2. 中国水产科学研究院黄海水产研究所 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 农业农村部海水养殖病害防治重点实验室 青岛市海水养殖流行病学与生物安保重点实验室 山东 青岛 266071;
3. 江苏数丰水产种业有限公司 江苏 高邮 225654;
4. 天津科技大学海洋与环境学院 天津 300457
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center; Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs; Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao 266071, China;
3. Jiangsu Shufeng Prawn Breeding Co. Ltd., Gaoyou 225654, China;
4. College of Marine and Environmental Science, Tianjin University of Science and Technology, Tianjin 300457, China
罗氏沼虾(Macrobrachium rosenbergii)是一种具有重要经济价值的淡水养殖虾类,隶属于节肢动物门(Arthropoda)、软甲纲(Malacostraca)、十足目(Decapoda)、长臂虾科(Palaemonidae)、沼虾属(Macrobrachium)。因其优良的生物学特性,罗氏沼虾被许多国家和地区作为主要淡水养殖品种进行养殖(FAO, 2004—2020)。我国罗氏沼虾年育苗量约250亿尾,养殖面积约30 000 hm2,据《中国渔业年鉴统计》数据,2021年养殖总产量达171 263 t (农业农村部渔业渔政管理局等, 2022)。从苗种、饲料、成虾、加工到国内外贸易总产值超过100亿元,形成一条巨大的产业链,我国已成为世界上罗氏沼虾养殖产量最大的国家。然而,自2010年开始,江苏、浙江、广东和广西等省份的罗氏沼虾养殖广泛出现以性早熟、甲壳硬和生长缓慢为主要特征的铁虾综合征(也称“铁虾”或“铁壳虾”) (Yang, 2014; Zhou et al, 2017)。“铁虾”导致经济损失达50%以上,但其病因一直不明且无有效的解决方案,严重制约了罗氏沼虾养殖业的绿色高质量发展。Dong等(2021)运用病毒宏转录组技术在性早熟罗氏沼虾中发现一种新的属于黄病毒科(Flaviviridae)的病毒,并证明了感染该病毒可导致正常罗氏沼虾性早熟和与之相关的生长缓慢,命名为传染性早熟病毒(infectious precocity virus, IPV)。Zhou等(2022)对生长缓慢罗氏沼虾和正常罗氏沼虾进行病毒组分析,同样在2组虾之间观察到黄病毒丰度存在明显差异。Dong等(2021)、Zhao等(2023)和Wang等(2023)分别建立了IPV的检测方法。Zhao等(2023)从罗氏沼虾、青虾(Macrobrachium nipponense)、克氏原螯虾(Procambarus clarkii)、凡纳对虾(Penaeus vannamei)、斑节对虾(Penaeus monodon)、口虾蛄(Oratosquilla oratoria)和直角小仰蝽(Anisops kuroiwae)中均检测出IPV阳性。Wang等(2023)利用建立的一步法实时荧光定量PCR (RT-qPCR)方法,连续3年在我国罗氏沼虾的主要养殖区进行流行病学调查,发现IPV与“铁虾”高度相关。Wang等(2023)和Zhao等(2023)进一步发现,IPV分布具有组织特异性,在眼柄和脑等神经相关组织中具有较高的病毒载量,提示IPV有嗜神经性。
目前,在脊椎动物和昆虫病毒方面所取得的重大进展显示,细胞等体外研究平台对认识病毒、解析疾病的发病机制以及药物筛选等具有重要作用和意义(Wu et al, 2022; 汪尊冬等, 2022)。细胞培养相对活体动物实验具有以下优势:首先,细胞培养无需大型养殖场地和设备,不受季节的影响,可节约大量的成本和时间;其次,细胞实验更易控制实验条件,不易受外界气温、溶氧量以及动物病害的影响。目前,对哺乳动物的细胞培养技术已趋成熟,但甲壳动物尤其是虾类还缺少稳定的细胞系,大部分细胞仍为原代培养,如多个团队分别建立了红鳌螯虾(Cherax quadricarinatus) (段虎, 2014)、拟穴青蟹(Scylla paramamosain) (乔琨等, 2014)、克氏原螯虾(魏静等, 1999)、罗氏沼虾(Thansa et al, 2021; Jie et al, 2012)和中华绒螯蟹(Eriocheir sinensis) (杨鹤等, 2019)等的血细胞体外培养技术。除此之外,罗氏沼虾已成功建立肌细胞体外培养技术(王军霞等, 2005)。有研究报道,对虾白斑综合征病毒(white spot syndrome virus, WSSV)可感染鳌虾血淋巴细胞,并在血细胞内大量增殖(李钫等, 2002)。在甲壳动物神经细胞体外培养方面,有不少学者先后开展了红螯螯虾(Cherax quadricarinatus) (张岩等, 2016)、中华绒螯蟹(孙金生等, 2000)和中国对虾(Fenneropenaeus chinensis) (Gao et al, 2003)等甲壳动物眼柄X器官–窦腺复合体的体外培养,但目前缺少罗氏沼虾神经细胞体外培养的报道。
前期研究证实,IPV具有神经嗜性(Wang et al, 2023; Zhao et al, 2023),因此,建立罗氏沼虾神经细胞原代培养技术对研究IPV与神经细胞的互作机制以及药物筛选等具有积极影响。本研究选择罗氏沼虾脑、眼柄、胸神经节和腹神经节共4个神经组织,通过酶解法获取神经细胞,并利用L-15培养基进行离体培养,建立一种操作简便且稳定的原代细胞培养技术,并初步建立IPV体外感染模型,可为罗氏沼虾嗜神经病毒研究和神经内分泌研究提供基础和平台。
1 材料与方法 1.1 实验用虾实验用罗氏沼虾由江苏数丰水产种业有限公司提供,运回实验室后,于50 L养殖箱中暂养7 d,水温为28 ℃,24 h不间断充气,每日投喂3次人工配合饲料。暂养期间进行IPV检测(方法见1.5),确定阴性后,挑选肢体健全、活力良好的健康个体供实验用。
1.2 细胞的分离与培养选取10只体长在10~12 cm之间、处于蜕皮间期的健康罗氏沼虾个体,置于冰上使其昏迷。先将头胸甲及眼柄部位用75%酒精仔细喷洒,再用浸泡75%酒精的脱脂棉球仔细擦拭体表。在超净台中,用无菌剪刀和眼科镊剥离甲壳、肌肉及其他结缔组织,依次取出眼柄X器官–窦腺复合体、脑、胸神经节和腹神经节组织,将分离出的神经组织在加有适量抗生素的PBS缓冲液(100 U/mL青链霉素、100 U/mL两性霉素和80 U/mL庆大霉素)中再次清洗2~3遍,放入PBS缓冲液等待消化。
将分离出的眼柄X器官–窦腺复合体、脑、胸腹神经节组织分别放入5 mL用PBS稀释至浓度为0.5%的木瓜蛋白酶溶液中,室温下消化5 min,期间用移液枪轻轻吹打。消化完成后,加入等体积1× L-15培养基终止消化,获得解离的细胞悬液。培养基在使用前加入15%的胎牛血清、140 mmol/L的D-葡萄糖以及抗生素(100 U/mL青链霉素、100 U/mL两性霉素和80 U/mL庆大霉素)。
轻轻吹打混匀细胞,将其转移至24孔细胞培养板中,黑暗室温下静置贴壁45 min。贴壁完成后,将细胞转移至细胞培养箱中,在黑暗中28 ℃继续培养。此后,将不同时间阶段的神经细胞于倒置显微镜下观察,记录其形态变化。
1.3 IPV病毒粗提液的制备称取10 g左右超低温冰箱保存的感染IPV罗氏沼虾眼柄组织,加入100 mL预冷的SM缓冲液(含0.35 mmol/L丝氨酸抑制剂)中,冰浴下,用匀浆器10 000 r/min匀浆10 s,连续匀浆3次。匀浆液于高速离心机4 ℃下10 000 g离心30 min,保留上清液。向沉淀中加入50 mL SM缓冲液,重悬沉淀。匀浆液于高速离心机4 ℃下8 000 g离心20 min,保留上清液。向沉淀中加入50 mL SM缓冲液,重悬沉淀,匀浆液于高速离心机4 ℃下6 000 g离心15 min,保留上清液,得到病毒粗提液。
1.4 IPV侵染细胞选择细胞数量较多、培养效果较好的脑细胞进行IPV攻毒。在细胞体外培养第5天时,开始接毒。将得到的病毒粗提液用0.22 μm滤膜过滤,按照病毒粗提液∶无血清培养基=1∶9的比例混合,实验组每个培养孔中加入2 mL混合液,对照组中加入2 mL无血清培养基孵育。在病毒感染后的0、6、12、24、48和96 h取样,对照组和实验组各选一个细胞孔,取200 μL上清液,–80 ℃超低温冰箱保存。
1.5 IPV的定量检测按照试剂盒说明书,用SPARKeasy组织/细胞RNA快速提取试剂盒(思科捷,山东)从200 μL细胞液中提取RNA。用NanoDrop 2000 (Thermo Scientific, 美国)测量RNA的浓度和质量。参照Wang等(2023)建立的方法,将1 μL RNA样本加入19 μL反应液体系中,使用CFX-96定量荧光仪(Bio Rad, 美国)检测罗氏沼虾脑细胞内IPV病毒载量。
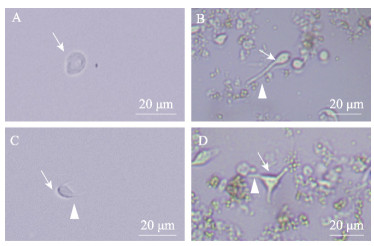
2 结果 2.1 脑细胞的形态学观察倒置显微镜下观察发现,从罗氏沼虾脑组织分离的细胞在培养24 h后大量贴壁,有些成团,胞体透亮,形态不一,在显微镜下观察到4种不同的形态,将其鉴定为以下4类:球形颗粒细胞(图 1A)、类假单极神经元(图 1B)、类双极神经元(图 1C)和类多极神经元(图 1D)。球形颗粒细胞无树突或轴突,胞体为透亮的圆形或椭圆形,直径不到10 μm。类假单极神经元胞体呈透亮的椭圆形,直径不到20 μm,一端带有长的轴突(> 20 μm)。类双极神经元胞体长轴约10 μm,短轴约5 μm,胞体的两极带有轴突,长度不超过10 μm。类多级神经元数量最少,其胞体带有3个及以上轴突。在含有谷氨酰胺的L-15培养基中,脑神经细胞可存活15 d。
|
图 1 脑细胞的类型 Fig.1 Types of neural cells of the brain A:培养1 d的球形颗粒细胞;B:培养2 d的类假单极神经元;C:培养2 d的类双极神经元;D:培养3 d的类多极神经元。箭头:细胞;三角箭头:轴突。下同。 A: Spherical granule cells cultured for 1 day; B: Unipolar neuron-like cultured for 2 days; C: Bipolar neuron-like cultured for 2 days; D: Multipolar neuron-like cultured for 3 days. Arrow: Cell; Triangular arrow: Axon. The same below. |
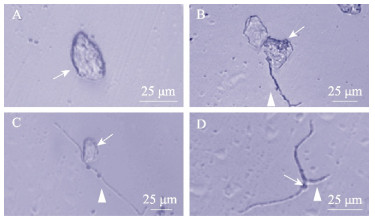
光镜镜检表明,体外培养的罗氏沼虾眼柄X器官–窦腺复合体细胞在24 h后开始贴壁。根据其在显微镜下观察到的形态特征,同样可将其分为4类:球形颗粒细胞(图 2A)、假单极神经元(图 2B)、双极神经元(图 2C)和多极神经元(图 2D)。球形颗粒细胞无树突或轴突,胞体内含较多折光性强的物质,多为椭圆形,胞体长轴直径在25 μm左右,短轴直径约为10 μm。假单极神经元胞体呈透亮的椭圆形,直径不到20 μm,一级连有树突,长度在30 μm左右。双极神经元胞体带有2条轴突,长度在50 μm以上。多级神经元数量最少,其胞体带有3个及以上轴突,胞体较小。在含有谷氨酰胺的L-15培养基中可在体外存活15 d。
|
图 2 XO-SG复合体细胞形态 Fig.2 Cell types of X organ-sinus gland complex of the eye stalk A:培养3 d的球形颗粒细胞;B:培养5 d的假单极神经元;C:培养7 d的双极神经元;D:培养7 d的多极神经元 A: Spherical granule cells cultured for 3 days; B: Unipolar neurons cultured for 5 days; C: Bipolar neurons cultured for 7 days; D: Multipolar neurons cultured for 7 days |
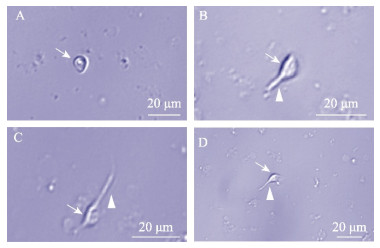
从罗氏沼虾胸神经节分离出的细胞,依据形态学特点,判断其分别为球形颗粒细胞(图 3A)、类假单极神经元(图 3B)、类双极神经元(图 3C)和类多极神经元(图 3D)。球形颗粒细胞无树突或轴突,胞体为透亮的圆形或椭圆形,直径不到5 μm。类假单极神经元和假单极神经元形态相似,胞体呈透亮的椭圆形,一端带有轴突。类双极神经元胞体的两极带有轴突,长度不超过5 μm。类多极神经元胞体带有3条及以上轴突,长短不一。能在体外培养条件下存活9 d。
|
图 3 胸神经节细胞的类型 Fig.3 Types of neural cell of Thoracic ganglion A~D分别为培养3 d的球形颗粒细胞、类假单极神经元、类双极神经元和类多级神经元。 A~D represent spherical granule cells, unipolar neuron-like, bipolar neuron-like, and multipolar neuron-like cultured for 3 days, respectively. |
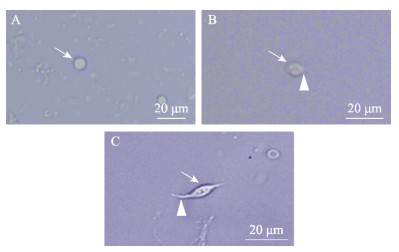
腹神经节细胞在显微镜下可观察到圆形(图 4A)、椭圆形的神经细胞(图 4B)和类双极神经元(图 4C),神经细胞胞体透亮,直径在5 μm左右,无树突或轴突,无再生趋势。类双极神经元胞体透亮,两端伸出轴突,在体外培养可存活9 d。
|
图 4 腹神经节细胞类型 Fig.4 Types of neural cell of the abdominal ganglion A:培养1 d的圆形神经细胞;B:培养1 d的椭圆形神经细胞;C:培养2 d的类双极神经元 A: Neurosecretory cells cultured for 1 day; B: Unipolar neurons cultured for 1 day; C: Bipolar neuron-like cultured for 2 days |
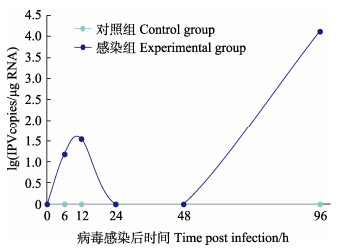
将IPV接种罗氏沼虾脑细胞,与对照组相比,感染组细胞贴壁率下降,多数细胞随感染时间的增加逐步破裂死亡,显微镜下观察到大量细胞碎片。RT-qPCR检测结果显示(图 5),感染IPV的脑细胞在6 h和12 h的病毒载量分别为15.30和35.59 copies/μg RNA,24 h和48 h的结果为阴性,然后,在96 h时检测到高的IPV载量,达到104 copies/μg RNA。
|
图 5 接毒后脑细胞IPV病毒载量变化 Fig.5 Changes of IPV viral load in the experimental group cells after infection |
为建立罗氏沼虾病毒–宿主互作的体外研究平台,本研究利用L-15培养基对罗氏沼虾神经细胞进行离体培养,从脑组织和眼柄X器官–窦腺复合体中分离出的细胞在体外存活时间可达15 d。选择培养效果最佳的脑细胞进行IPV体外感染,在感染后96 h可检测到高载量的IPV。
通过RT-qPCR对感染IPV的罗氏沼虾不同组织进行检测,发现IPV感染具有嗜神经性,在眼柄、脑、胸神经节等组织中的载量较高(Wang et al, 2023; Zhao et al, 2023)。同时,转录组测序发现,催乳素、雌激素、胰岛素、促性腺激素释放激素、胰高血糖素、催产素等与生殖调控相关激素的代谢过程在患病虾与正常虾眼柄之间存在差异(王国浩等, 2022)。故本研究选择罗氏沼虾眼柄X器官–窦腺复合体组织、脑组织、胸神经节组织和腹神经节组织分离出来的细胞进行分离培养。原代细胞培养成功的关键因素之一为组织处理,温和的处理可以保证良好的细胞活性和数量。多数哺乳动物的组织分离是借助胰蛋白酶获得解离的神经细胞(邓超等, 2018; 郭慧等, 2016)。姜茜等(2009)在分离大鼠(Rattus norvegicus)皮层神经元单细胞悬液时,采用了木瓜蛋白酶化学消化与机械吹打相结合的方法,所得到的细胞与胰蛋白酶化学消化法相比,数量多且损伤小,杂质少,纯度高,树突、突触发育正常,可长期存活。本研究选择消化效果更为温和的木瓜蛋白酶和机械吹打相结合的方法处理从罗氏沼虾体内剥离出的神经组织,从而降低消化对细胞造成的损伤,所得细胞可正常发育。本研究在罗晓雯等(2022)建立的鳜(Siniperca chuatsi)脑组织细胞系的研究和张岩等(2016)建立的红螯螯虾眼柄X器官–窦腺复合体细胞原代培养方法的基础上,选择L-15作为基础培养基,使用前加入神经细胞生长所需的D-葡萄糖促进细胞生长的胎牛血清和防止染菌的抗生素。本研究所培养的脑细胞和眼柄X器官–窦腺复合体细胞可在该培养基中存活15 d,而胸腹神经节细胞可在该培养基中存活9 d。
潘鹏宇等(2023)对传染性法氏囊病病毒疫苗株B87在DF-1细胞和Vero细胞感染性研究中,使用DMEM维持液,将病毒液稀释10倍后孵育DF-1细胞和Vero细胞,成功感染2种不同的细胞系。故本研究在进行IPV感染实验时,同样用维持液将IPV病毒液稀释10倍,取2 mL稀释的IPV病毒液孵育脑细胞,感染后的96 h,可在显微镜下观察到感染的细胞贴壁率下降,多数细胞失去贴壁能力,漂浮在培养基中。在Tamás等(2005)使用同为黄病毒科的尤苏它病毒(Usutu virus)感染原代细胞时也出现了类似的现象。RT-qPCR检测细胞液结果显示,在接毒96 h时,可在脑细胞内检测到较高的IPV病毒载量,浓度达到1.31×104 copies/μg。在王文广等(2023)的研究中,通过对肠道病毒71型(EV71)体外感染树鼬(Tupaia belangeri)肾细胞的特性观察,发现细胞在接种病毒的12 h内,未观察到细胞的明显病变效应,且检测出较低水平的病毒载量。本研究病毒载量的变化也表现出相同的趋势,在病毒感染后的24 h和48 h时,未检测到病毒,表明病毒在接种后有一定的适应期,在动物感染实验中我们也观察到类似的结果(未发表数据)。而在此期间,病毒如何侵染神经细胞,结合了哪些关键受体,这些科学问题或将可以依靠细胞感染模型得以深入探讨。由于剩余细胞的量无法满足取样需求,致使检测时间点截止到96 h。受到细胞量的限制,96 h以后病毒在细胞内载量的变化及其他几种细胞对IPV的易感性还需进一步探究。本研究分离出的罗氏沼虾腹神经节细胞贴壁缓慢,胞体为圆形或椭圆形,直径不超过20 μm,可在体外存活9 d左右。
综上所述,本研究建立了一种健康罗氏沼虾来源的神经细胞原代培养方法,为罗氏沼虾神经内分泌研究和嗜神经病毒–宿主互作提供了研究基础和平台。
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2022. Beijing: China Agriculture Press, 2022 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2022中国渔业统计年鉴. 北京: 中国农业出版社, 2022]
|
DENG C, ZHANG H, XUE J L, et al. Primary culture and identification of trigeminal neurons in neonatal SD rats. Journal of Oral Science Research, 2018, 34: 3 [新生SD大鼠三叉神经元细胞的体外原代培养及鉴定. 口腔医学研究, 2018, 34: 3] |
DONG X, WANG G, HU T, et al. A novel virus of Flaviviridae associated with sexual precocity in Macrobrachium rosenbergii. mSystems, 2021, 6: e0000321 DOI:10.1128/mSystems.00003-21 |
DUAN H. Studies on cell culture of hemocytes and cellular immunology of red claw crayfish, Cherax quadricarinatus. Doctoral dissertations of University of Chinese Academy of Sciences, Oceanography Institute, 2014 [段虎. 红螯螯虾血细胞培养及细胞免疫学研究. 中国科学院研究生院(海洋研究所)博士研究生学位论文, 2014]
|
FAO. Cultured Aquatic Species Information Programme. Macrobrachium rosenbergii. Text by New M B. In: FAO Fisheries and Aquaculture Department. Rome. http://www.fao.org/fishery/culturedspecies/Macrobrachium_rosenbergii/en, 2004–2020
|
GAO C L, SUN J S, XIANG J H. Primary culture and characteristic morphologies of medulla terminalis neurons in the eyestalks of Chinese shrimp, Fenneropenaeus chinensis. Journal of Experimental Marine Biology and Ecology, 2003, 290: 71-80 DOI:10.1016/S0022-0981(03)00069-8 |
GUO H, YU D, ZHOU H, et al. Research on the improved culture of hipocampus neurons from rat in vitro. Chinese Journal of Obstetrics & Gynecology and Pediatrics (Electronic Edition), 2016, 12: 5 [新生大鼠海马神经元细胞体外培养方法改良研究. 中华妇幼临床医学杂志(电子版), 2016, 12: 5] |
JIANG Q, JIANG W W, WANG J M, et al. An improved method for primary culture of rat cortical neuron and cell identification. Journal of Peking University (Health Science), 2009, 41(2): 212-216 [一种改进的大鼠皮层神经元原代培养方法及其性质鉴定. 北京大学学报(医学版), 2009, 41(2): 212-216 DOI:10.3969/j.issn.1671-167X.2009.02.019] |
JIE D, OU J, LI W, et al. Primary hemocyte culture of the freshwater prawn Macrobrachium rosenbergii and its susceptibility to the novel pathogen spiroplasma strain MR-1008. Aquaculture, 2012, 330/331/332/333: 21–28
|
LI F, YANG F. Proliferation of white spot bacilliform virus in the primary cell culture from haemocytes of crayfish Cambarus clarkii. Chinese High Technology Letters, 2002, 12: 4 [对虾白斑杆状病毒对螯虾血淋巴原代细胞的感染. 高技术通讯, 2002, 12: 4] |
LUO X W, ZENG L B, JIANG N, et al. Establishment of a cell line derived from the brain tissue of mandarin fish Siniperca chuatsi and its susceptibility to infection by fish viruses. Progress in Fishery Sciences, 2022, 43(5): 179-188 [鳜脑组织细胞系的建立及其病毒敏感性研究. 渔业科学进展, 2022, 43(5): 179-188 DOI:10.19663/j.issn2095-9869.20211021001] |
PAN P Y, HAN J Z, YIN J H, et al. Study on infectivity of IBDV vaccine strain B87 in DF-1 cells and Vero cells. Progress in Veterinary Medicine, 2023, 44(2): 42-45 [传染性法氏囊病病毒疫苗株B87在DF-1细胞和Vero细胞感染性研究. 动物医学进展, 2023, 44(2): 42-45] |
QIAO K, ZHANG Y Q, WANG S P, et al. The optimization of primary hemocyte culture of Scylla paramamosain. China Animal Husbandry and Veterinary Medicine, 2014, 41: 5 [拟穴青蟹血细胞原代培养及条件优化. 中国畜牧兽医, 2014, 41: 5] |
TAMÁS B, HELGA L, HERBERT W, et al. In vitro host-cell susceptibility to Usutu virus. Emerging Infectious Diseases, 2005, 11(2): 298-301 DOI:10.3201/eid1102.041016 |
THANSA K, KRUANGKUM T, PUDGERD A, et al. Establishment of hematopoietic tissue primary cell cultures from the giant freshwater prawn Macrobrachium rosenbergii. Cytotechnology, 2021, 73(2): 141-157 DOI:10.1007/s10616-021-00451-w |
WANG G H, DONG X, WANG Y T, et al. Study on the eyestalk transcriptome of Macrobrachium rosenbergii suffering from iron prawn syndrome. Progress in Fishery Sciences, 2022, 43(3): 64-74 [患铁虾综合征罗氏沼虾眼柄转录组研究. 渔业科学进展, 2022, 43(3): 64-74 DOI:10.19663/j.issn2095-9869.20210206001] |
WANG G, GUO X, HUANG X, et al. The quantitative characteristics of infection with infectious precocity virus (IPV) revealed with a new TaqMan probe-based real-time RT-PCR method. Aquaculture, 2023, 566: 739179 DOI:10.1016/j.aquaculture.2022.739179 |
WANG J X, LIU J, WANG W N, et al. Effect of Cu2+ and Zn2+ on cultured muscle cells of Macrobrachium rosenbergii. Marine Sciences, 2005, 29: 38-43 [Cu2+和Zn2+对罗氏沼虾体外培养肌细胞的影响. 海洋科学, 2005, 29: 38-43] |
SUN J S, LIU A X, CHEN J T. Cytology and culture of neurosecretory cell in the eyestalk of Eriocheir sinensis. Acta Hydrobiologica Sinica, 2000, 24: 6 [河蟹MTXO细胞的离体培养和细胞学研究. 水生生物学报, 2000, 24: 6] |
WANG W G, KUANG D X, YI B W, et al. Study on infectivity of EV71 in kidney cells of tree shrew. Laboratory Animal and Comparative Medicine, 2023, 37(2): 123-129 [肠道病毒71型体外感染树鼩肾细胞特性观察. 实验动物与比较医学, 2023, 37(2): 123-129] |
WANG Z D, LI W, ZENG Z, et al. Improved primary culture of atrial myocytes in neonatal SD rats. International Journal of Cardiovascular Disease, 2022, 49: 4 [新生SD大鼠心房肌细胞的原代培养及改良. 国际心血管病杂志, 2022, 49: 4] |
WEI J, YANG C H. The primary cell culture of crawfish hemocytes. Animal Husbandry and Veterinary Medicine, 1999, 31: 11-12 [克氏原螯虾的血淋巴细胞原代培养. 畜牧与兽医, 1999, 31: 11-12] |
WU C, PAN Y, WANG L, et al. A new method for primary culture of microglia in rats with spinal cord injury. Biochemical and Biophysical Research Communications, 2022, 599: 63-68 |
YANG H, YUE W C, HOU X, et al. Primary hematocyte cultivation and house-keeping gene detection of Chinese mitten crab (Eriocheir sinensis). Journal of Agricultural Biotechnology, 2019, 27: 8 [中华绒螯蟹血细胞的原代培养及其管家基因检测. 农业生物技术学报, 2019, 27: 8] |
YANG M. Farming of giant freshwater prawn in China. World Aquaculture, 2014, 48-51 |
ZHANG Y, DUAN H, JIN S J, et al. Primary cell culture of XO-SG from red claw crayfish (Cherax quadricarinatus). Journal of Fisheries of China, 2016, 40: 7 [红螯螯虾XO-SG神经细胞的原代培养. 水产学报, 2016, 40: 7] |
ZHAO C, MIU Q, LIU S, et al. Detection methods, epidemiological investigation, and host ranges of infectious precocity virus. Aquaculture, 2023, 562: 738818 |
ZHOU D, LIU S, GUO G, et al. Virome analysis of normal and growth retardation disease-affected Macrobrachium rosenbergii. Microbiology Spectrum, 2022, 10(6): e0146222 |
ZHOU J M, DAI X L, JIANG F, et al. The preliminary analysis of the reasons for the poor growth of Macrobrachium rosenbergii in pond. Journal of Shanghai Ocean University, 2017, 26: 853-861 |



