2. 海水养殖育种与可持续产出全国重点实验室(中国水产科学研究院黄海水产研究所) 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 农业农村部海水养殖病害防治重点实验室 青岛市海水养殖流行病学与生物安保重点实验室 山东 青岛 266071;
3. 天津农学院水产学院 天津 300392;
4. 山东大学生命科学学院 山东 青岛 266237;
5. 亚太水产养殖中心网 泰国 曼谷 10900
2. State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center; Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs; Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao 266071, China;
3. Fisheries School, Tianjin Agriculture College, Tianjin 300392, China;
4. College of Life Science, Shandong University, Qingdao 266237, China;
5. Network of Aquaculture Centres in Asia-Pacific, Bangkok 10900, Thailand
急性肝胰腺坏死病(acute hepatopancreatic necrosis disease, AHPND)是影响我国乃至全球对虾养殖业的细菌性疾病,临床病症为肝胰腺发白、萎缩和空肠空胃等(Lightner et al, 2012)。AHPND自2010年暴发以来,
每年给全球多国的对虾养殖业造成超过约70亿美元的经济损失(张宝存等, 2012; Tran et al, 2013; Gomez-Gil et al, 2014; de la Peña et al, 2015; Dabu et al, 2017; Tang et al, 2020; FAO, 2013)。研究表明,AHPND病原菌的毒力源于其携带的约70 kb的可编码二元毒力蛋白PirAB的毒力质粒(pVA1型毒力质粒) (Lee et al, 2015; Tinwongger et al, 2016; Han et al, 2017)。AHPND的致病菌存在多样性,包括副溶血弧菌(Vibrio parahaemolyticus) (张宝存等, 2012)、欧文氏弧菌(V. owensii) (Liu et al, 2015)、坎贝氏弧菌(V. campbellii) (Dong et al, 2017)、普纳弧菌(V. punensis) (Restrepo et al, 2018)、哈维氏弧菌(V. harveyi) (Muthukrishnan et al, 2019)和鳗弧菌(V. anguillarum) (Shen et al, 2021)等,这些病原菌中均含有pVA1型毒力质粒。
已有研究发现,pVA1型毒力质粒可通过接合转移引起AHPND致病菌多样性(Dong et al, 2019a、b)。与pVA1型毒力质粒接合转移有关的功能基因簇被注释为trb型Ⅳ型分泌系统(type Ⅳ secretion system, T4SS) (Dong et al, 2019b; Wang et al, 2022)。革兰氏阴性菌中的T4SS重要组分包括通道复合物(Darbari et al, 2015; Low et al, 2014; Costa et al, 2021)、Ⅳ型偶联蛋白及松弛酶复合体(Grohmann et al, 2018)等。供受体菌通过碰撞接触形成接合通道(Costa et al, 2021),松弛酶复合体被激活后识别质粒oriT序列上的nic位点,将质粒一条链切割为线性单链DNA (T链),单链DNA与松弛酶形成蛋白质-DNA复合体,在T4偶联蛋白作用下将单链DNA通过接合转移通道转移至受体菌中。受体菌中T链滚环复制合成第2条DNA链形成完整质粒,至此完成质粒水平转移(Llosa et al, 2002; Draper et al, 2005; Garcillán-Barcia et al, 2007)。副溶血弧菌20130629002S01 (Vp2S01)菌株已被证明携带新的trb型T4SS,且仅在高细菌密度(1012 CFU/mL)条件下检测到接合转移发生(Wang et al, 2022)。细菌高细胞密度可增加供受体菌间相互接触的概率,同时也能激活细菌群体感应(quorum sensing, QS)的高密度调控因子OpaR,因此,推测接合转移可能与群体感应系统的高密度调控子相关。
群体感应系统也称密度感应系统,是指细菌细胞间的信息交流过程,可调控多种基因的表达,以响应周围环境中自诱导剂(autoinducer, AI)信号分子的浓度变化(Ng et al, 2009)。副溶血弧菌的群体感应的2个中心调控子为AphA和OpaR,分别在低细胞密度(low cell density, LCD)和高细胞密度(high cell density, HCD)下发挥作用。在LCD时,胞外信号分子启动受体蛋白LuxN和LuxU的级联磷酸化通路,促进AphA的表达,同时抑制OpaR表达(Ball et al, 2017);在HCD时,高浓度群体感应信号分子抑制受体蛋白LuxN和LuxU的级联磷酸化通路,从而解除对OpaR的抑制,OpaR得以正常表达(Lenz et al, 2004)。研究表明,OpaR参与副溶血弧菌中的多种生物学过程,如OpaR可参与激活副溶血弧菌荚膜多糖产生和T6SS2系统的表达(王丽, 2014),促进生物被膜的形成(Zhang et al, 2021),抑制泳动运动(Lu et al, 2021)和群集运动(Lu et al, 2019),抑制T3SS (Henke et al, 2004)和T6SS1 (Ma et al, 2012; 董新波, 2015)的表达。但OpaR对AHPND致病菌中T4SS的调控研究目前尚未见报道。
本研究以高密度调控子opaR基因作为研究对象,构建副溶血弧菌Vp2S01::cat株opaR基因敲除株(Vp2S01::catΔopaR),通过生长性能、生物被膜形成及接合转移等结果,探究高密度调控子OpaR对副溶血弧菌生物学特性以及T4SS的影响。
1 材料与方法 1.1 菌株和质粒副溶血弧菌Vp2S01分离自中国广西AHPND患病对虾,Vp2S01:cat是由同源重组技术在pVPGX1质粒上插入氯霉素抗性基因所得(Dong et al, 2019b)。坎贝氏弧菌VcLMB29分离自深圳美国红鱼(Sciaenops ocellatus)皮肤溃疡样品,由河海大学海洋学院赵哲教授惠赠。菌株现均保存于中国水产科学研究院黄海水产研究所养殖生物疾病控制与分子病理学研究室;质粒pMAD、自杀质粒pCVD442和自杀质粒中间宿主大肠杆菌(Escherichia coli) β2155均为实验室质粒和菌株(表 1)。
|
|
表 1 本研究所使用的菌株和质粒 Tab.1 Strains and plasmids used in this study |
超保真DNA聚合酶PrimeSTAR Max Premix (2×)和T4 DNA连接酶购自TaKaRa公司,细菌质粒小量提取试剂盒、细菌总RNA提取试剂盒均购自天根生化科技(北京)有限公司,胶回收试剂盒购自莫纳生物科技有限公司,氯霉素(chloramphenicol, Chl)和利福平(rifampicin, Rif)购自北京索莱宝科技有限公司,红霉素(erythromycin, Em)购自上海阿拉丁生化科技股份有限公司,第1链合成试剂盒和2×SYBR Green qPCR Mix试剂盒购自山东思科捷生物技术有限公司,8%红色SDS-PAGE凝胶制备试剂盒购自Genscript (中国)金斯瑞生物科技有限公司,脱氧核糖核酸酶DNaseⅠ(无RNase)购自New England Biolabs。
1.3 opaR基因缺失株的构建基因缺失株的构建参考王小鹿等(2021)的实验方法。通过同源重组技术在Vp2S01::cat基础上,以红霉素抗性基因(ermB)替代opaR基因构建缺失株Vp2S01::catΔopaR。以副溶血弧菌Vp2S01基因组和pMAD质粒为模板,使用引物opaR-5F/5R、opaR-3F/3R、opaR-ermBF/ermBR (表 2)分别进行扩增得到opaR基因上、下游同源臂及红霉素抗性基因,并通过融合PCR技术将3个片段按照上游同源臂–红霉素抗性基因–下游同源臂的顺序连接并克隆入自杀质粒pCVD442,获得打靶质粒pCVD442-ΔopaR: : ermB。将重组质粒电转化至E. coli β2155,获得供体菌β2155/pCVD442-ΔopaR: : ermB。以Vp2S01::cat为受体菌进行接合实验,在红霉素抗性平板上筛选获得抗性的阳性克隆,称为Vp/pCVD442-ΔopaR: : ermB。取数个Vp/pCVD442-ΔopaR: : ermB克隆菌液铺在含10%蔗糖的LB平板上,培养至单克隆形成。通过PCR技术筛选获得opaR基因被ermB基因取代的克隆,将其命名为Vp2S01::catΔopaR。
|
|
表 2 opaR缺失突变株构建所使用的引物 Tab.2 Primers used in the construction of opaR mutation |
将Vp2S01::cat和Vp2S01::catΔopaR单菌落转接至2216E液体培养基中,28 ℃、180 r/min震荡培养至对数生长期,以1%接种量转接至200 mL新鲜的2216E液体培养基,每隔2 h取样后使用全波长酶标仪测定在600 nm处的吸光值(OD600),绘制各菌株的生长曲线并进行差异性分析。
1.5 生物被膜形成能力测定参考杨金龙等(2021)报道的方法检测菌株的生物被膜形成能力。将Vp2S01::cat和Vp2S01::catΔopaR培养至OD600为0.5左右后分别稀释100倍。以96孔酶标板为实验容器,每个孔内加入180 μL 2216E液及20 μL稀释菌液,放置于28℃培养箱中静置培养24 h,无菌2216E培养液作为阴性对照,每个菌株平行对照9组。培养完成后,将酶标板从培养箱中取出,吸出每个孔中的菌液后,加入200 μL的1×PBS缓冲液洗涤3次,晾干约20 min,加入1%的结晶紫(crystal violet, CV)染液200 μL,染色15 min。将酶标孔中的结晶紫染液吸净,再加入200 μL的1×PBS轻轻洗涤3次。吸净PBS,每孔中加入200 μL无水乙醇后常温静置10 min以洗脱生物被膜,待结晶紫完全溶解后,检测595 nm处的吸光值(OD595)。
| $ \begin{array}{l} 特异性生物膜形成({\rm{SBF}})指数=\\ \;\;\;\;\; \;\;\;\;\;\;\;\;{\text{(CV adherence - CV control)}}/G \end{array} $ |
式中,G为细胞在600 nm处的吸光值。
1.6 运动性测定运动性实验参考邓益琴等(2019)和李洋洋等(2021)的实验方法。
泳动运动(swimming motility):将Vp2S01::cat (Chl 20 μg/mL)和Vp2S01::catΔopaR (Chl 20 μg/mL、Em 100 μg/mL)转接至含对应抗生素的2216E液体培养基中,28 ℃、180 r/min振荡培养至对数生长期,调整各菌液浓度为OD600=0.3左右,吸取2 μL菌液垂直点样于0.3%的LB半固体泳动平板中。将其置于28 ℃培养箱中静置培养约8 h,测量细菌泳动圈直径,并对菌株泳动能力进行分析。
群集运动(swarming motility):菌株培养条件同泳动运动实验,吸取2 μL菌液垂直点样于1.5%的LB固体群集平板中,将其放置于28 ℃培养箱中培养约12 h,测量细菌圆形运动直径并进行拍照记录,实验平行3次。
1.7 opaR基因缺失对毒力质粒接合转移的影响参考Dong等(2019b)的接合转移实验方法,分别检测Vp2S01::cat和Vp2S01::catΔopaR的接合转移效率。将Vp2S01::cat、Vp2S01::catΔopaR和VcLMB29 (Rif 20 μg/mL)在28 ℃、180 r/min分别培养至OD600为0.5时,收集菌体。将Vp2S01、Vp2S01::catΔopaR分别与VcLMB29混合,4 ℃、6 000 r/min离心10 min后弃上清液,5 mL无抗2216E液体相同条件洗涤1次,100 mL混合菌体加入5 mL 2216E液体(含DNaseⅠ: 20 U/mL),相同条件洗涤后,用100 μL 2216E (含DNaseⅠ:200 U/mL)液体重悬于铺有0.22 μm滤膜的2216E平板上,28℃进行接合,培养时间为12、24和48 h,每个时间点3组平行。将滤膜上的菌体洗脱后进行梯度稀释至10–9,吸取100 μL高浓度菌液(10–1、10–2和10–3)涂布于双抗平板,低浓度(10–7、10–8和10–9)涂布于单抗平板,28 ℃培养至单克隆形成,并进行计数和检测。
1.8 实时荧光定量PCR (RT-qPCR)鉴定将Vp2S01::cat和Vp2S01::catΔopaR培养至OD600为0.5左右,使用细菌总RNA试剂盒提取细菌总RNA,利用第1链合成试剂盒将其逆转录为cDNA。以gyrB为内参引物,利用aphA-qRT (张义全, 2014)、opaR-qRT以及T4SS-qRT引物(表 3),按照2×SYBR Green qPCR Mix试剂盒在罗氏LC96荧光定量PCR仪中进行RT-qPCR,每个反应重复3次。使用2–ΔΔCt法计算opaR、aphA和T4SS基因的相对表达量(王旭江等, 2022)。
|
|
表 3 实时荧光定量PCR引物 Tab.3 Real-time quantitative PCR primers |
所有实验均设置3个生物学重复。所有实验数据使用SPSS 28.0统计软件和Excel软件中的独立样本T检验进行统计学差异分析,数据用平均值±标准差(Mean±SD)表示,P < 0.05表示具有显著性差异,P < 0.01表示具有极显著性差异。使用Origin 2022软件绘图。
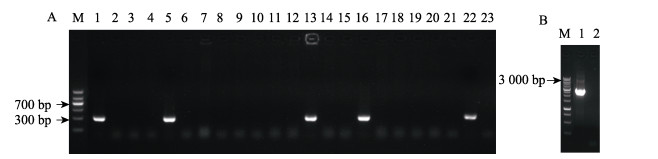
2 结果与分析 2.1 副溶血弧菌Vp2S01::catΔopaR基因缺失突变株的鉴定结果通过特异性引物设计、重组载体构建及细菌的转化与筛选,成功构建了副溶血弧菌基因缺失突变株Vp2S01::catΔopaR。在opaR基因序列的内侧设计引物opaR-in-F/R,突变株的PCR检测电泳结果见图 1。出发菌株Vp2S01::cat的检测片段大小为311 bp (图 1A,泳道22),阴性对照株无条带(图 1A,泳道23),成功突变株Vp2S01::catΔopaR无条带(图 1A,泳道1~21)。选取21号菌株,利用opaR的外侧引物opaR-out-F/R进行PCR扩增验证,突变株Vp2S01::catΔopaR扩增片段大小为2 535 bp (图 1B,泳道1)。将外侧引物扩增条带送测序,测序结果表明,基因突变株Vp2S01::catΔopaR成功构建。
|
图 1 opaR基因缺失株Vp2S01::catΔopaR的PCR鉴定结果 Fig.1 PCR identification results of mutant strain Vp2S01::catΔopaR A:opaR基因内部引物opaR-in-F/R扩增结果;M:DNA Marker DL1 200;1~21:第1~21号菌落;22:Vp2S01::cat菌株,产物长度:311 bp;23:阴性对照。B:opaR基因外部引物opaR-out-F/R鉴定;M:DNA Marker DL10 000;1:第21号菌落;2:阴性对照。 A: Amplification results of the internal primer opaR-in-F/R of opaR gene; M: DNA Marker DL1 200; 1–21: The colony of No.1–21; 22: Vp2S01::cat, and the product length is 311 bp; 23: Negative control. B: Amplification results of the external primer opaR-out-F/R of opaR gene; M: DNA Marker DL10 000; 1: The colony of No.21; 2: Negative control. |
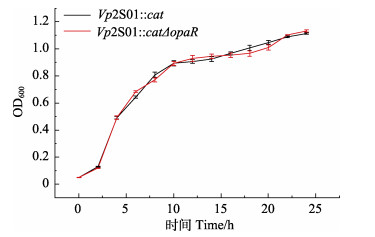
对Vp2S01::cat和Vp2S01::catΔopaR的生长曲线进行分析,结果表明,出发菌株Vp2S01::cat与缺失株Vp2S01::catΔopaR的生长情况无显著差异(图 2),表明opaR基因缺失不影响副溶血弧菌的生长。
|
图 2 菌株Vp2S01::cat和Vp2S01::catΔopaR的生长曲线 Fig.2 The growth curves of the Vp2S01::cat and Vp2S01::catΔopaR 图中各取样时间点中2菌株生长曲线均无显著性差异(P > 0.05)。 There was no significant difference in the growth curves of the two strains at each sampling time (P > 0.05). |
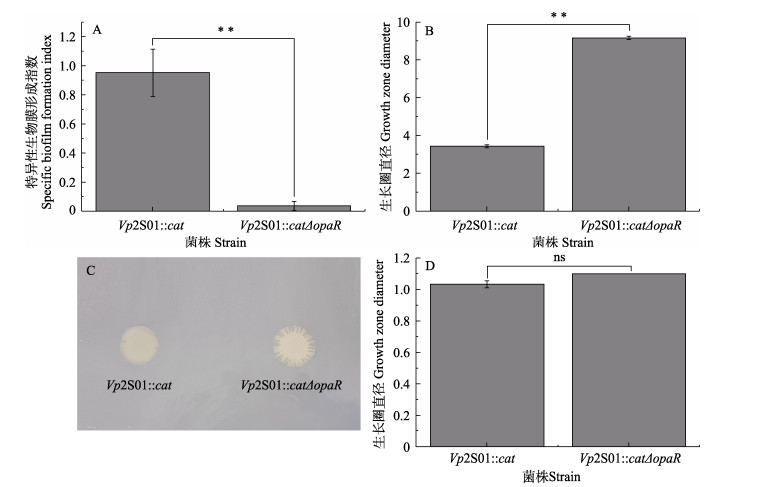
以96孔酶标板为实验容器,采用结晶紫染色法比较Vp2S01::cat和缺失株Vp2S01::catΔopaR的生物被膜形成能力的差异。结果显示,基因缺失株Vp2S01::catΔopaR (0.04±0.03)的生物被膜形成指数极显著降低,仅为Vp2S01::cat (0.95±0.16)的3.90% (图 3A),表明opaR基因可参与调控副溶血弧菌生物被膜的形成。
|
图 3 菌株Vp2S01::cat和Vp2S01::catΔopaR的生物被膜形成能力和运动能力的差异分析 Fig.3 Analysis of differences in biofilm formation and motility of Vp2S01::cat and Vp2S01::catΔopaR A:菌株的生物被膜形成能力差异;B:菌株的泳动直径差异;C:群集平板菌落;D:菌株的群集直径差异。*: P < 0.05;**: P < 0.01;ns:无显著差异。下同。 A: The difference in biofilm-forming ability; B: The difference in growth zone diameter of swimming; C: The colony of strains in the 1.5% LB plate; D: The difference in growth zone diameter of swarming. *: P < 0.05; **: P < 0.01; ns: No significant difference. The same below. |
为探究opaR基因是否影响细菌运动性,通过LB半固体培养基和固体培养基对菌株运动能力进行测定。泳动实验结果显示,培养约8 h左右,缺失株Vp2S01::catΔopaR的泳动圈直径[(9.17±0.09) cm]大于Vp2S01::cat的泳动圈直径[(3.43±0.08) cm],泳动能力显著增加了约2.67倍(图 3B)。群集运动实验结果表明,培养约12 h左右,Vp2S01::catΔopaR的生长圈直径(1.10 cm)与Vp2S01::cat [(1.03±0.02) cm]相比无显著性差异,但Vp2S01::catΔopaR的生长圈边缘多缺刻(图 3C、D)。综上所述,opaR基因缺失不影响副溶血弧菌Vp2S01::cat的群集运动能力,但可显著提高其泳动运动能力。
2.4 opaR基因缺失对毒力质粒接合转移和Ⅳ型分泌系统基因表达的影响使用接合转移模型对不同时间点的接合转移效率进行测定。实验结果显示,在12 h时,Vp2S01::catΔopaR转移效率[(8.77±3.82)×10–8]较Vp2S01::cat [(1.55±0.42)×10–8]增加约5.66倍。在24 h时,Vp2S01::catΔopaR的转移效率[(1.01±0.40)×10–7]较Vp2S01::cat [(1.10±0.48)×10–9]增加约96.20倍。但在48 h时,未检测到Vp2S01::catΔopaR实验组存在接合子(图 4A)。

|
图 4 菌株Vp2S01::cat和Vp2S01::catΔopaR不同时间点的接合转移效率(A)和接合转移24 h时Ⅳ型分泌系统基因的表达水平(B) Fig.4 The conjugative transfer efficiency at different time points (A) and the relative transcription level at conjugative transfer 24 h of Vp2S01::cat and Vp2S01::catΔopaR (B) |
使用相对定量法检测接合转移24 h后菌体的T4SS基因的转录水平(图 4B)。在基因缺失株Vp2S01::catΔopaR中,opaR基因无表达,T4SS基因的相对表达水平比菌株Vp2S01::cat增加了约1.13~3.21倍。其中,接合转移关键基因traF、trbE和traG的相对表达水平分别显著提高1.96、1.92和3.21倍。综上所述,opaR基因可通过调控T4SS基因的表达进而调控接合转移效率。
3 讨论与结论AHPND致病菌的pVA1型毒力质粒上携带新型trb型T4SS,可介导毒力质粒的接合转移引起AHPND致病菌多样性(Dong et al, 2019a、b; Wang et al, 2022),本团队前期研究发现,接合转移效率与细菌密度密切相关(Wang et al, 2022)。opaR基因编码的高细胞密度调控子OpaR可参与调控多种基因表达,在副溶血弧菌致病过程中发挥重要作用(Zhong et al, 2013; Wu et al, 2019; Lu et al, 2021)。本研究以opaR基因为研究对象构建了缺失株,并探索其对副溶血弧菌Vp2S01::cat生物学功能和pVA1型毒力质粒接合转移效率的影响。
副溶血弧菌的生物被膜能够保护细菌细胞免受环境变化及降低对抗生素的敏感度,促进其在宿主中的定殖和生存(Yildiz et al, 2009; Li et al, 2020)。成熟生物被膜的形成受鞭毛、菌毛和荚膜多糖(capsular polysaccharide, CPS)等多因素的影响。群体感应高密度调控子OpaR可通过激活CPS操纵子以抑制生物被膜形成量(McCarter, 1998; Boles et al, 2002; Kim et al, 2007; Kalburge et al, 2017);且Zhang等(2021)研究发现,OpaR可通过降低环鸟苷二磷酸(cyclic-di-GMP, c-di-GMP)的浓度以抑制副溶血弧菌RIMD2210633中生物被膜形成量。但Zhong等(2021)研究发现,副溶血弧菌HZ株的ΔopaR菌株的生物被膜形成弱于野生菌株。这些结果表明,高密度调控子OpaR是副溶血弧菌生物被膜形成的重要调控因子,但其对生物被膜形成的调控机制存在菌株差异性。本研究中,opaR基因缺失株的生物被膜形成能力显著下降,表明opaR基因可能参与调控副溶血弧菌Vp2S01::cat生物被膜形成,但OpaR对AHPND致病菌生物被膜形成的调控机制还有待研究。
副溶血弧菌的极生鞭毛(polar flagella, Pof)和侧生鞭毛(lateral flagella, Laf)可帮助其在不同环境中运动,以适应周围环境帮助弧菌定殖(Stewart et al, 2003)。极生鞭毛与泳动运动相关,可推动副溶血弧菌在液体介质中运动;侧生鞭毛与群集运动相关,使弧菌在固体介质中移动(McCarter, 1995)。本研究发现,opaR基因缺失株Vp2S01::catΔopaR的泳动能力显著增强,但群集能力无显著改变。Lu等(2021)发现,OpaR是副溶血弧菌泳动运动和pof基因转录的负调控因子;泳动实验结果与其研究结果一致,表明OpaR是Vp2S01::cat泳动运动的负调控因子。当菌体处于固体介质时,侧生鞭毛合成且细胞达到阈值时产生群集运动,细菌间连接形成圆形微菌落(Aagesen et al, 2013)。已有研究表明,OpaR可通过抑制laf基因表达以调控群集运动,但在opaR基因缺失情况下,群集运动仍受到严格表型调控(Jaques et al, 2006)。研究结果显示,OpaR不影响Vp2S01::cat的群集运动能力,但缺失株Vp2S01::catΔopaR的群集菌落多缺刻。因此,opaR基因对于Vp2S01::cat侧生鞭毛和菌落缺刻的相关影响机制还需进一步研究。
接合转移是基因水平转移的重要方式之一,前期已被证实是AHPND致病菌多样性的重要原因(Liu et al, 2015; Dong et al, 2017、2019a、2019b)。已有的研究显示,OpaR可参与调控副溶血弧菌T3SS和T6SS等多种分泌系统(Zhong et al, 2013; Henke et al, 2004; 王丽, 2014),但目前尚未有关于OpaR对副溶血弧菌T4SS的调控研究的报道。接合转移实验以及RT-qPCR检测结果表明,当opaR基因缺失时,T4SS基因表达率和pVA1型毒力质粒接合转移效率增加。与大部分单一菌的群体感应实验相比,本研究中使用的接合转移模型含有2种菌,供体菌为副溶血弧菌(Vp2S01::cat和Vp2S01::catΔopaR),受体菌为坎贝氏弧菌(VcLMB29),供体菌和受体菌中均含有高细胞密度调控子。因此,opaR基因对T4SS基因表达和接合转移的关系还需构建opaR基因回补株或以更多AHPND致病菌的接合转移实验来进行确认。
研究结果表明,高细胞密度调控基因opaR对副溶血弧菌Vp2S01::cat的生长特性及群集运动能力无显著影响,但对Vp2S01::cat的泳动能力、生物被膜形成能力、Ⅳ型分泌系统基因的表达水平及接合转移效率均有显著影响,由此揭示,OpaR是副溶血弧菌Vp2S01::cat毒力质粒接合转移的重要调控因子。但细菌的调控通路极为复杂,目前尚未有研究报道群体感应与pVA1型毒力质粒接合转移的调控关系,后续将结合基因回补株、原核转录组测序技术及蛋白表达等实验以厘清高密度调控子OpaR对pVA1型毒力质粒pVA1型毒力质粒接合转移的调控通路及机制。本研究为解析群体感应系统调控致AHPND致病菌T4SS表达以及毒力质粒接合转移机制提供了基础数据,可为控制AHPND致病菌毒力质粒传播提供技术支撑。
AAGESEN A M, PHUVASATE S, SU Y C, et al. Persistence of Vibrio parahaemolyticus in the Pacific oyster, Crassostrea gigas, is a multifactorial process involving pili and flagella but not type Ⅲ secretion systems or phase variation. Applied and Environmental Microbiology, 2013, 79(10): 3303-3305 DOI:10.1128/AEM.00314-13 |
BALL A S, CHAPARIAN R R, VAN KESSEL J C, et al. Quorum sensing gene regulation by LuxR/HapR master regulators in Vibrios. Journal of Bacteriology, 2017, 199(19): e00105-17 |
BOLES B R, MCCARTER L L. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. Journal of Bacteriology, 2002, 184(21): 5946-5954 DOI:10.1128/JB.184.21.5946-5954.2002 |
COSTA T R D, HARB L, KHARA P, et al. Type Ⅳ secretion systems: Advances in structure, function, and activation. Molecular Microbiology, 2021, 115(3): 436-452 DOI:10.1111/mmi.14670 |
DABU I M, LIM J J, ARABIT P M T, et al. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquaculture Research, 2017, 48(3): 792-799 DOI:10.1111/are.12923 |
DARBARI V C, WAKSMAN G. Structural biology of bacterial type Ⅳ secretion systems. Annual Review of Biochemistry, 2015, 84(1): 603-629 DOI:10.1146/annurev-biochem-062911-102821 |
DE LA PEÑAL D, CABILLON N A, CATEDRAL D D, et al. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Diseases of Aquatic Organisms, 2015, 116(3): 251-254 DOI:10.3354/dao02919 |
DENG Y Q, CHEN C, SU Y L, et al. Construction and characterization of the sRNA srvg23535 knock-out mutant of Vibrio alginolyticus ZJ-T. Microbiology China, 2019, 46(4): 829-841 [邓益琴, 陈偿, 苏友禄, 等. 溶藻弧菌ZJ-T小RNA srvg23535基因突变株的构建及其功能初探. 微生物学通报, 2019, 46(4): 829-841] |
DONG X B, Quorum sensing-dependent regulation of type Ⅵ secretion system Ⅰ in Vibrio parahaemolyticus. Master´s Thesis of Anhui Agricultural University, 2015 [董新波. 副溶血性弧菌中密度感应系统依赖的T6SS1调控机制研究. 安徽农业大学硕士研究生学位论文, 2015]
|
DONG X, CHEN J Y, SONG J P, et al. Evidence of the horizontal transfer of pVA1-type plasmid from AHPND-causing V. campbellii to non-AHPND V. owensii. Aquaculture, 2019a, 503: 396-402 DOI:10.1016/j.aquaculture.2019.01.016 |
DONG X, SONG J P, CHEN J Y, et al. Conjugative transfer of the pVA1-type plasmid carrying the pirABvp genes results in the formation of new AHPND-causing Vibrio. Frontiers in Cellular and Infection Microbiology, 2019b, 9(1): 195 |
DONG X, WANG H L, XIE G S, et al. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerging Microbes and Infections, 2017, 6(1): e2 |
DRAPER O, CÉSAR C E, MACHÓN C, et al. Site-specific recombinase and integrase activities of a conjugative relaxase in recipient cells. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(45): 16385-16390 |
FAO. Report of the FAO/MARD technical workshop on early mortality syndrome (EMS) or acute hepatopancreatic necrosis syndrome (AHPND) of cultured shrimp (under TCP/VIE/ 3304): FAO Fisheries and Aquaculture Report, Hanoi, Vietnam, 2013
|
GARCILLÁN-BARCIA M P, JURADO P, GONZÁLEZ-PÉREZ B, et al. Conjugative transfer can be inhibited by blocking relaxase activity within recipient cells with intrabodies. Molecular Microbiology, 2007, 63(2): 404-416 DOI:10.1111/j.1365-2958.2006.05523.x |
GOMEZ-GIL B, SOTO-RODRIGUEZ S, LOZANO R, et al. Draft genome sequence of Vibrio parahaemolyticus strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announcements, 2014, 2(2): e00055-14 |
GROHMANN E, CHRISTIE P J, WAKSMAN G, et al. Type Ⅳ secretion in gram-negative and gram-positive bacteria. Molecular Microbiology, 2018, 107(4): 455-471 DOI:10.1111/mmi.13896 |
HAN J E, TANG K F J, ARANGUREN L F, et al. Characterization and pathogenicity of acute hepatopancreatic necrosis disease natural mutants, pirABvp (–) V. parahaemolyticus, and pirABvp (+) V. campbellii strains. Aquaculture, 2017, 470: 84-90 DOI:10.1016/j.aquaculture.2016.12.022 |
HENKE J M, BASSLER B L. Quorum sensing regulates type Ⅲ secretion in Vibrio harveyi and Vibrio parahaemolyticus. Journal of Bacteriology, 2004, 186(12): 3794-3805 DOI:10.1128/JB.186.12.3794-3805.2004 |
JAQUES S, MCCARTER L L. Three new regulators of swarming in Vibrio parahaemolyticus. Journal of Bacteriology, 2006, 188(7): 2625-2635 DOI:10.1128/JB.188.7.2625-2635.2006 |
KALBURGE S S, CARPENTER M R, ROZOVSKY S, et al. Quorum sensing regulators are required for metabolic fitness in Vibrio parahaemolyticus. Infection and Immunity, 2017, 85(3): e00930-16 |
KIM Y K, MCCARTER L L. SCRG, a GGDEF-EAL Protein, participates in regulating swarming and sticking in Vibrio parahaemolyticus. Journal of Bacteriology, 2007, 189(11): 4094-4107 DOI:10.1128/JB.01510-06 |
LEE C T, CHEN I T, YANG Y T, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(34): 10798-10803 |
LENZ D H, MOK K C, LILLEY B N, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell, 2004, 118(1): 69-82 DOI:10.1016/j.cell.2004.06.009 |
LI W, WANG J J, QIAN H, et al. Insights into the role of extracellular DNA and extracellular proteins in biofilm formation of Vibrio parahaemolyticus. Frontiers in Microbiology, 2020, 11: 813 DOI:10.3389/fmicb.2020.00813 |
LI Y Y, WANG Q, GUO R, et al. Effects of VPA1500 gene deletion on biological characteristics and pathogenicity of Vibrio parahaemolyticus. Acta Microbiologica Sinica, 2021, 61(12): 3937-3951 [李洋洋, 王权, 郭容, 等. VPA1500基因缺失对副溶血弧菌生物学特性和致病性的影响. 微生物学报, 2021, 61(12): 3937-3951] |
LIGHTNER D V, REDMAN R M, PANTOJA C R, et al. Early mortality syndrome affects shrimp in Asia. Global Aquaculture Advocate, 2012, 15(1): 40 |
LIU L Y, XIAO J Z, XIA X M, et al. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announcements, 2015, 3(6): e01395-15 |
LLOSA M, GOMIS-RÜTH F X, COLL M, et al. Bacterial conjugation: A two-step mechanism for DNA transport. Molecular Microbiology, 2002, 45(1): 1-8 DOI:10.1046/j.1365-2958.2002.03014.x |
LOW H H, GUBELLINI F, RIVERA-CALZADA A, et al. Structure of a type Ⅳ secretion system. Nature, 2014, 508(7497): 550-553 DOI:10.1038/nature13081 |
LU R F, SUN J F, QIU Y, et al. The quorum sensing regulator OpaR is a repressor of polar flagellum genes in Vibrio parahaemolyticus. Journal of Microbiology, 2021, 59: 651-657 DOI:10.1007/s12275-021-0629-3 |
LU R F, TANG H, QIU Y, et al. Quorum sensing regulates the transcription of lateral flagellar genes in Vibrio parahaemolyticus. Future Microbiology, 2019, 14(12): 1043-1053 DOI:10.2217/fmb-2019-0048 |
MA L Z, ZHANG Y Q, YAN X J, et al. Expression of the type Ⅵ secretion system 1 component Hcp1 is indirectly repressed by OpaR in Vibrio parahaemolyticus. The Scientific World Journal, 2012, 1: 982140 |
MCCARTER L L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. Journal of Bacteriology, 1998, 180(12): 3166-3173 DOI:10.1128/JB.180.12.3166-3173.1998 |
MCCARTER L L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. Journal of Bacteriology, 1995, 177(6): 1595-1609 DOI:10.1128/jb.177.6.1595-1609.1995 |
MUTHUKRISHNAN S, Defoirdt T, INA-SALWANY M Y, et al. Vibrio parahaemolyticus and Vibrio harveyi causing acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds. Aquaculture, 2019, 511: 734227 DOI:10.1016/j.aquaculture.2019.734227 |
NG W, BASSLER B L. Bacterial quorum-sensing network architectures. Annual Review of Genetics, 2009, 43: 197-222 DOI:10.1146/annurev-genet-102108-134304 |
RESTREPO L, BAYOT B, ARCINIEGAS S, et al. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Scientific Reports, 2018, 8(1): 13080 DOI:10.1038/s41598-018-30903-x |
SHEN H Y, SONG T T, LU J Q, et al. Shrimp AHPND causing Vibrio anguillarum infection: Quantitative diagnosis and identifying antagonistic bacteria. Marine Biotechnology, 2021, 23(6): 964-975 DOI:10.1007/s10126-021-10079-8 |
STEWART B J, MCCARTER L L. Lateral flagellar gene system of Vibrio parahaemolyticus. 2003, 185(15): 4508–4518
|
TANG K F J, BONDAD-REANTASO M G, ARTHUR J R, et al. Shrimp acute hepatopancreatic necrosis disease strategy manual. FAO Fisheries and Aquaculture Circular NFIA/C1190 (En), Rome, FAO, 2020
|
TINWONGGER S, NOCHIRI Y, THAWONSUWAN J, et al. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. Journal of Applied Microbiology, 2016, 121(6): 1755-1765 DOI:10.1111/jam.13256 |
TRAN L, NUNAN L, REDMAN R M, et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting Penaeid shrimp. Diseases of Aquatic Organisms, 2013, 105(1): 45-55 DOI:10.3354/dao02621 |
WANG D H, WANG L Y, BI D X, et al. Conjugative transfer of acute hepatopancreatic necrosis disease-causing pVA1-type plasmid is mediated by a novel self-encoded type Ⅳ secretion system. Microbiology Spectrum, 2022, 10(5): e01702-22 |
WANG L. Quorum sensing-dependent regulation of type Ⅲ secretion system 1 and type Ⅵ secretion system 2 in Vibrio parahaemolyticus. Doctoral Dissertation of Chongqing Medical University, 2014 [王丽. 副溶血性弧菌中密度感应系统依赖的T3SS1和T6SS2调控机制研究. 重庆医科大学博士研究生学位论文, 2014]
|
WANG X J, YAO Z L, LAI Q F, et al. Transcriptomic analysis of gene expression of Litopenaeus vannamei during long-term exposure to high alkaline water. Progress in Fishery Sciences, 2022, 43(4): 22-32 [王旭江, 么宗利, 来琦芳, 等. 长期高碱胁迫下凡纳滨对虾基因表达差异研究. 渔业科学进展, 2022, 43(4): 22-32] |
WANG X L, LI J, LI G Y, et al. The effect of rpoS on Hcp expression and bactericidal activity in Vibrio anguillarum MHK3. Progress in Fishery Sciences, 2021, 42(6): 125-134 [王小鹿, 李杰, 李贵阳, 等. rpoS对鳗弧菌MHK3株Hcp表达和杀菌能力的影响. 渔业科学进展, 2021, 42(6): 125-134] |
WU K, ZHENG Y Y, WU Q P, et al. Vibrio parahaemolyticus cqsA controls production of quorum sensing signal molecule 3-hydroxyundecan-4-one and regulates colony morphology. Journal of Microbiology, 2019, 57(12): 1105-1114 DOI:10.1007/s12275-019-9379-x |
YANG J L, DUAN Z H, DING W Y, et al. Effects of VB7 and VB12 on biofilm formation and larval metamorphosis of the mussel Mytilus coruscus. 2021, 42(5): 113–123 [杨金龙, 段志鸿, 丁文扬, 等. 维生素B7和B12对细菌生物被膜形成及厚壳贻贝幼虫变态的影响. 渔业科学进展, 2021, 42(5): 113–123]
|
YILDIZ F H, VISICK K L. Vibrio biofilms: So much the same yet so different. Trends in Microbiology, 2009, 17(3): 109-118 DOI:10.1016/j.tim.2008.12.004 |
ZHANG B C, LIU F, BIAN H H, et al. Isolation, identification, and pathogenicity analysis of a Vibrio parahaemolyticus strain from Litopenaeus vannamei. Progress in Fishery Sciences, 2012, 33(2): 56-62 [张宝存, 刘飞, 边慧慧, 等. 一株凡纳滨对虾病原菌的分离、鉴定及其致病力分析. 渔业科学进展, 2012, 33(2): 56-62 DOI:10.3969/j.issn.1000-7075.2012.02.009] |
ZHANG Y Q. Transcriptional regulation of the genes associated with quorum sensing system by AphA and OpaR in Vibrio parahaemolyticus. Doctoral Dissertation of Academy of Military Medical Sciences, 2014 [张义全. AphA和OpaR对副溶血弧菌密度感应系统相关基因的转录调控机制研究. 中国人民解放军军事医学科学院博士研究生学位论文, 2014]
|
ZHANG Y, QIU Y, GAO H, et al. OpaR controls the metabolism of c-di-GMP in Vibrio parahaemolyticus. Frontiers in Microbiology, 2021, 12: 676436 DOI:10.3389/fmicb.2021.676436 |
ZHONG X J, LU R R, LIU F W, et al. Identification of LuxR family regulators that integrate into quorum sensing circuit in Vibrio parahaemolyticus. Frontiers in Microbiology, 2021, 12: 691842 DOI:10.3389/fmicb.2021.691842 |



