2. 海水养殖生物育种与可持续产出全国重点实验室 中国水产科学研究院黄海水产研究所 中国水产科学研究院碳汇渔业重点实验室 山东 青岛 266071;
3. 青岛海洋科技中心海洋渔业科学与食物产出过程功能实验室 山东 青岛 266237;
4. 荣成楮岛水产有限公司 山东 荣成 264312
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Key Laboratory of Carbon Sink Fisheries, Qingdao 266071, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao 266071, China;
4. Rongcheng Chudao Aquaculture Corporation, Rongcheng 264312, China
长牡蛎(Crassostrea gigas)又称太平洋牡蛎,是世界上养殖范围最广、产量最高的经济贝类,也是我国最重要的海水养殖贝类种类。然而,近几十年来,在国内外沿海区域均出现了夏季长牡蛎大量死亡的现象。例如,1995年大连沿海养殖的长牡蛎死亡率达到40%~50%,有的甚至达到70%以上,仅存的个体也表现出生长缓慢、出肉率低的情况(隋锡林等, 2002);2008年法国养殖的长牡蛎死亡率达到40%~ 100% (Li et al, 2009);2009年桑沟湾崖头长牡蛎养殖区死亡率高达51% (廉伟等, 2010);2019年乳山养殖区死亡率达50%~90% (魏钰恒等, 2020)。死亡高峰期多发生在8月中下旬。诸多研究表明,造成长牡蛎大规模死亡是温度、溶氧、盐度、疾病、饵料和繁殖等因素共同作用的结果(Soletchnik et al, 2007; 林思恒, 2016)。其中,高温是一个最重要的非生物胁迫因素(Gagnaire et al, 2006),夏季高温使长牡蛎酶代谢水平发生紊乱,导致长牡蛎生长缓慢或停止生长(王如才等, 2008);另外,性成熟个体的繁殖活动造成了糖原的大量消耗,体质虚弱叠加高温胁迫易诱发长牡蛎大量死亡(毛玉泽等, 2005; Royer et al, 2007)。
长牡蛎三倍体因其高度不育性,具有生长速度快、抗逆能力强、经济性状优等特点(Amiard et al, 2004; Nell et al, 2005; Guo et al, 2008),近些年来,在我国尤其是北方沿海地区形成了一定的养殖规模。有关长牡蛎三倍体与二倍体之间的生物学和生理学等差异在国内外已有较多的研究,多集中在长牡蛎三倍体与二倍体生长特性(王昭萍等, 2002)、软组织成分(Laing et al, 1994; 曾志南等, 1999; 孔令锋等, 2001; Lin et al, 2002)、性腺发育(Normand et al, 2009)、抗病力(Gagnaire et al, 2006; Azema et al, 2016; Haure et al, 2021)、免疫性能(Duchemin et al, 2007)和鳃结构等差异方面(孔令锋等, 2003)。但关于长牡蛎三倍体与二倍体摄食、代谢生理与能量收支以及碳收支比较的研究尚未见报道。本研究聚焦夏季高温期这一特殊时段,采用现场流水法,研究长牡蛎三倍体与二倍体的摄食和代谢生理特征,对比分析三倍体与二倍体应对高温环境的能量分配策略。研究结果可揭示长牡蛎倍性效应而引发的生理差异,进而服务于养殖容量评估提供数据支撑。
1 材料与方法 1.1 实验材料实验于2022年8月15—30日在山东省荣成市楮岛码头实验室进行。实验用三倍体及二倍体长牡蛎均取自山东荣成桑沟湾近海养殖区,这2种牡蛎均为2022年5月放苗的个体,初始壳高均为10 mm左右,实验用三倍体长牡蛎壳高为(63.07±6.21) mm (n=30),二倍体长牡蛎壳高为(52.95±4.12) mm (n=30)。挑选健康、形态和大小一致的个体各30只,清洗壳表面的污物和附着生物后,将其暂养于6.6×103 L养殖池内;养殖池海水由桑沟湾近海持续泵入,自然海水保持流水状态,暂养3 d后,选取9只牡蛎开始进行相关实验。
1.2 实验方法 1.2.1 实验系统搭建与设计实验采用现场流水法测定2种长牡蛎的滤水率、同化效率、耗氧率和排氨率。实验系统搭建于桑沟湾楮岛码头实验室,搭建方法参考王晓芹等(2017),如图 1所示。
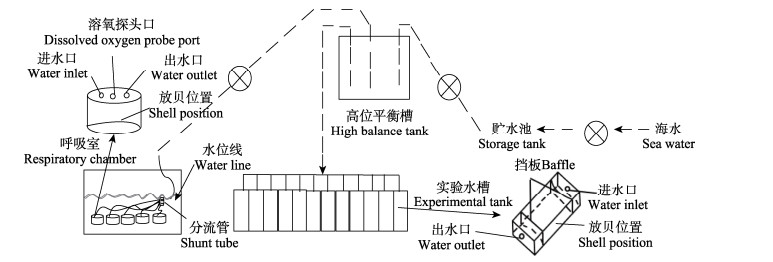
|
图 1 现场流水系统示意图 Fig.1 Diagram of experimental flow-through system |
实验期间,于每日08:00、12:00和18:00测定实验海水的温度(T)、盐度(S)、溶解氧(DO)、pH、叶绿素a (Chl-a)。T、S、DO和pH采用便捷式水质参数分析仪(YSI WTW, 美国)测定,Chl-a采用叶绿素浊度仪(ACLW-USB, 日本)测定。实验期间水温为(24.84±0.56) ℃,盐度为30.12±0.17,pH为8.17±0.16,DO为(7.68±0.78) mg/L,Chl-a为(2.36±0.11) μg/L,颗粒有机物(POM)为(6.20±1.11) mg/L,颗粒有机碳(POC)为(0.49±0.09) mg/L。
1.2.2 实验方法滤水率及同化效率测定:实验共布设12个流水槽,实验前,先将流速调节到180~200 mL/min,流速稳定之后,将已标记号码的长牡蛎逐一放入流水槽中,每个流水槽放置1个长牡蛎,形成9个实验组(放置牡蛎)、3个对照组(不放牡蛎),每隔3个实验组放置1个对照组,保证实验用水的均匀性。观察流水槽内牡蛎的状态,在牡蛎开口的那一刻开始计时,在牡蛎适应好的第1、2、3小时从流水槽出水口接水样100 mL,利用便携式颗粒计数器PAMAS (测定粒径范围为2~200 μm, S4031GO, 德国)测定水样瓶中的水体颗粒物数量,并在程序中设定每个水样测定3次,即每个水样可得到3组数据。同时,在入水口取水样1 000 mL,每个时间点取2个平行样,用预先450 ℃灼烧6 h并称重(W0)的Whatman GF/F玻璃纤维滤膜(孔径为0.7 μm,直径为47 mm)抽滤,抽滤时用0.5 mol/L的甲酸铵冲洗,将带有样品的滤膜在60 ℃烘干48 h称重(W60),再经450 ℃灼烧6 h后称重(W450)(精确至0.000 1 g),测定水体中悬浮颗粒物(TPM)和POM的浓度;另取,1 000 mL的入水口海水,抽滤至预先450 ℃灼烧6 h的Whatman GF/F滤膜(孔径为0.7 μm, 直径为25 mm)上,将带有样品的滤膜经雾化的盐酸酸化,60 ℃烘干至恒重后,采用Elementar EL型元素分析仪(Elementar公司, 美国)测定水体中POC的浓度。
粪便的收集:滤水实验结束后,用吸管虹吸粪便至预先450 ℃灼烧6 h并称重的Whatman GF/F玻璃纤维滤膜(孔径为0.7 μm, 直径为47 mm)上,抽滤时用0.5 mol/L的甲酸铵冲洗,将带有样品的滤膜在60 ℃烘干48 h称重(W60),再经450 ℃灼烧6 h后称重(W450)。以此测定粪便中TPM和POM的浓度。
耗氧率及排氨率测定:实验共布设12个呼吸室,将已标记号码的长牡蛎逐一放入呼吸室中,每个呼吸室放置1只长牡蛎,形成9个实验组(放置牡蛎)、3个对照组(不放牡蛎)。开始实验前,将牡蛎在呼吸室中流水驯化至开口。随后,关闭呼吸室进出水口,开始正式实验,采用十通道实时溶氧测定仪(PreSens Precision Sensing, 德国)连续监测各个呼吸室的溶解氧动态变化情况,以“呼吸室内溶氧含量变化率大于初始溶氧的10%,但溶氧含量不能低于5 mg/L”作为实验结束判断标准,既保证呼吸室内溶氧的差异性,又避免实验动物受溶氧胁迫现象的发生。耗氧实验结束后,用聚乙烯塑料瓶取100 mL海水,用玻璃纤维滤膜(孔径为0.45 μm,直径为47 mm)过滤掉浮游生物,加入2~3滴三氯甲烷以隔绝空气,用于氨氮的测定,氨氮的测定依据《海洋调查规范》(GB/T12763 -2007)中次溴酸钠氧化法。
实验结束后,用游标卡尺测量长牡蛎的壳长、壳高和壳宽;用电子天平称量长牡蛎的壳湿重,解剖软组织、称量其湿重,之后将其放入烘箱中60 ℃烘干72 h,称其壳干重和软组织干重(精确至0.01 g)。
1.3 实验指标的计算 1.3.1 生理率指标| $ \text { 滤水率 }(\mathrm{CR}, \mathrm{L} / \mathrm{h})=\mathrm{FR} \times\left(C_0-C_t\right) / \mathrm{C}_0 $ | (1) |
式中,FR为流水槽的流速(L/h),C0和Ct分别为对照组和实验组实验水槽中的颗粒物数量。
| $ \begin{array}{l} 同化效率({\rm{AE}}, \%)=\\ \;\;\;\;\;\;\;\;\;\; (F-E) /[(1-E) \times F] \times 100 \% \text { (Conover, 1966) } \end{array} $ | (2) |
式中,E = W1/W2,W1 = W60 – W450,W2 = W60 – W0,F和E计算方法相同,F为饵料中有机物干重的比例,E为粪便中有机物干重的比例。
| $ \text { 耗氧率 }\left(\mathrm{OR}, \mathrm{mgO}_2 / \mathrm{h}\right)=\left(\mathrm{DO}_0-\mathrm{DO}_t\right) \times V / t $ | (3) |
式中,DO0和DOt分别为实验结束时对照组和实验组水中溶解氧浓度(mg/L),V为呼吸室体积(L),t为耗氧用时(h)。
| $ \text { 排氨率 }(\mathrm{RE}, \mathrm{mgN} / \mathrm{h})=\left(N_t-N_0\right) \times V / t $ | (4) |
式中,N0和Nt分别为实验结束时对照组和实验组水中的氨氮浓度(mg/L),V为呼吸室体积(L),t为实验持续时间(h)。
| $ \text { 组织干重标准化 } Y_s=\left(W_s / W_e\right)^{\rm{b}} \times Y_e $ | (5) |
式中,Ys是生物软组织干重标准化后的生理参数,Ws是标准重量(1 g),We为测得的动物软组织干重(g),Ye是未标准化的生理参数,b为0.67 (Wang et al, 2015)。
| $ \text { 氧氮比=(OR/16)/(RE/14)(Widdows} \; et\; al,\; 1988) $ | (6) |
| $ \text { 贝类能量收支方程: } C=P+R+U+F $ | (7) |
式中,C为摄食能,P为生长能(生长余力),R为呼吸能,U为排泄能,F为排粪能。
| $ \text { 生长余力 }(\mathrm{SFG})[\mathrm{P}, \mathrm{J} /(\mathrm{h} \cdot \mathrm{g})]=A-(R+U) $ | (8) |
式中,同化能(A)=C × AE,C = CR [L/(h⋅g)] × POM(mg/L) × 23 (J/mg POM),本研究在计算能量收支时,采用如下能量转换因子:1 mg POM=23 J,1 mg O2=14.25 J,1 mg NH4+-N= 24.93 J (Slobodkin et al, 1961; Widdows et al, 1979; Gnaiger, 1983)。
由能量收支方程转换为碳收支方程:
| $ C_c=P_c+R_c+U_c+F_c $ | (9) |
式中,Cc为摄食碳,Pc为生长碳(生长余力),Rc为呼吸碳,Uc为排泄碳,Fc为粪便碳。
| $ \text { 生长余力 }\left[\mathrm{P}_{\mathrm{c}}, \mathrm{mg} \mathrm{POC} /(\mathrm{h} \cdot \mathrm{g})\right]=A_c-\left(R_c+U_c\right) $ | (10) |
式中,同化碳(Ac)=Cc ×AE,Cc =CR[L/(h⋅g)]×POC(mg/L),Fc = Cc × (1–AE),Rc = 0.85 × (12/32) × OR,Uc = (12/28) ×RE (Bayne et al, 1983)。
1.4 数据统计与分析实验数据用Excel 2010软件整理,实验结果以平均值±标准差(Mean±SD)表示;采用SPSS 26统计软件进行单因子方差分析(one-way ANOVA)的基础上,采用Duncan´s多重比较检验组间差异,P < 0.05为差异显著,P < 0.01为差异极显著;采用Graphpad prism 7.0软件作图。
2 结果与分析 2.1 三倍体和二倍体长牡蛎的摄食生理比较三倍体和二倍体长牡蛎的滤水率和同化效率见图 2。三倍体长牡蛎的单位软组织干重滤水率和同化效率分别为(5.66±1.90) L/(h⋅g)和52.98%,二倍体长牡蛎的单位软组织干重滤水率和同化效率分别为(4.76±1.70) L/(h⋅g)和47.02%;三倍体长牡蛎的滤水率和同化效率高于二倍体长牡蛎,但均无显著差异(P > 0.05)。
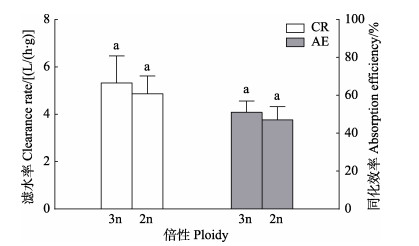
|
图 2 三倍体和二倍体长牡蛎的滤水率和同化效率 Fig.2 Clearance rate and absorption efficiency of triploid and diploid C. gigas 同种生理率不同倍性长牡蛎之间标有的不同字母的数据之间差异显著(P < 0.05)。下同。 There are significant differences between the data labeled with different letters for the same physiological rate and different ploidy of C. gigas (P < 0.05). The same below. |
三倍体和二倍体长牡蛎的耗氧率和排氨率见图 3。三倍体长牡蛎的单位软组织干重耗氧率和排氨率分别为(1.15±0.15) mg/(h⋅g)和(0.091±0.011) mg/(h⋅g),二倍体长牡蛎的单位软组织干重耗氧率和排氨率分别为(1.76±0.19) mg/(h⋅g)和(0.020±0.002) mg/(h⋅g);三倍体长牡蛎的耗氧率显著低于二倍体长牡蛎(P < 0.05),排氨率显著高于二倍体长牡蛎(P < 0.01)。
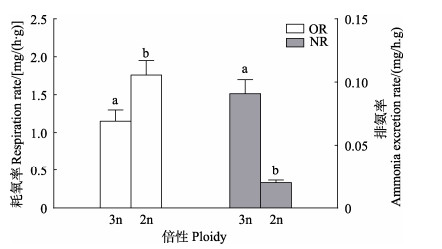
|
图 3 三倍体与二倍体长牡蛎的耗氧率和排氨率 Fig.3 Respiration rate and ammonia excretion rate of triploid and diploid C. gigas |
三倍体和二倍体长牡蛎的氧氮比见图 4。三倍体和二倍体长牡蛎的O/N值分别为(11.36±3.15)%和(77.00±17.19)%,二倍体长牡蛎的O/N值显著高于三倍体长牡蛎(P < 0.01)。
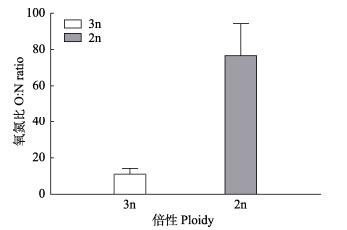
|
图 4 三倍体与二倍体长牡蛎的氧氮比 Fig.4 Oxygen to nitrogen ratio of triploid to diploid C. gigas |
三倍体和二倍体长牡蛎的能量收支见表 1。从表 1可以看出,三倍体长牡蛎的摄食能、同化能均高于二倍体长牡蛎,但无显著差异(P > 0.05);三倍体与二倍体的呼吸能、排泄能、生长余力存在显著差异性,三倍体长牡蛎的耗氧能显著低于二倍体长牡蛎(P < 0.05),但排氨能、生长余力显著高于二倍体长牡蛎(P < 0.05)。
|
|
表 1 三倍体与二倍体长牡蛎的能量收支(以软组织干重计) Tab.1 Energy budget of triploid and diploid C. gigas (based on dry weight of soft tissue) |
三倍体和二倍体长牡蛎的碳收支结果见表 2。可以看出,三倍体长牡蛎从食物中摄取并同化的碳主要用于自身生长,代谢碳占比较少;而二倍体长牡蛎的同化碳主要用于呼吸消耗,其次用于生长,排泄碳最少。三倍体长牡蛎的摄食碳和同化碳均高于二倍体长牡蛎,但无显著差异(P > 0.05);三倍体与二倍体长牡蛎的代谢碳和生长余力存在显著差异(P < 0.05),三倍体长牡蛎的呼吸碳显著低于二倍体长牡蛎(P < 0.05),但排泄碳、生长余力显著高于二倍体长牡蛎(P < 0.01)。
|
|
表 2 三倍体与二倍体长牡蛎的碳收支(以软组织干重计) Tab.2 Carbon budget of triploid and diploid C. gigas (based on dry weight of soft tissue) |
滤水率是反映贝类滤水能力的一项重要的动态指标,受多种因素影响(张媛等, 2015; 王冲等, 2020)。本研究发现,同一区域同一时期投苗的三倍体和二倍体长牡蛎在夏季高温期的滤水率无显著性差异,但三倍体长牡蛎的滤水率高于二倍体长牡蛎。Mizuta等(2021)研究发现,在美国Gloucester Point海域,同种规格的三倍体与二倍体美洲牡蛎(Crassostra virginica)的滤水率分别为(5.63±0.98)和(4.69±0.43) L/(h⋅g),美洲牡蛎的滤水率与倍性无显著相关性,但三倍体美洲牡蛎均高于二倍体,这与本研究结果一致。已有研究表明,三倍体与二倍体长牡蛎滤水率的差异和鳃面积与干肉重的比值有关,三倍体长牡蛎较高的鳃面积与干肉重比使得鳃丝对海水颗粒物的截留效率高于二倍体长牡蛎(Haure et al, 2003)。
同化效率是反映滤食性贝类对食物颗粒消化吸收能力的重要指标。本研究中,三倍体与二倍体长牡蛎的同化效率无显著差异,但三倍体长牡蛎同化效率高于二倍体长牡蛎。Osterheld等(2023)研究发现,三倍体和二倍体紫贻贝(Mytilus galloprovincialis)的同化效率分别为(86.0±2.4)%和(84.8±1.4)%,紫贻贝的同化效率与倍性无显著相关性,这与本研究结果一致。已有研究表明,滤食性贝类的同化效率主要是由纤毛和消化酶活性控制(MacDonald et al, 1998),三倍体长牡蛎较高的消化酶活性,使得机体能够较大限度地对食物消化吸收(Kesarcodi-Watson et al, 2001b)。
3.2 三倍体和二倍体长牡蛎的代谢生理比较耗氧率和排氨率是维持滤食性贝类正常新陈代谢和其他生命活动的重要指标。目前,对长牡蛎代谢生理的研究多集中于二倍体,且发现处于不同发育阶段的长牡蛎生理代谢存在差别,尤其是对于性成熟的个体(毛玉泽等, 2005)。本研究聚焦夏季高温这一重要时段,对比分析了二倍体和三倍体的代谢生理差异。结果表明,在繁殖季节,三倍体和二倍体长牡蛎的耗氧率和排氨率具有显著差异,三倍体长牡蛎耗氧率显著低于二倍体,但排氨率显著高于二倍体。Kesarcodi-Watson等(2001a)研究发现,三倍体与二倍体悉尼岩牡蛎(Saccostrea commercialis)的耗氧率分别为0.57 mg/(h⋅g)和0.70 mg/(h⋅g),排氨率分别为0.042 mg/(h⋅g)和0.021 mg/(h⋅g),三倍体悉尼岩牡蛎的耗氧率显著低于二倍体,但排氨率显著高于二倍体,这与本研究结果一致。已有研究结果表明,贝类呼吸代谢与杂合度呈负相关关系,杂合度越高的贝类,其呼吸代谢越低(Tremblay et al, 2016);贝类杂合度与基因的剂量状态效应无关,而与等位基因多样性有关,其中与等位基因多样性相关的多态位点酶主要有6种,如酯酶-1、酯酶-3、磷酸葡萄糖变位酶-1、6-磷酸葡萄糖酸脱氢酶和磷酸葡萄糖异构酶,多态位点酶浓度越高,杂合度越高(Hawkins et al, 1994)。此外,贝类氨氮排泄量较高与体内存在较强的蛋白质代谢和周转有关,主要机理在于体内天冬氨酸转氨酶(Aat)浓度较高,使氨基酸的脱氨基作用加快,促进了蛋白质的代谢和周转(Hawkins et al, 1996; 周钱森等, 2022)。
氧氮比(O/N)是生物利用代谢底物的一个重要参数,用于评估生物体对外界环境的适应能力,它反映生物在特定状态下蛋白质、糖类、脂肪代谢之间的比例关系(任黎华等, 2014; 姜娓娓等, 2017)。当外界环境发生变化时,生物体供能的代谢底物会发生相应的变化,当O/N≈7.0~9.3时,生物体对能量需求较低,主要以蛋白质代谢为主(霍恩泽等, 2021; 周建聪等, 2022);当O/N > 24时,生物体主要以糖类和脂肪代谢为主(Mayzaud et al, 1973)。本研究发现,在夏季高温环境下,三倍体和二倍体长牡蛎的O/N值的波动范围分别为7.91~14.11和59.81~94.19,因此,三倍体长牡蛎的主要供能物质为蛋白质,二倍体长牡蛎的主要供能物质为糖类和脂肪,这种代谢底物的差异使得处于该时期的三倍体长牡蛎糖原含量明显高于二倍体。许多研究发现,三倍体双壳类在繁殖期的糖原含量明显高于二倍体(Berthelin et al, 2000; Ojea et al, 2004; Qin et al, 2019),这与本研究结果一致。已有研究表明,贝类体内蛋白质代谢较高与体内氨氮排泄量增加及对能量需求低有关(Hawkins et al, 1996);贝类体内糖类和脂肪代谢较高与产卵期能量需求高有关(毛玉泽等, 2005)。
3.3 三倍体和二倍体长牡蛎的能量/碳分配比较生长余力是评估生物整体生理活动状态的一项重要的生理指标(Bayne, 2017)。通过解析倍性效应对长牡蛎摄食能/碳在体内分配的情况,可以反映倍性效应对长牡蛎生长余力的影响。本研究中,倍性对长牡蛎的摄食能/碳、同化能/碳均无显著影响,但三倍体长牡蛎的摄食能/碳、同化能/碳均大于二倍体长牡蛎,倍性对长牡蛎的代谢能/碳有显著影响,三倍体长牡蛎的呼吸能/碳显著低于二倍体长牡蛎,而排泄能/碳显著高于二倍体长牡蛎;经此能量/碳分配,三倍体长牡蛎的生长余力显著高于二倍体长牡蛎。张国范等(2000)对长牡蛎和Qin等(2019)对香港牡蛎(Crassostrea hongkongensis)的研究发现,三倍体贝类的生长速度快于二倍体,养殖1年后,三倍体贝类的体重为二倍体的1.5倍左右。Osterheld等(2023)研究发现,与二倍体相比,三倍体紫贻贝具有更高的生长潜力,三倍体紫贻贝的生长余力约为二倍体的3.5倍。这与本研究结果一致。已有研究表明,三倍体双壳类较高的生长余力与机体低代谢水平有关(Shpigel et al, 1992; Hawkins et al, 2000; Qin et al, 2019);此外,三倍体长牡蛎的性腺发育程度低,其性腺指数为二倍体长牡蛎的1/5 (周一兵等, 2000);与二倍体相比,三倍体双壳类的生长优势在于软组织部储备的能量大多用于生长,少数用于配子发育(Ruiz-Verdugo et al, 2000; Normand et al, 2008)。
本研究从个体水平生理生态学层面研究发现,与二倍体相比,在夏季高温季节,三倍体长牡蛎通过调整摄食和代谢生理行为,在能量分配策略上表现出一定的优势,但三倍体长牡蛎应对不利环境所采取的响应策略内在的分子机制尚不明确,后续尚需结合组学等系统生物学技术从分子层面深入诠释。
AMIARD H, PERRERN-ETTAGANI H, GRARD A. Influence of ploidy and metal-metal interactions on the accumulation of Ag, Cd, and Cu in oysters Crassostrea gigas Thunberg. Archives of Environmental Contamination and Toxicology, 2004, 48: 68-74 DOI:10.1007/s00244-003-0180-8 |
AZEMA P, TRAVERS M A, BENABDELMOUNA A, et al. Single or dual experimental infections with Vibrio aestuarianus and OsHV-1 in diploid and triploid Crassostrea gigas at the spat, juvenile and adult stages. Journal of Invertebrate Pathology, 2016, 139: 92-101 DOI:10.1016/j.jip.2016.08.002 |
BAYNE B L, NEWELL R C. Physiological energetics of marine molluscs//The mollusca. Academic Press, 1983, 407–515
|
BAYNE B L. Biology of oysters. Academic Press, 2017
|
BERTHELIN C, KELLNER K, MATHIEU M. Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2000, 125(3): 359-369 DOI:10.1016/S0305-0491(99)00187-X |
CONOVER R J. Assimilation of organic matter by zooplankton. Limnology and Oceanography, 1966, 11(3): 338-345 DOI:10.4319/lo.1966.11.3.0338 |
DUCHEMIN M B, FOURNIER M, AUFFRET M. Seasonal variations of immune parameters in diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg). Aquaculture, 2007, 264(1/2/3/4): 73-81 |
GAGNAIRE B, SOLETCHNIK P, MADEC P, et al. Diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg), reared at two heights above sediment in Marennes-Oleron Basin, France: Difference in mortality, sexual maturation and hemocyte parameters. Aquaculture, 2006, 254(1/2/3/4): 606-616 |
GNAIGER E. Calculation of energetic and biochemical equivalents of respiratory oxygen consumption//Polarographic oxygen sensors: Aquatic and physiological applications. Berlin, Heidelberg: Springer, 1983, 337–345
|
GUO X, WANG Y, DEBROSSE G A, et al. Building a superior oyster for aquaculture. Jersey Shoreline, 2008, 25: 7-9 |
HAWKINS A J S, DAY A J, GERARD A, et al. A genetic and metabolic basis for faster growth among triploids induced by blocking meiosis Ⅰ but not meiosis Ⅱ in the larviparous European flat oyster, Ostrea edulis L. Journal of Experimental Marine Biology and Ecology, 1994, 184(1): 21-40 DOI:10.1016/0022-0981(94)90164-3 |
HAWKINS A J S, SMITH R F M, BAYNE B L, et al. Novel observations underlying the fast growth of suspension-feeding shellfish in turbid environments: Mytilus edulis. Marine Ecology Progress Series, 1996, 131: 179-190 DOI:10.3354/meps131179 |
HAWKINS A J S, MAGOULAS A, HERAL M, et al. Separate effects of triploidy, parentage and genomic diversity upon feeding behaviour, metabolic efficiency and net energy balance in the Pacific oyster Crassostrea gigas. Genetics Research, 2000, 76(3): 273-284 DOI:10.1017/S0016672300004766 |
HAURE J, FRANCOIS C, DEGREMONT L, et al. Physiological comparisons of Pacific cupped oysters at different levels of ploidy and selection to OsHV-1 tolerance. Aquaculture, 2021, 544: 737111 DOI:10.1016/j.aquaculture.2021.737111 |
HAURE J, FORTIN A, DUPUY B, et al. Etude comparative des caractéristiques écophysiologiques et des performances de croissance de l'huître creuse Crassostrea gigas diploïde et triploïde en milieu contrôlé. Rapport, Ifremer, Laboratoire Conchylicole des Pays de Loire, 2003, 34 |
HUO E Z, ZHANG W W, LI J Q, et al. Effects of sudden salinity drop on energy budget of Crassostrea chinensis and Crassostrea gigas. Progress in Fishery Sciences, 2021, 42(2): 132-138 [霍恩泽, 张雯雯, 李加琦, 等. 盐度骤降对近江牡蛎和长牡蛎能量收支的影响. 渔业科学进展, 2021, 42(2): 132-138] |
JIANG W W, FANG J G, JIANG Z J, et al. Effects of temperature stress on physiological and biochemical activities of Haliotis discus hannai. Journal of Fishery Sciences of China, 2017, 24(2): 220-230 [姜娓娓, 方建光, 蒋增杰, 等. 温度胁迫对皱纹盘鲍生理和生化活动的影响. 中国水产科学, 2017, 24(2): 220-230] |
KESARCODI-WATSON A, LUCAS J S, KLUMPP D W. Comparative feeding and physiological energetics of diploid and triploid Sydney rock oysters, Saccostrea commercialis: Ⅰ. Effects of oyster size. Aquaculture, 2001a, 203(1/2): 177-193 |
KESARCODI-WATSON A, KLUMPP D W, LUCAS J S. Comparative feeding and physiological energetics in diploid and triploid Sydney rock oysters (Saccostrea commercialis): Ⅱ. Influences of food concentration and tissue energy distribution. Aquaculture, 2001b, 203(1/2): 195-216 |
KONG L F, WANG S P, YU R H, et al. Comparative study on Biochemical composition and amino acid composition of diploid and triploid Crassostrea gigas before and after reproduction. Journal of Oceanology and Limnology, 2001(4): 44-49 [孔令锋, 王昭萍, 于瑞海, 等. 二倍体和三倍体太平洋牡蛎繁殖前后生化成分及氨基酸组成的比较研究. 海洋与湖沼, 2001(4): 44-49] |
KONG L F, WANG S P, YU R H, et al. Comparison of gill scanning electron microscopy between diploid and triploid Crassostrea giga. Journal of Zoology, 2003, 38(4): 2-5 [孔令锋, 王昭萍, 于瑞海, 等. 二倍体和三倍体太平洋牡蛎鳃扫描电镜的比较. 动物学杂志, 2003, 38(4): 2-5] |
LAING I, UTTING S D. The physiology and biochemistry of diploid and triploid Manila clam (Tapes philippinarum Adams & Reeve) larvae and juveniles. Journal of Experimental Marine Biology and Ecology, 1994, 184(2): 159-169 |
LIAN W, WEN H S, MAO Y Z, et al. Preliminary study on the relationship between summer death of Crassostrea gigas and culture environment and body constitution. Progress in Fishery Sciences, 2010, 31(4): 92-100 [廉伟, 温海深, 毛玉泽, 等. 长牡蛎夏季死亡与养殖环境及自身体质关系的初步研究. 渔业科学进展, 2010, 31(4): 92-100] |
LIN H, WANG X X, ZHANG B. Comparison of taste components between triploid and diploid oyster. Journal of Ocean University of Oingdao, 2002, 1(1): 55-58 |
LIN S H. Study on molecular mechanism of Haliotis discus hannai crease die in summer and response to high temperature stress. Master's Thesis of Graduate School of Chinese Academy of Sciences (Institute of Oceanography), 2016 [林思恒. 皱纹盘鲍度夏死亡与高温胁迫响应的分子机制研究. 中国科学院研究生院(海洋研究所)硕士研究生学位论文, 2016]
|
LI Y, QIN J G, LI X, et al. Spawning-dependent stress response to food deprivation in Pacific oyster Crassostrea gigas. Aquaculture, 2009, 286(3/4): 309-317 |
MACDONALD B A, BACON G S, WARD J E. Physiological responses of infaunal (Mya arenaria) and epifaunal (Placopecten magellanicus) bivalves to variations in the concentration and quality of suspended particles: Ⅱ. Absorption efficiency and scope for growth. Journal of Experimental Marine Biology and Ecology, 1998, 219(1/2): 127-141 |
MAO Y Z, ZHOU Y, YANG H S, et al. Study on the seasonal variation of metabolic rate and its relationship with summer death of Crassostrea gigas. Journal of Oceanology and Limnology, 2005(5): 445-451 [毛玉泽, 周毅, 杨红生, 等. 长牡蛎代谢率的季节变化及其与夏季死亡关系的探讨. 海洋与湖沼, 2005(5): 445-451] |
MIZUTA D D, WIKFORS G H, MESECK S L, et al. Use of natural trophic resources by Eastern oysters and Pacific oysters of different ploidy. Aquaculture and Fisheries, 2021, 6(1): 75-83 |
MAYZAUD P. Respiration and nitrogen excretion of zooplankton. Ⅱ. Studies of the metabolic characteristics of starved animals. Marine Biology, 1973, 21(1): 19-28 |
NELL J A, PERKINS B. Studies on triploid oysters in Australia: Farming potential of all-triploid Pacific oysters, Crassostrea gigas (Thunberg), in Port Stephens, New South Wales, Australia. Aquaculture Research, 2005, 36(6): 530-536 |
NORMAND J, ERNANDE B, HAURE J, et al. Reproductive effort and growth in Crassostrea gigas: Comparison of young diploid and triploid oysters issued from natural crosses or chemical induction. Aquatic Biology, 2009, 7(3): 229-241 |
NORMAND J, LE PENNEC M, BOUDRY P. Comparative histological study of gametogenesis in diploid and triploid Pacific oysters (Crassostrea gigas) reared in an estuarine farming site in France during the 2003 heatwave. Aquaculture, 2008, 282(1/2/3/4): 124-129 |
OJEA J, PAZOS A J, MARTINEZ D, et al. Seasonal variation in weight and biochemical composition of the tissues of Ruditapes decussatus in relation to the gametogenic cycle. Aquaculture, 2004, 238(1/2/3/4): 451-468 |
OSTERHELD K, DAVIDSON J, COMEAU L A, et al. Triploidy in Mytilus edulis impacts the mechanical properties of byssal threads. Aquaculture, 2023, 566: 739191 |
QIN Y, ZHANG Y, MO R, et al. Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oysters Crassostrea hongkongensis in southern China. Aquaculture, 2019, 507: 108-118 |
REN L H, ZHANG J H, FANG J G, et al. Effects of calcification on respiratory entropy of cultured Crassostrea gigas and its attached organismsv. Journal of Applied Ecology, 2014, 25(6): 1785-1790 [任黎华, 张继红, 方建光, 等. 钙化作用对养殖长牡蛎及其附着生物呼吸熵的影响. 应用生态学报, 2014, 25(6): 1785-1790] |
ROYER J, ROPERT M, COSTIL K. Spatio-temporal changes in mortality, growth and condition of the Pacific oyster, Crassostrea gigas, in Normandy (France). Journal of Shellfish Research, 2007, 26(4): 973-984 |
RUIZ-VERDUGO C A, RAMIREZ J L, ALLEN J S K, et al. Triploid Catarina scallop (Argopecten ventricosus Sowerby Ⅱ, 1842): growth, gametogenesis, and suppression of functional hermaphroditism. Aquaculture, 2000, 186(1/2): 13-32 |
SHPIGEL M, BARBER B J, MANN R. Effects of elevated temperature on growth, gametogenesis, physiology, and biochemical composition in diploid and triploid Pacific oysters, Crassostrea gigas Thunberg. Journal of experimental Marine Biology and Ecology, 1992, 161(1): 15-25 |
SLOBODKIN L B, RICHMAN S. Calories/gm. in species of animals. Nature, 1961, 191(4785): 299-299 |
SOLETCHNIK P, ROPERT M, MAZURIE J, et al. Relationships between oyster mortality patterns and environmental data from monitoring databases along the coasts of France. Aquaculture, 2007, 271(1/2/3/4): 384-400 |
SUI X L, SUN J W, WANG F G, et al. Analysis of the causes of death of a large number of Crassostrea gigas the coast of Dalian. Journal of Dalian Fisheries University, 2002(4): 272-278 [隋锡林, 孙景伟, 王富贵, 等. 大连沿海太平洋牡蛎大量死亡原因解析. 大连水产学院学报, 2002(4): 272-278] |
TREMBLAY R, LANDRY T. The implication of metabolic performance of Mytilus edulis, Mytilus trossulus, and hybrids for mussel aquaculture in eastern Canadian waters. Marine Biology Aquaculte, 2016, 2(1): 1-7 |
WANG X Q, RASTRICK S P S, WU Y L, et al. Impact of seawater acidification on the energy budget of Mytilus galloprovincialis. Progress in Fishery Sciences, 2019, 40(3): 21-30 [王晓芹, Samuel P. S. Rastrick, 吴亚林, 等. 海水酸化胁迫对紫贻贝能量分配的影响. 渔业科学进展, 2019, 40(3): 21-30] |
WANG C, LI Y M, WANG Z Z, et al. Temperature, salinity and the influence of the quality of Anadara uropygimelana filtering rate. Aquatic Science and Technology Information, 2020, 47(6): 301-305 [王冲, 李永明, 王志忠, 等. 温度、盐度和体质量对魁蚶滤水率的影响. 水产科技情报, 2020, 47(6): 301-305] |
WANG R C, WANG Z P. Science of marine shellfish culture. Qingdao: China Ocean University Press, 2008: 125-126 [王如才, 王昭萍. 海水贝类养殖学. 青岛: 中国海洋大学出版社, 2008: 125-126]
|
WANG Z P, LI Y, YU R H, et al. Study on growth of triploid oyster during breeding season. Journal of Ocean University of Qingdao (Natural Science), 2002(5): 701-706 [王昭萍, 李赟, 于瑞海, 等. 三倍体牡蛎在繁殖季节的生长研究. 青岛海洋大学学报(自然科学版), 2002(5): 701-706] |
WANG Y J, LI L S, HU M H, et al. Physiological energetics of the thick shell mussel Mytilus coruscus, exposed to seawater acidification and thermal stress. Science of the Total Environment, 2015, 514: 261-272 |
WEI Y H, PAN Y, LIU S, et al. Seasonal differences in resistance gene expression and living environment of Crassostrea gigas of Rushan. Marine bulletin, 2020, 39(5): 601-608 [魏钰恒, 潘英, 刘圣, 等. 乳山长牡蛎的抗性基因表达和生存环境的季节差异. 海洋通报, 2020, 39(5): 601-608] |
WIDDOWS J, JOHNSON D. Physiological energetics of Mytilus edulis: Scope for growth. Marine Ecology Progress Series, 1988, 113-121 |
WIDDOWS J, BAYNE B L, LIVINGSTONE D R, et al. Physiological and biochemical responses of bivalve molluscs to exposure to air. Comparative Biochemistry and Physiology Part A: Physiology, 1979, 62(2): 301-308 |
ZENG Z N, LIN Q, WU J S, et al. Annual changes in meat weight and biochemical composition of triploid and diploid Crassostrea gigas. Marine Science, 1999(5): 54-56 [曾志南, 林琪, 吴建绍, 等. 三倍体和二倍体太平洋牡蛎肉重和生化成分的周年变化. 海洋科学, 1999(5): 54-56] |
ZHANG G F, WANG Z C, CHANG Y Q, et al. Study on floating raft culture technology of triploid Crassostrea gigas. Journal of Fishery Sciences of China, 2000(1): 68-72 [张国范, 王子臣, 常亚青, 等. 三倍体长牡蛎浮筏养殖技术的研究. 中国水产科学, 2000(1): 68-72] |
ZHOU Q S, REN X Y, XU Y, et al. Effects of ammonia nitrogen stress on metabolic enzyme and antioxidant enzyme activities of Lobsterus fortune. Progress in Fishery Sciences, 2022, 43(2): 147-156 [周钱森, 任宪云, 徐垚, 等. 氨氮胁迫对锦绣龙虾代谢酶和抗氧化酶活力的影响. 渔业科学进展, 2022, 43(2): 147-156] |
ZHOU J C, CAI L, YANG J R, et al. Effects of temperature and ammonia nitrogen on oxygen consumption rate and ammonia expulsion rate of Red Chelicarten with different sizes. Progress in Fishery Sciences, 2022, 43(3): 95-102 [周建聪, 蔡利, 杨静茹, 等. 温度和氨氮对不同规格红螯螯虾耗氧率与排氨率的影响. 渔业科学进展, 2022, 43(3): 95-102] |
ZHOU Y B, SONG J, LI X Y, et al. Comparison of bioenergetics between triploid and diploid at Crassostrea gigas different temperatures. Journal of Fisheries of China, 2000(6): 504-509 [周一兵, 宋坚, 李晓艳, 等. 不同温度下太平洋牡蛎三倍体和二倍体生物能量学比较. 水产学报, 2000(6): 504-509] |
ZHANG Y, FANG J G, MAO Y Z, et al. Effects of water temperature, salinity and feed density on the leachability of Olive granarca granosa. Marine Science, 2015, 39(9): 39-43 [张媛, 方建光, 毛玉泽, 等. 水温、盐度和饵料密度对橄榄蚶滤水率的影响. 海洋科学, 2015, 39(9): 39-43] |



