2. 中国水产科学研究院黄海水产研究所 农业农村部海水养殖病害防治重点实验室 青岛市海水养殖流行病学与生物安保重点实验室 山东 青岛 266071;
3. 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 山东 青岛 266237;
4. 海南中正水产科技有限公司 海南 东方 572632
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs; Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao 266071, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
4. Hainan Zhongzheng Aquatic Technology Co., Ltd., Dongfang 572632, China
凡纳对虾(Penaeus vannamei)具有生长速度快、环境适应能力强的特点,是我国养殖产量最高的对虾品种,产量占所有养殖对虾总量的90%以上(农业农村部渔业渔政管理局等, 2020)。近年来,在对虾养殖业快速发展的同时,许多制约养殖产业发展的病害、种质及饲料问题也逐渐显现(邓伟等, 2013; Watanabe, 2010; 农业农村部渔业渔政管理局等, 2020)。在对虾饲料方面,因国际鱼粉价格连续多年居高,导致养殖成本急剧增加。受饲料蛋白原料来源及成本的限制,对虾人工饲料中通常添加大量的植物蛋白,对虾食用后易导致生长受限、消化免疫功能受损(杨奇慧, 2010; 孟阳, 2019)。为了从增强营养、提高免疫及减少病原感染等途径系统地提高水产养殖动物的健康水平,应用发酵饲料的技术逐渐受到了研究者的关注(付豪等, 2023; 杨耐德等, 2008; 袁春营等, 2018)。但关于枯草芽孢杆菌(Bacillus subtilis)、酿酒酵母(Saccharomyces cerevisiae)和嗜酸乳杆菌(Lactobacillus acidophilus)用于发酵饲料的应用效果差异研究尚未见报道,产业中缺少相关技术的支撑。
益生菌可以抑制病原微生物的增殖,饲料中添加益生菌能够提高养殖动物免疫能力,维持肠道微生物菌群平衡,促进养殖动物生长(杜文勇等, 2023; 张盛静等, 2016; Balcazar et al, 2006)。研究表明,饲料中含有巨大芽孢杆菌(B. megeterium)、植物乳杆菌(L. plantarum)和酿酒酵母能够显著提高对虾存活率及非特异性免疫力,增强对虾抗病力(孙博超等, 2019; Vieira et al, 2010; Afsharnasab et al, 2016)。在我国允许用于水产养殖的益生菌主要为芽孢杆菌、乳酸菌、酵母菌及光合细菌中的部分菌类。
对虾养殖用的益生菌种类较多、效果各异,本研究选取对虾养殖中常用的枯草芽孢杆菌、酿酒酵母、嗜酸乳杆菌3种益生菌进行单一及联合菌种的饲料发酵及应用,通过养殖实验比较不同发酵饲料投喂凡纳对虾的养殖效果,同时分析对虾体内外的弧菌含量,以期为益生菌发酵饲料在对虾养殖中的应用提供参考。
1 材料与方法 1.1 实验材料实验用凡纳对虾来源于海南省某育苗场,虾体长为(3.14±0.33) cm,平均体重为(0.26±0.02) g。实验用枯草芽孢杆菌(编号20130720002)分离自健康对虾体内,酿酒酵母(编号20211027001)和嗜酸乳杆菌(编号20211027002)分离自市场销售的水产养殖用益生菌产品,3株菌均为实验室保存。
1.2 饲料制备枯草芽孢杆菌及酿酒酵母分别接种于溶菌肉汤(lysogeny broth, LB)、酵母膏胨葡萄糖培养基(yeast peptone dextrose medium, YPD)中,并置于摇床中28 ℃、180 r/min震荡培养24 h;在MRS肉汤培养基(de Man, Rogosa and Sharp broth)中接种嗜酸乳杆菌,在无氧条件下37 ℃、180 r/min震荡培养24 h。平板涂布发酵液确定枯草芽孢杆菌、酿酒酵母、嗜酸乳杆菌的浓度分别为7.5×108、7.8×108和6.4×108 CFU/mL。将3种菌分别离心后弃上清液,PBS稀释菌体,使3种菌的浓度均为1×109 CFU/mL,同时,将浓度均为1×109 CFU/mL的3种菌液等体积混合作为复合菌液。取商用对虾饲料(广东深圳市澳华集团股份有限公司生产,主要成分:粗蛋白质≥42.0%、粗纤维≤5.0%、粗灰分≤15.0%、粗脂肪≥5.0%、总磷≥1.0%、赖氨酸≥2.5%、水分≤12.0%) 4份,分别添加制备的枯草芽孢杆菌、酿酒酵母、嗜酸乳杆菌及复合菌液,添加量为1% (W/W),为保证菌液分散均匀且保持一定的湿度,菌液用25倍(W/W)的水稀释后,边喷洒边搅拌,于室温密封发酵24 h后4 ℃保存,每3天制备1次,对照组饲料不进行任何处理。
1.3 实验分组及养殖管理实验分别设置枯草芽孢杆菌、酿酒酵母、嗜酸乳杆菌、复合益生菌发酵饲料组及空白对照组,每个处理组分别设置4个重复,其中1个组用于补齐其他3组采样后的对虾数量。各平行组的对虾养殖于体积为60 L的水族箱中,有效水体40 L,每箱内随机放养30尾健康虾。各处理组每日投喂3次,日投喂量为对虾体重的3%,并记录每日饲料投喂量。日换水30%,养殖期间水温为(25.6±1.5) ℃,盐度为31.6±0.7,连续充气,养殖实验共进行28 d。
1.4 发酵饲料中目标菌的总菌数取1 g各组益生菌发酵饲料,加入10倍体积PBS缓冲液,充分研磨后,以10倍浓度梯度稀释至合适倍数,分别涂布于酵母培养基YPD及嗜酸乳杆菌MRS平板中,取部分稀释液于80 ℃水浴15 min后涂布于LB平板中(用于枯草芽孢杆菌的计数)(Roy et al, 2013),每组3个平行。YPD、LB平板于28 ℃培养箱中培养24 h后计数,MRS平板于37 ℃培养24 h后计数。
1.5 水质指标的测定养殖第7、14、21、28天时,用无菌瓶采集每组中层水样100 mL,每组取3个平行,低温带回实验室,测定养殖水体中氨氮、亚硝氮浓度,测定方法参考海洋监测规范第4部分:海水分析中的靛酚蓝分光光度法和萘乙二胺分光光度法。
1.6 生长指标的测定养殖结束时记录各处理组的对虾存活数、体长、体重,并记录实验过程中的投喂量,计算存活率、特定生长率及饵料系数。
存活率=存活对虾尾数/初始对虾尾数×100%;
存活提高率=(各实验组存活率–对照组存活率)/对照组存活率×100%;
体长增长率=(对虾末体长–对虾初体长)/对虾初体长×100%;
特定生长率=[ln(对虾末体重)–ln(对虾初体重)]/实验周期×100%;
饵料系数=饲料投喂量/(对虾末体重–对虾初体重)。
1.7 对虾肝胰腺中可培养细菌及弧菌定量在养殖第7、14、21天,随机取各处理组的对虾3尾(取出后,从备用组中取规格一致的对虾补齐实验组),无菌操作分离对虾肝胰腺并精确称重,加入10倍质量的PBS缓冲液研磨,以10倍梯度稀释至合适倍数,各梯度取0.1 mL涂布于2216E海水细菌培养基(zobell marine broth 2216)和硫代硫酸盐柠檬酸盐胆盐蔗糖琼脂培养基(thiosulfate citrate bile salts sucrose agar culture medium, TCBS)平板中,每组3个平行,28 ℃培养24 h,观察记录可培养细菌数及弧菌数。
1.8 养殖水体中可培养细菌及弧菌定量在养殖第7、14、21天,取各处理组的水样,以10倍梯度稀释至合适倍数,涂布于2216E和TCBS平板中,每组3个平行,28 ℃培养24 h,观察记录可培养细菌数及弧菌数。
1.9 免疫相关酶活测定实验至第28天时,在各处理组的每个平行组中随机挑选5尾对虾采集血清,测定超氧化物歧化酶(SOD)、过氧化物酶(POD)、酚氧化酶(PO)、溶菌酶(LZM)活性以及总蛋白(TP)浓度,相关试剂盒购自南京建成生物工程研究所,测定方法参考说明书。
1.10 数据处理及统计分析实验数据用Excel和SPSS软件进行统计分析,数据取平均值±标准差(Mean±SD),差异显著性检验使用SPSS分析软件对数据进行单因素方差分析(one-way ANOVA),当P < 0.05时为差异显著。
2 结果 2.1 饲料发酵完成后目标菌的数量各实验组饲料发酵完成后,目标菌的数量如表 1所示,枯草芽孢杆菌、嗜酸乳杆菌及复合益生菌发酵饲料中的各目标菌均达到107 CFU/g数量级,酿酒酵母发酵饲料中酿酒酵母的含量为106 CFU/g数量级。表明,枯草芽孢杆菌及嗜酸乳杆菌的活菌数量经24 h发酵,提高3倍左右,而酿酒酵母组活菌数量较添加数量降低50%左右。复合益生菌发酵组中枯草芽孢杆菌及嗜酸乳杆菌的活菌增殖数量与单菌发酵相似,提高2倍左右,而酿酒酵母增殖数量最高,达到添加剂量的6倍左右。
|
|
表 1 饲料中目标菌的数量(平均值±标准差, n=3) Tab.1 Total number of target bacteria in feed (Mean±SD, n=3) |
投喂不同益生菌发酵后,各组对虾存活率如表 2所示,实验组中对虾存活率最高的组为复合益生菌组,存活率为100%;枯草芽孢杆菌与嗜酸乳杆菌组的存活率相同,均为(98.89±0.02)%;酿酒酵母组较低,仅为(95.56±0.05)%;与对照组存活率(91.11±0.02)%相比,实验组中除了酿酒酵母组与其他各组无差异外(P > 0.05),其他各组的存活率均显著高于对照组(P < 0.05),其中,复合益生菌、枯草芽孢杆菌、嗜酸乳杆菌及酿酒酵母组的存活提高率分别达到9.76%、8.54%、8.54%和4.88%。
|
|
表 2 各组对虾存活率(平均值±标准差, n=3) Tab.2 Survival rates of shrimp in different groups (Mean±SD, n=3) |
养殖实验结束后,测量对虾体长、体重,计算饵料系数,结果见表 3,对虾体长增长率最高的2组为嗜酸乳杆菌和枯草芽孢杆菌组,2组差异不显著(P > 0.05),但均显著高于对照组(P < 0.05),其他各组间差异不显著(P > 0.05);各实验组对虾特定生长率高于对照组,但差异不显著(P > 0.05),嗜酸乳杆菌组饵料系数显著低于对照组(P < 0.05),其他组间差异不显著(P > 0.05)。研究表明,在提高对虾饲料利用率及促生长方面,嗜酸乳杆菌组最佳。
|
|
表 3 各组对虾体长体重及饵料系数(平均值±标准差, n=3) Tab.3 Body length, body weight and feed conversion rate of shrimp (Mean±SD, n=3) |
在投喂发酵饲料的第7、14、21天,检测各组对虾肝胰腺组织中可培养细菌总数及弧菌数量,结果如表 4所示,在养殖第7天,枯草芽孢杆菌发酵饲料组中,对虾肝胰腺细菌总数显著高于其他各组(P < 0.05),而弧菌数量最低组为枯草芽孢杆菌组与酿酒酵母组,这2组差异不显著,但均显著低于嗜酸乳杆菌组、复合益生菌组及空白组(P < 0.05),对照组中弧菌数量分别是枯草芽孢杆菌和酿酒酵母发酵饲料组的20.0和27.3倍,枯草芽孢杆菌组弧菌占比最低仅为0.01%;养殖至第14天,各实验组对虾肝胰腺细菌总数及弧菌数量均显著低于对照组(P < 0.05),复合益生菌组弧菌数量最低为(2.3±1.5)×103 CFU/g;养殖至第21天,复合益生菌组对虾肝胰腺细菌总数显著高于对照组(P < 0.05),但该组中肝胰腺中的弧菌占比最低,仅为0.06%,其余各组对虾肝胰腺细菌总数显著低于对照组(P < 0.05),各实验组弧菌数量均显著低于对照组(P < 0.05)。
|
|
表 4 对虾肝胰腺可培养细菌及弧菌总数(平均值±标准差, n=3) Tab.4 Total bacterium counts (TBC) and Vibrio bacterium counts (VBC) in shrimp hepatopancreas (Mean±SD, n=3) |
检测养殖期间各组养殖水体中的细菌及弧菌数量如表 5所示,在养殖第7天,枯草芽孢杆菌组水体细菌总数与对照组无显著差异,其余各组水体细菌总数显著低于对照组(P < 0.05),枯草芽孢杆菌组和嗜酸乳杆菌组水体中弧菌数量与对照组差异不显著,但酿酒酵母及复合益生菌组水体中的弧菌数显著高于对照组(P < 0.05),弧菌占比最低组为枯草芽孢杆菌组;养殖第14天,各组水体细菌总数显著低于对照组(P < 0.05),除了复合益生菌组水体中的弧菌数显著高于其他各组外,其他各组间差异不显著(P > 0.05),弧菌占比最低组为枯草芽孢杆菌及空白对照组;养殖至第21天,各实验组水体细菌总数显著高于对照组(P < 0.05),枯草芽孢杆菌和嗜酸乳杆菌组水体弧菌数量显著低于对照组(P < 0.05),而弧菌占比最高组为空白对照组。
|
|
表 5 养殖水体中细菌总数及弧菌数量(平均值±标准差, n=3) Tab.5 Total bacterium counts (TBC) and Vibrio bacterium counts (VBC) in the shrimp aquaculture water (Mean±SD, n=3) |
投喂发酵饲料后,各实验组对虾血清中免疫相关酶活性的检测结果如图 1所示,血清LZM活性在各实验组中,仅有复合菌组显著高于对照组(P < 0.05),其他各组间差异不显著(P > 0.05) (图 1A);POD活性最高组为嗜酸乳杆菌组,显著高于其他各组(P < 0.05),次高组为酿酒酵母组,显著高于对照组、枯草芽孢杆菌组和复合益生菌组(P < 0.05),复合益生菌组与枯草芽孢杆菌组差异不显著(P > 0.05),但高于空白对照组(P < 0.05) (图 1B)。SOD活性最高组为嗜酸乳杆菌组,显著高于其他各组(P < 0.05),最低组为复合益生菌组,显著低于其他各组(P < 0.05),枯草芽孢杆菌、酿酒酵母及空白组差别不显著(P > 0.05) (图 1C);PO活性最高组为嗜酸乳杆菌,次高组为复合益生菌组,2组差异显著,但均显著高于酿酒酵母组(P < 0.05),枯草芽孢杆菌组与对照组差异不显著(P > 0.05),但均显著低于其他各组(P < 0.05) (图 1D);血清总蛋白含量各实验组均高于对照组,但嗜酸乳杆菌组显著高于其他实验组(P < 0.05) (图 1E)。可以看出,在所有的实验组中,嗜酸乳杆菌增强对虾非特异性免疫力的效果最为突出。
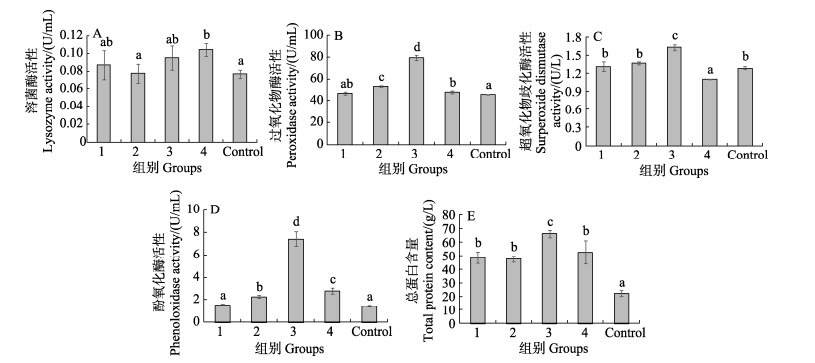
|
图 1 各组对虾血清中溶菌酶(A)、过氧化物酶(B)、超氧化物歧化酶(C)、酚氧化酶(D)活性及总蛋白含量(E) (平均值±标准差, n=3)
Fig.1 Activity of LZM(A), POD(B), SOD(C), PO(D) and content of TP(E) in serum of shrimp in each group (Mean±SD, n=3)
1:枯草芽孢杆菌;2:酿酒酵母;3:嗜酸乳杆菌;4:复合益生菌。下同。 不同字母表示组间存在显著差异(P < 0.05)。 1: B. subtilis; 2: S. cerevisiae; 3: L. acidophilus; 4: Complex-probiotics. The same below. Groups with different letters are significantly different (P < 0.05). |
养殖期间各组养殖水体中NH4+-N浓度如图 2A所示,在养殖的第7和14天,各实验组养殖水体中的NH4+-N浓度均高于对照组(P < 0.05),第7和14天的最高浓度组分别为复合益生菌组和酿酒酵母组;养殖第21和28天,各实验组养殖水体NH4+-N浓度均显著低于对照组(P < 0.05),最低组为酿酒酵母组;养殖期间各组养殖水体中NO2–-N浓度如图 2B所示,养殖至第7和14天时,各组养殖水体NO2–-N浓度均较低,在第7天时,各实验组均低于对照组,而到第14天时,最高组为酿酒酵母组,显著高于对照组、枯草芽孢杆菌组和嗜酸乳杆菌组(P < 0.05);养殖至第21和28天时,各组的NO2–-N浓度普遍上升,2个时间点的最低浓度组均为嗜酸乳杆菌组,显著低于其他各组(P < 0.05),最高浓度组分别为酿酒酵母组和空白对照组,均显著高于其他各组(P < 0.05)。至28 d时,各实验组NO2–-N浓度均低于对照组(P < 0.05)。
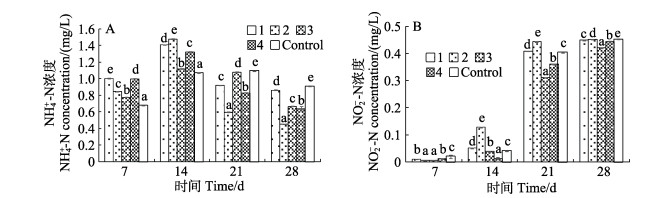
|
图 2 养殖水体中NH4+-N(A)和NO2–-N(B)的浓度(平均值±标准差, n=3) Fig.2 The concentration of NH4+-N (A) and NO2–-N (B) in the water in each group (Mean±SD, n=3) 同一时间段内,字母不同表示差异显著(P < 0.05)。 Groups with different letters in the same day are significantly different (P < 0.05). |
益生菌种类较多,水产养殖中应用广泛的有光合细菌、乳酸菌及芽孢杆菌类等,使用方式多为水体泼洒及口服。近年来,由于对虾易暴发消化道疾病,如急性肝胰腺坏死病和虾肝肠胞虫病等,导致对虾消化系统的组织结构受损。为改善对虾消化系统功能、减少饲料中植物性蛋白抗营养因子对机体产生危害,采用发酵饲料进行对虾养殖的技术逐渐被认可。研究表明,用乳酸菌发酵饲料能够将饲料蛋白分解为小分子的活性肽和氨基酸,提高饲料的营养水平和利用率(Missotten et al, 2016)。将对虾饲料用植物乳杆菌、酿酒酵母和沙福芽孢杆菌(B. safensis) 3个菌株按照1∶3∶3混合发酵,能够显著提高饲料中天冬氨酸、苏氨酸、丝氨酸、甘氨酸和丙氨酸等12种氨基酸的含量,投喂对虾后表现出显著的促生长效果(Zhang et al, 2021)。用酿酒酵母发酵豆粕替代50%鱼粉制备虾饲料投喂印度对虾(Penaeus indicus)可获得与非替代组相似的对虾产量及生长速度(Sharawy et al, 2016)。本研究发现,投喂枯草芽孢杆菌、嗜酸乳杆菌、复合益生菌发酵饲料的3个组对虾的存活率显著提高,而乳酸杆菌发酵饲料组的饵料系数最低,表明不同益生菌发酵饲料的应用效果不同。综合分析各组饲料的营养效果,嗜酸乳杆菌促生长及降低饵料系数的效果最佳。
3.2 益生菌发酵饲料对对虾肝胰腺弧菌抑制效果对虾的肝胰腺与消化道相通,环境中病原菌易通过摄食进入肝胰腺的管腔中,控制肝胰腺中病原菌的数量能降低对虾的发病率。本研究发现,酿酒酵母发酵饲料在应用前期即可显著降低对虾肝胰腺中弧菌的数量,效果与枯草芽孢杆菌相近,均优于嗜酸乳杆菌,而在养殖后期,枯草芽孢杆菌组对降低肝胰腺弧菌载量的效果减弱,嗜酸乳杆菌组降低肝胰腺弧菌载量的效果增强。枯草芽孢杆菌可分泌细菌素、酶类、抗菌肽和脂肽类等化合物(卢昱茜等, 2022; Chakraborty et al, 2018),对病原菌具有抑制效果。同时,摄食枯草芽孢杆菌发酵的饲料能提高对虾脂多糖和β-1, 3-葡聚糖结合蛋白及血蓝蛋白等的表达水平,有助于提高对虾对弧菌的抵抗力(Vogeley et al, 2019)。酵母菌发酵饲料的应用在家畜及鱼类养殖中有较多报道,饲料经过发酵后,能够提高其利用率,促进养殖动物的生长(Maamouri et al, 2022; 崔正贺等, 2022)。水产中,应用酿酒酵母联合植物乳杆菌和沙福芽孢杆菌发酵对虾饲料,能够改善对虾肠道微生态环境,进而改善对虾健康状况(Zhang et al, 2021),但应用于养殖对虾抗弧菌效果的研究报道较少,对虾摄食后其降低对虾肝胰腺弧菌载量的作用机制尚不清楚。在发酵底物的过程中,乳酸菌能够产生抗菌肽、大量乳酸和其他多种酸性化合物,在杀菌、调节肠道菌群方面具有显著效果(Karthik et al, 2014; 刘迪等, 2016)。
3.3 益生菌发酵饲料对对虾血清免疫相关酶活的影响血清中LZM、过氧化氢酶(CAT)、SOD和PO等非特异性免疫相关酶活性是衡量对虾免疫水平的重要指标(Mai et al, 2010; Wang et al, 2018)。Lee等(2021)研究发现,在饲料中添加枯草芽孢杆菌和短小芽孢杆菌(B. pumilus)可提高对虾PO和SOD活性。李军亮等(2018)研究表明,饲料中适量添加嗜酸乳杆菌,能够提高凡纳对虾CAT、LZM和SOD活性,增强其非特异免疫力。在饲料中添加1%的酵母水解物或酿酒酵母,能够提高凡纳对虾的免疫力(Jin et al, 2018)。酵母菌细胞壁含有对虾非特异免疫增强剂葡聚糖,饲料中添加0.1% (W/W)分子量为15 kDa的低聚β-葡聚糖,能够显著提高斑节对虾(P. monodon)血细胞的吞噬活性,增强POD和PO活性(Luan et al, 2021)。本研究发现,将枯草芽孢杆菌及嗜酸乳杆菌添加到对虾饲料中进行单一菌株发酵后,菌体的浓度得到明显的提高,而经过复合菌发酵后,3种菌的浓度均得到提高。且不同益生菌发酵饲料后,对提高对虾免疫相关酶活性的效果不同。研究表明,枯草芽孢杆菌、酿酒酵母和嗜酸乳杆菌促进免疫力的途径不同。枯草芽孢杆菌可促进对虾饲料中谷氨酰胺的消化率,而谷氨酰胺可作为免疫细胞的燃料或通过己糖胺生物合成途径(HBP)调节免疫分子表达和O-糖基化,从而改善对虾的健康状况(Chine et al, 2020)。酿酒酵母细胞壁中的β-葡聚糖能够通过下调细胞因子的表达来减轻炎症反应,也可与白细胞上的几种受体结合并激活先天免疫机制,从而增强免疫反应(侯冬强等, 2022)。乳杆菌是良好的免疫激活剂和非特异性免疫调节因子,能够增加抗病相关基因的表达量,从而提高机体非特异性免疫力(张家国等, 2014)。
3.4 发酵饲料对养殖水体的脱氮作用残饵和粪便会导致养殖水体中氨氮和亚硝氮等有害物质的累积,影响动物的生长、代谢及免疫等功能,降低养殖动物的存活率和生长(Zhao et al, 2020; 肖威等, 2020)。益生菌能够调节水质,降低养殖水体中的氨氮等有害物质的浓度(Zhou et al, 2009)。Mirbakhsh等(2021)将不同浓度的枯草芽孢杆菌喷洒在凡纳对虾基础饲料中,发现对养殖后期水体中的氨氮、亚硝氮具有去除效果,枯草芽孢杆菌的脱氮机制源于其含有moaA、moaD、moaE、mobB和moeA等多个脱氮基因参与氮的去除(Yang et al, 2021),同时,该菌可分泌多种降解有机物的酶类,促进养殖系统中有机质的分解,利于降低水体系统中的快速氨氮移除(Wang et al, 2022)。目前,尚未见对乳酸及酿酒酵母脱氮机制的报道,但有研究表明,将乳酸菌及酿酒酵母随着饲料释放到养殖水体,也可对环境中的氨氮和亚硝氮具有一定的降解作用(李咏梅等, 2021; 崔正贺等, 2022)。本研究也发现,在养殖后期投喂各种益生菌发酵饲料均可使水体氨氮(21 d后)和亚硝氮浓度(28 d)降低,推测可能与饲料经过益生菌发酵后,其中的大分子植物蛋白会随着发酵被降解为易于吸收的小分子物质,提高了饲料蛋白的吸收利用率,间接减少了对虾粪便中含氮物质的排放,进而达到降低养殖水体氨氮和亚硝氮的效果(Madani et al, 2018)。另外,菌体自身也可能存在一定的脱氮机能,在养殖持续一段过程后,益生菌定植在养殖系统中发挥脱氮作用。
4 结论在各实验组中,嗜酸乳杆菌、枯草芽孢杆菌和复合菌发酵饲料均能显著提高养殖对虾存活率,而嗜酸乳杆菌和枯草芽孢杆菌对提高对虾体长增长率也具有显著效果,且嗜酸乳杆菌组的饵料系数最低。实验中后期(14 d和21 d),各实验组发酵饲料对降低对虾肝胰腺弧菌数量均有显著效果,但在提高对虾血清POD、SOD和PO活性及总蛋白含量方面,嗜酸乳杆菌组效果最佳。综合分析,采用嗜酸乳杆菌发酵饲料投喂对虾效果最佳。
AFSHARNASAB M, KAKOOLAKI S, MOHAMMADIDOST M. Immunity enhancement with administration of Gracilaria corticata and Saccharomyces cerevisiae compared to gamma irradiation in expose to WSSV in shrimp, in juvenile Litopenaeus vannamei: A comparative study. Fish and Shellfish Immunology, 2016, 56: 21-33 DOI:10.1016/j.fsi.2016.06.052 |
BALCAZAR J L, BLAS I, RUIZ-ZARZUELA I, et al. The role of probiotics in aquaculture. Veterinary Microbiology, 2006, 114(3/4): 173-186 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2020. 2020, 22 [农业农村部渔业渔政管理局. 中国渔业统计年鉴. 北京: 中国农业出版社, 2020, 22]
|
CHAKRABORTY K, THILAKAN B, KIZHAKKEKALAM V K. Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. Journal of Applied Microbiology, 2018, 124(1): 108-125 DOI:10.1111/jam.13627 |
CHINE C C, LIN T Y, CHI C C, et al. Probiotic, Bacillus subtilis E20 alters the immunity of white shrimp, Litopenaeus vannamei via glutamine metabolism and hexosamine biosynthetic pathway. Fish and Shellfish Immunology, 2020, 98: 176-185 DOI:10.1016/j.fsi.2020.01.014 |
CUI Z H, YU C, LI Y M, et al. Effect of fermented brewer's yeast (Saccharomyces cerevisiae) supplementation on growth, feed utilization, and water quality in large-mouth bass (Micropterus salmoides) farming. Journal of Fishery Sciences of China, 2022, 29(2): 274-283 [崔正贺, 余聪, 李云梦, 等. 饲料中发酵啤酒酵母添加水平对大口黑鲈生长、饲料利用效率和水质的影响. 中国水产科学, 2022, 29(2): 274-283] |
DENG W, HUANG T S, ZHANG Z D. Development status and countermeasure of shrimp seed industry in white shrimp. China Fisheries, 2013, 12: 22-25 [邓伟, 黄太寿, 张振东. 我国南美白对虾种业发展现状及对策建议. 中国水产, 2013, 12: 22-25] |
DU W Y, WANG T T, HAN H Z, et al. Isolation, identification, and characterization of two potential probiotics from marine fish. Progress in Fishery Sciences, 2023, 44(3): 188-199 [杜文勇, 王腾腾, 韩慧宗, 等. 2株海水鱼源潜在益生菌的分离、鉴定及特性分析. 渔业科学进展, 2023, 44(3): 188-199] |
FU H, SONG H M, MU X D, et al. Effects of fish meal replacement with fermented Antarctic krill meal on growth performance, body color and serum biochemical indexes of koi carp (Cyprinus carpio L.). Progress in Fishery Sciences, 2023, 44(5): 80-89 [付豪, 宋红梅, 牟希东, 等. 发酵南极磷虾粉替代鱼粉对锦鲤生长、体色及血清生化指标的影响. 渔业科学进展, 2023, 44(5): 80-89] |
HOU D Q, ZHAO H X, PENG K, et al. Biological function of β-glucan and its application in aquaculture. China Animal Husbandry and Veterinary Medicine, 2022, 49(12): 4625-4634 [侯冬强, 赵红霞, 彭凯, 等. β-葡聚糖的生物学功能及在水产养殖中的应用. 中国畜牧兽医, 2022, 49(12): 4625-4634] |
JIN M, XIONG J, ZHOU Q C, et al. Dietary yeast hydrolysate and brewer's yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish and Shellfish Immunology, 2018, 82: 121-129 DOI:10.1016/j.fsi.2018.08.020 |
KARTHIK R, JAFFAR HUSSAIN A, MUTHEZHILAN R. Effectiveness of Lactobacillus sp (AMET1506) as probiotic against Vibriosis in Penaeus monodon and Litopenaeus vannamei shrimp aquaculture. Biosciences Biotechnology Research Asia, 2014, 11: 297-305 |
LEE C, KIM S, SHIN J, et al. Dietary supplementations of Bacillus probiotic improve digestibility, growth performance, innate immunity, and water ammonia level for Pacific white shrimp, Litopenaeus vannamei. Aquaculture International, 2021, 29(6): 2463-2475 DOI:10.1007/s10499-021-00760-z |
LI J L, YANG Q H, TAN B P, et al. Effects of Lactobacillus acidophilus supplementation on the growth, enzyme activities, and mRNA expression of disease-resistance related enzymes of juvenile Litopenaeus vannamei. Journal of Fishery Sciences of China, 2018, 25(5): 1022-1031 [李军亮, 杨奇慧, 谭北平, 等. 添加嗜酸乳杆菌对凡纳滨对虾生长、酶活性及相关酶mRNA表达的影响. 中国水产科学, 2018, 25(5): 1022-1031] |
LI Y M, LI T F, TIAN X L, et al. Effects of probiotics and polyhydroxybutyrate on growth and non-specific immunity of Litopenaeus vannamei and its culturing water quality. Periodical of Ocean University of China, 2021, 51(11): 22-31 [李咏梅, 李腾飞, 田相利, 等. 添加益生菌和PHBV对凡纳滨对虾养殖系统水质和对虾生长、非特异免疫指标的影响. 中国海洋大学学报(自然科学版), 2021, 51(11): 22-31] |
LIU D, DONG X H, TAN B P, et al. Effects of Lactobacillus acidophilus on growth performance, immunity and disease resistance in pacific white leg shrimp Litopenaeus vannamei. Fisheries Science, 2016, 35(1): 37-42 [刘迪, 董晓慧, 谭北平, 等. 嗜酸乳杆菌对凡纳滨对虾生长、免疫力和抗病力的影响. 水产科学, 2016, 35(1): 37-42] |
LU Y Q, WAN J W, WANG B, et al. Research progress on antibacterial active substances of Bacillus subtilis and its effect on disease resistance of aquatic animals. Feed Research, 2022, 45(22): 125-128 [卢昱茜, 万继武, 王博, 等. 枯草芽孢杆菌抗菌活性物质及其对水产动物抗病力影响的研究进展. 饲料研究, 2022, 45(22): 125-128] |
LUAN L Q, VU N T, NGHIA N T, et al. Synergic degradation of yeast β-glucan with a potential of immunostimulant and growth promotor for tiger shrimp. Aquaculture Reports, 2021, 21: 100858 |
MAAMOURI O, SALEM M B. The effect of live yeast Saccharomyces cerevisiae as probiotic supply on growth performance, feed intake, ruminal pH and fermentation in fattening calves. Veterinary Medicine and Science, 2022, 8: 398-404 |
MADANI N S H, ADORIAN T J, FARSANI H G, et al. The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquaculture Research, 2018, 49(5): 1926-1933 |
MAI W J, WANG W N. Protection of blue shrimp (Litopenaeus stylirostris) against the white spot syndrome virus (WSSV) when injected with shrimp lysozyme. Fish and Shellfish Immunology, 2010, 28(4): 727-733 |
MENG Y. Effect and mechanism of fermented feed on Penaeus vannamei. Master′s Thesis of Tianjin University of Science and Technology, 2019 [孟阳. 发酵饲料对凡纳滨对虾的作用效果与机理研究. 天津科技大学硕士研究生学位论文, 2019]
|
MIRBAKHSH M, MAHJOUB M, AFSHARNASAB M, et al. Effects of Bacillus subtilis on the water quality, stress tolerance, digestive enzymes, growth performance, immune gene expression, and disease resistance of white shrimp (Litopenaeus vannamei) during the early hatchery period. Aquaculture International, 2021, 29(6): 2489-2506 |
MISSOTTEN J A, MICHIELS J, DEGROOTE J, et al. Fermented liquid feed for pigs: An ancient technique for the future. Journal of Animal Science and Biotechnology, 2016, 6(1): 1-9 |
ROY T, BANERJEE G, DAN S K, et al. Optimization of fermentation conditions for phytase production by two strains of Bacillus licheniformis (LF1 and LH1) isolated from the intestine of rohu, Labeo rohita (Hamilton). Proceedings of the Zoological Society, 2013, 66(1): 27-35 |
SHARAWY Z, GODA A S, HASSAAN M S. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, postlarvae. Animal Feed Science and Technology, 2016, 212: 90-99 |
SUN B C, YANG K Y, LI Y H, et al. Effects of single or mixed Bacillus on WSSV infection and immune-related gene expression in Litopenaeus vannamei. Progress in Fishery Sciences, 2019, 40(3): 113-121 [孙博超, 杨运楷, 李玉宏, 等. 饲料中添加复合芽孢杆菌对凡纳滨对虾抗病毒感染能力及抗病基因表达的影响. 渔业科学进展, 2019, 40(3): 113-121] |
VIEIRA F N, BUGLIONE C C, MOURINO J P L, et al. Effect of probiotic supplemented diet on marine shrimp survival after challenge with Vibrio harveyi. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 2010, 62(3): 631-638 |
VOGELEY J L, INTERAMINENSE J A, BUARQUE D S, et al. Growth and immune gene expression of Litopenaeus vannamei fed Bacillus subtilis and Bacillus circulans supplemented diets and challenged with Vibrio parahaemolyticus. Aquaculture International, 2019, 27(5): 1-14 |
WANG Q, FU W L, LU R Q, et al. Characterization of Bacillus subtilis Ab03 for efficient ammonia nitrogen removal. Systems Microbiology and Biomanufacturing, 2022, 2: 580-588 |
WANG Y, BRANICKY R, NOE A, et al. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. The Journal of Cell Biology, 2018, 217(6): 1915-1928 |
WATANABE T. Strategies for further development of aquatic feed. Fisheries Science, 2010, 68(2): 242-252 |
XIAO W, SHEN H W, MA S et al. Effects of chronic nitrite stress on body composition and glucose metabolism of Litopenaeus vannamei. Progress in Fishery Sciences, 2020, 41(6): 74-81 [肖威, 单洪伟, 马甡, 等. 亚硝态氮慢性胁迫对凡纳滨对虾体成分和糖代谢的影响. 渔业科学进展, 2020, 41(6): 74-81] |
YANG N D, FU G C. Study on substituting fish meal with fermented soybean meal in diets of Litopenaeus vannamei. Feed Industry, 2008(10): 24-26 [杨耐德, 符广才. 凡纳滨对虾饲料中发酵豆粕替代鱼粉的研究. 饲料工业, 2008(10): 24-26] |
YANG Q H. The study of the effects of combination of plant protein sources and fish meal on nutritional physiology in juvenile white shrimp Litopenaeus vannamei, Boone. Doctoral Dissertation of Sichuan Agricultural University, 2010 [杨奇慧. 植物蛋白源与鱼粉组合对凡纳滨对虾(Litopenaeus vannamei, Boone)幼虾营养生理效应研究. 四川农业大学博士研究生学位论文, 2010]
|
YANG T, YANG Q, SHI Y, et al. Insight into the denitrification mechanism of Bacillus subtilis JD-014 and its application potential in bioremediation of nitrogen wastewater. Process Biochemistry, 2021, 103: 78-86 |
YUAN C Y, MENG Y, BI J C, et al. Effects of fermented feed on digestive enzyme activities and intestinal microflora of Penaeus vannamei. Feed Industry, 2018(24): 24-28 [袁春营, 孟阳, 毕建才, 等. 发酵饲料对南美白对虾消化酶活性与肠道菌群的影响. 饲料工业, 2018(24): 24-28] |
ZHANG J G, LIU C L. Application of lactic acid bacteria instead of antibiotics in aquaculture. China Fisheries, 2014, 464(7): 66-68 [张家国, 刘翠玲. 乳酸菌代替抗生素在水产养殖上的应用. 中国水产, 2014, 464(7): 66-68] |
ZHANG M Z, PAN L Q, FAN D P, et al. Study of fermented feed by mixed strains and their effects on the survival, growth, digestive enzyme activity and intestinal flora of Penaeus vannamei. Aquaculture, 2021, 530(5): 735703-735720 |
ZHANG S J, ZHAO X J, SONG X L, et al. Effects of adding probiotics on promoting intestinal bacteria, Toll receptors, and lysozyme immune gene expression and resistance to Vibrio harveyi in Litopenaeus vanname. Journal of Fishery Sciences of China, 2016, 23(4): 846-854 [张盛静, 赵小金, 宋晓玲, 等. 饲料添加益生菌对凡纳滨对虾肠道菌群、Toll受体及溶菌酶基因表达及抗感染的影响. 中国水产科学, 2016, 23(4): 846-854] |
ZHAO M M, YAO D F, LI S K, et al. Effects of ammonia on shrimp physiology and immunity: A review. Reviews in Aquaculture, 2020, 12: 2194-2211 |
ZHOU X X, WANG Y B, LI W F. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture, 2009, 287(3/4): 349-353 |



