 736277 |
CHU P, LUO S, WANG H Z, et al. Interactive effects of temperature and salinity on the apoptosis, antioxidant enzymes, and MAPK signaling pathway of juvenile pufferfish (Takifugu fasciatus). Aquaculture Reports, 2023, 29: 101483 DOI:10.1016/j.aqrep.2023.101483 |
CUI Z K, LIU Y, WANG W W, et al. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis). Scientific Reports, 2017, 7: 42213 DOI:10.1038/srep42213 |
GUAN Q D, YANG J, LIU Y Q, et al. Factors influencing detection of apoptosis by flow cytometry using Annexin V/PI staining. Biotechnology, 2022, 32(6): 710-714 [管强东, 杨健, 刘艳青, 等. Annexin V/PI法流式细胞术检测细胞凋亡的影响因素. 生物技术, 2022, 32(6): 710-714] |
GUO A N. The molecular mechanism of bmp10, dusp1 by cold stress and construction of gsdf transgenic line in zebrafish. Masterxs Thesis of Shanghai Ocean University, 2018 [郭安宁. 低温胁迫下斑马鱼bmp10、dusp1分子调控机制的研究及gsdf转基因品系的构建. 上海海洋大学硕士研究生学位论文, 2018]
|
GUO L, WANG Y M, LIANG S J, et al. Tissue-overlapping response of half-smooth tongue sole (Cynoglossus semilaevis) to thermostressing based on transcriptome profiles. Gene, 2016, 586(1): 97-104 DOI:10.1016/j.gene.2016.04.020 |
HE Y F, WU X B, ZHU Y J, et al. Effect of rearing temperature on growth and thermal tolerance of Schizothorax (Racoma) kozlovi larvae and juveniles. Journal of Thermal Biology, 2014, 46: 24-30 DOI:10.1016/j.jtherbio.2014.09.009 |
JIANG W W. Effects of temperature variation on physiological activities of scallops and abalone. Doctoral Dissertation of University of Chinese Academy of Sciences (Institution of Oceanology, Chinese Academy of Sciences), 2017 [姜娓娓. 扇贝和皱纹盘鲍对温度变化的生理响应研究. 中国科学院大学(中国科学院海洋研究所)博士研究生学位论文, 2017]
|
JIANG X Y, HUANG M, YANG X G, et al. Antioxidant enzyme activities of juvenile rainbow and steelhead trout (Oncorhynchus mykiss) in response to acute high-temperature stress. Journal of Fishery Sciences of China, 2021, 28(1): 57-65 [姜旭阳, 黄铭, 杨小刚, 等. 急性高温胁迫对虹鳟和硬头鳟幼鱼抗氧化酶活性的影响. 中国水产科学, 2021, 28(1): 57-65] |
KIM J H, KIM S K, HUR Y B. Temperature-mediated changes in stress responses, acetylcholinesterase, and immune responses of juvenile olive flounder Paralichthys olivaceus in a bio-floc environment. Aquaculture, 2019, 506: 453-458 DOI:10.1016/j.aquaculture.2019.03.045 |
KONG X H, WANG S S, DONG Y H, et al. Analysis of expression characteristics of related genes in response to acute thermal stress in the razor clam Sinonovacula constricta. Progress in Fishery Sciences, 2022, 43(2): 194-203 [孔祥辉, 王莎莎, 董迎辉, 等. 缢蛏急性高温胁迫应答主要候选基因的表达特征分析. 渔业科学进展, 2022, 43(2): 194-203] |
LIU B, XU P, BROWN P B, et al. The effect of hyperthermia on liver histology, oxidative stress and disease resistance of the Wuchang bream, Megalobrama amblycephala. Fish and Shellfish Immunology, 2016, 52: 317-324 DOI:10.1016/j.fsi.2016.03.018 |
LIU E G. Effects of acute heat stress on liver and gill tissue damage, apoptosis and immune response of pikeperch (Sander lucioperca). Masterxs Thesis of Suzhou University, 2021 [刘恩光. 急性热胁迫对白梭吻鲈肝脏和鳃组织损伤、细胞凋亡及免疫反应的影响. 苏州大学硕士研究生学位论文, 2021]
|
LIU Z M, ZHU X L, LU J, et al. Effect of high temperature stress on heat shock protein expression and antioxidant enzyme activity of two morphs of the mud crab Scylla paramamosain. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology, 2018, 223: 10 DOI:10.1016/j.cbpa.2018.04.016 |
LU J, ZHANG J J, ZHOU G Q, et al. Effect of acute high temperature stress on tissue damage and HSPs gene expression of largemouth bass Micropterus salmoides "Youlu No.3". Fisheries Science, 2021, 40(4): 508-515 [陆健, 张佳佳, 周国勤, 等. 急性高温胁迫对大口黑鲈"优鲈3号"组织损伤及HSPs基因表达的影响. 水产科学, 2021, 40(4): 508-515] |
LU S, LI Y Z, WANG L, et al. Application of genomic selection in the breeding of new variety of Chinese tongue sole (Cynoglossus semilaevis) "Tayou No.1". Journal of Fisheries of China, 2022, 46(8): 1305-1312 [卢昇, 李仰真, 王磊, 等. 基因组选择技术在半滑舌鳎"鳎优1号"新品种培育中的应用研究. 水产学报, 2022, 46(8): 1305-1312] |
LU S, ZHOU Q, CHEN Y D, et al. Development of a 38K single nucleotide polymorphism array and application in genomic selection for resistance against Vibrio harveyi in Chinese tongue sole, Cynoglossus semilaevis. Genomics, 2021, 113: 1838-1844 DOI:10.1016/j.ygeno.2021.03.034 |
LUO S W, WANG W N, SUN Z M, et al. Molecular cloning, characterization and expression analysis of (B-cell lymphoma-2 associated X protein) Bax in the orange-spotted grouper (Epinephelus coioides) after the Vibrio alginolyticus challenge. Developmental and Comparative Immunology, 2016, 60: 66-79 DOI:10.1016/j.dci.2016.02.017 |
NING R H, ZHANG M, CHEN Z Y, et al. Molecular cloning and expression of GPX gene from Siniperca chuatsi. Fish and Shellfish Immunology, 2016(53): 53 |
NIU H B, HU P, CHENG P L, et al. The role of dusp1 downregulation in apoptosis of zebrafish ZF4 cells under cold stress. Journal of Fishery Sciences of China, 2017, 24(5): 995-1002 DOI:10.3724/SP.J.1118.2017.16358 |
SHARIFI M, SOURINEJAD I, HOSSEINI S J, et al. Application of AFLP molecular marker for genetic analysis of black pomfret Parastromateus niger from the Persian Gulf. Iranian Journal of Fisheries Sciences, 2015, 14(4): 857-875 |
SHI C Y, ZHAO C P, HU Y X, et al. Effects of high temperature stress on physiology and expression of related gene in Pampus argenteus. Journal of Applied Oceanography, 2022, 41(1): 1-7 [史琛榆, 赵淳朴, 胡艺潇, 等. 银鲳应对高温胁迫的生理响应及其相关基因表达研究. 应用海洋学学报, 2022, 41(1): 1-7 DOI:10.3969/J.ISSN.2095-4972.2022.01.001] |
SHI M J, ZHANG Q X, LI Y M, et al. Global gene expression profile under low-temperature conditions in the brain of the grass carp (Ctenopharyngodon idellus). PLoS One, 2020, 15(9): e0239730 DOI:10.1371/journal.pone.0239730 |
SUN X H, BING X W, DING W D, et al. Effects of high-temperature stress on serum biochemical indexes, liver sod gene and heat shock protein gene expression of juvenile Siniperca chuatsi. Journal of Southern Agriculture, 2022, 53(12): 3539-3547 DOI:10.3969/j.issn.2095-1191.2022.12.025 |
孙旋辉, 邴旭文, 丁炜东, 等. 高温应激对鳜幼鱼血清生化指标及肝脏sod基因和热休克蛋白基因表达的影响. 南方农业学报, 2023, 53(12): 3539-3547 |
WANG G C. Effect of high temperature stress on physiology and biochemistry, HSC70 gene cloning and expression of pikeperch (Sander lucioperca). Masterxs Thesis of Suzhou University, 2017 [王国成. 高温胁迫对白梭吻鲈生理生化的影响及其HSC70基因的克隆与表达. 苏州大学硕士研究生学位论文, 2017]
|
WANG X G. Effect of deltamethrin on gills and livers in Danio rerio. Masterxs Thesis of Shandong Normal University, 2015 [王晓光. 溴氰菊酯对斑马鱼鳃、肝脏的影响. 山东师范大学硕士研究生学位论文, 2015]
|
WANG X Y, HU C L, CHENG P L, et al. Effects of cold acclimation on the apoptosis and ROS of zebrafish ZF4 cells. Genomics and Applied Biology, 2020, 39(10): 4475-4480 [王晓煜, 胡春兰, 程鹏丽, 等. 低温驯化对斑马鱼ZF4细胞凋亡和ROS的影响. 基因组学与应用生物学, 2020, 39(10): 4475-4480] |
WANG Y Z. Expression characteristics of p38MAPK and its role in regulating apoptosis under high temperature stress in Sinonovacula constricta. Masterxs Thesis of Shanghai Ocean University, 2022 [王艺臻. 高温胁迫下缢蛏p38MAPK基因的表达特征以及对细胞凋亡的调控作用. 上海海洋大学硕士研究生学位论文, 2022]
|
WANG Y, WANG H M, ZHOU Y, et al. Dusp1 regulates thermal tolerance limits in zebrafish by maintaining mitochondrial integrity. Zoological Research, 2023, 44(1): 126-141 DOI:10.24272/j.issn.2095-8137.2022.397 |
XIA B P, LIU Z, ZHOU Y J, et al. Effects of chronic heat stress on part of serum non-specific immunity parameters in rainbow trout (Oncorhynchus mykiss). Journal of Agricultural Biotechnology, 2017, 25(7): 1078-1085 [夏斌鹏, 刘哲, 周彦静, 等. 慢性热应激对虹鳟部分血清非特异性免疫指标的影响. 农业生物技术学报, 2017, 25(7): 1078-1085] |
XU D D, LOU B, ZHAN W, et al. Effect of high temperature stress on growth performance and activities of antioxidant enzymes in liver of olive flounder Paralichthys olivaceus. Journal of Fisheries of China, 2010, 34(7): 1099-1105 [徐冬冬, 楼宝, 詹炜, 等. 高温胁迫对褐牙鲆生长及肝脏抗氧化酶活性的影响. 水产学报, 2010, 34(7): 1099-1105] |
XU D X, ZHOU S, SUN L N. RNA-seq based transcriptional analysis reveals dynamic genes expression profiles and immune-associated regulation under heat stress in Apostichopus japonicus. Fish and Shellfish Immunology, 2018, 78: 169-176 DOI:10.1016/j.fsi.2018.04.037 |
YANAR M, ERDOĞAN, ERHAN, KUMLU M. Thermal tolerance of thirteen popular ornamental fish species. Aquaculture, 2019, 501: 382-386 DOI:10.1016/j.aquaculture.2018.11.041 |
YANG M, JIANG F, SHI Y H, et al. Effect of high temperature stress on activities of digestive enzymes in Alosa sapidissima. Journal of Northwest A&F University (Natural Science), 2020, 48(10): 1-8 [杨明, 蒋飞, 施永海, 等. 高温胁迫对美洲鲥消化酶活性的影响. 西北农林科技大学学报(自然科学版), 2020, 48(10): 1-8] |
YEBRA-PIMENTEL E S, GEBERT M, JANSEN H J, et al. Deep transcriptome analysis of the heat shock response in an Atlantic sturgeon (Acipenser oxyrinchus) cell line. Fish and Shellfish Immunology, 2019, 88: 508-517 DOI:10.1016/j.fsi.2019.03.014 |
ZANG Y Q, TIAN X L, DONG S L, et al. Growth, metabolism and immune responses to evisceration and the regeneration of viscera in sea cucumber, Apostichopus japonicus. Aquaculture, 2012, 358/359: 50-60 DOI:10.1016/j.aquaculture.2012.06.007 |
ZHAI S H, FU H T, QIAO H, et al. Effects of high temperature on heat shock proteins, antioxidant enzyme activity, and histology of oriental river prawn Macrobrachium nipponense. Journal of Fishery Sciences of China, 2022, 29(5): 684-695 [翟书华, 傅洪拓, 乔慧, 等. 高温胁迫对日本沼虾热休克蛋白基因的表达、抗氧化酶活力及组织结构的影响. 中国水产科学, 2022, 29(5): 684-695] |
ZHANG C G, DING W D, CAO Z M, et al. Effects of acute high temperature stress on antioxidant enzymes activity, digestive enzymes activity and gene expression of heat shock proteins in mandarin fish (Siniperca chuatsi). Journal of Southern Agriculture, 2021, 52(3): 815-826 [张晨光, 丁炜东, 曹哲明, 等. 急性高温胁迫对翘嘴鳜幼鱼抗氧化酶和消化酶活性及热休克蛋白基因表达的影响. 南方农业学报, 2021, 52(3): 815-826] |
ZHANG S M, LI J F, WEN H S, et al. Effect of acute temperature stress on liver metabolism of black rockfish Sebastes schlegelii and associating physiological mechanism. Periodical of Ocean University of China (Natural Science), 2018, 48(5): 32-38 [张思敏, 李吉方, 温海深, 等. 急性温度胁迫对许氏平鲉肝脏代谢机能和血液指标的影响及生理机制. 中国海洋大学学报(自然科学版), 2018, 48(5): 32-38] |



