2. 农业部水产品质量安全检测与评价重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 山东省海洋生态修复重点实验室 山东省海洋资源与环境研究院 山东 烟台 264006;
4. 中国海洋大学食品科学与工程学院 山东 青岛 266003;
5. 海水养殖生物育种与可持续产出全国重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071
2. Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Affairs, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China;
3. Shandong Key Laboratory of Marine Ecological Restoration, Shandong Marine Resources and Environment Research Institute, Yantai 264006, China;
4. College of Food Science and Engineering, Ocean University of China, Qingdao, 266003, China;
5. State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
麻痹性贝毒(paralytic shellfish toxins, PSTs)是一类急性神经毒素,具有近60种同系物(Qiu et al, 2018),根据R4位点取代基的差异,可将PSTs分为3类:氨基甲酸酯类毒素(STX、NEO、GTX1&4和GTX2&3)、脱氨甲酰基类毒素(dcSTX、dcNEO、dcGTX1&4和dcGTX2&3),N-磺酰氨甲酰基类毒素(C1&2、C3&4、GTX5和GTX6)。PSTs主要由海洋甲藻和淡水蓝绿藻产生,其中,海洋甲藻包括亚历山大藻属(Alexandrium)多个物种、链状裸甲藻(Gymnodinium catenatum)、巴哈马梨甲藻(Pyrodinium bahamense)(Yu et al, 2021)。双壳贝类滤食产毒藻后,造成PSTs在体内积累,食用有毒贝类对人体健康造成巨大威胁。PSTs中毒后会出现头晕、恶心、肌肉麻痹、呼吸困难等症状,甚至死亡(Liu et al, 2017)。据估计,全球每年约发生2 000起PSTs中毒事件(Wang et al, 2016; Brown et al, 2019)。我国是世界上最大的水产品养殖国家,2022年贝类产量约为1589万t (农业农村部渔业渔政管理局等, 2023),同时也有庞大的贝类消费者。近几年因食用有毒贝类造成的中毒事件频发,例如福建贻贝中毒事件和香港虾夷扇贝中毒事件等(梁玉波等, 2019)。我国PSTs中毒事件发生呈现出分布区域广、区域性风险反复、产毒藻多样性、季节性差异明显等特点,可造成多人出现急性中毒症状,甚至死亡,致死率较高,不仅严重威胁我国消费者健康,也不利于地区的社会稳定(包振民等, 2021; 范礼强等, 2021)。
河北省位于我国华北地区,海岸线长484 km,具有丰富的渔业资源,其中,2022年海水养殖产量约为58万t (农业农村部渔业渔政管理局等, 2023)。近年来,河北近岸尤其是秦皇岛沿岸,多次在双壳贝类中检测到PSTs。其中,2016年秦皇岛发生严重的贝类中毒事件,毒素含量超出安全限量标准800 μg STXeq/kg的65倍,引起该中毒事件的主要肇事藻种为链状亚历山大藻(Alexandrium catenella)(Yu et al, 2021)。Wu等(2022)研究发现,近5年来,每年4月份秦皇岛地区易发生链状亚历山大藻大量繁殖,并在贻贝、扇贝中多次检出PSTs。Cao等(2023)研究表明,2021年5月和2022年5月秦皇岛地区紫贻贝(Mytilus galloprovincialis)、栉孔扇贝(Azumapecten farreri)和脉红螺(Rapana venosa)样品均检出PSTs。
目前,对河北省PSTs污染情况调查主要集中在秦皇岛山海关海域,且主要是贻贝和扇贝样品,还缺少对河北省近岸其他城市以及多种不同贝类样品的调查,风险评估研究也较少(Liu et al, 2017; 郑旭颖等, 2023)。本研究在2022年对唐山和秦皇岛两地多个海域采集的贻贝、扇贝、牡蛎、蚶类、蛤类5类6个品种贝类进行连续PSTs调查,通过液相色谱-质谱联用法(LC-MS/MS)检测,探究河北省近岸PSTs污染特征以及对消费者的危害,旨在为我国贝类毒素监管和食用安全提供基础数据。
1 材料与方法 1.1 样品采集2022年3—6月份在秦皇岛山海关海域(39.93°~ 39.96°N, 119.77°~119.81°E)(39个贝样)、北戴河海域(39.74°~39.84°N, 119.40°~119.54°E)(37个贝样)、南戴河海域(39.78°~39.79°N, 119.44°~119.51°E) (3个贝样)和河北唐山曹妃甸海域(39.02°~39.97°N, 118.33°~ 118.62°E)(24个贝样) 4个海域自行网捕采集5类6个品种贝类样品,包括紫贻贝、海湾扇贝(Argopecten irradians)、太平洋牡蛎(Crassostrea gigas)、毛蚶(Scapharca subcrenata)、魁蚶(Scapharca broughtonii)和菲律宾蛤仔(Ruditapes philippinarum),共103个样品,依照《水产品抽样规范》(GB/T 30891-2014)要求进行贝类样品采样,贻贝、扇贝、蚶类和蛤类样品质量不少于3 kg,牡蛎样品质量不少于5 kg。去除贝壳后,能够用于检测的样品量不少于700 g。采集样品后在0~4 ℃冷藏情况下运至实验室后,于–20 ℃保存待测。
1.2 实验材料AB-5500 QTRAP液相色谱–四极杆/离子阱复合质谱(AB SCIEX公司,美国),配有电喷雾离子源(ESI);Himac CR 22GⅡ高速离心机(Hitachi公司,日本);XW-80A旋涡混合器(上海医大仪器厂);恒温水浴锅(上海蓝凯仪器仪表有限公司);Milli-Q超纯水仪(Millipore公司,美国);固相萃取装置(Supelco公司,美国)。
甲酸、甲酸铵(色谱级,瑞士Fluka公司);乙酸(色谱级,Thermo公司,美国);乙腈(质谱级,Merck公司,美国);超纯水(18.2 MΩ cm);氨水(色谱级,Sigma公司,美国);石墨化碳黑固相萃取柱(ENVI-CarbTM,250 mg/3 mL,Supelco公司,美国);其他未作特殊说明试剂均为分析纯。
毒素标准溶液(STX、NEO、dcSTX、dcNEO、dcGTX2&3、GTX1&4、GTX2&3、GTX5和C1&2)购自加拿大国家研究理事会海洋生物研究所。
1.3 毒素检测 1.3.1 贝类样品前处理在室温下,使冷冻的样品呈半冷冻状态,用清水将贝壳外表洗净后去壳匀浆,准确称取(5.00±0.05) g试样于50 mL离心管中,加入5 mL 1%乙酸水溶液,涡旋混合90 s。将样品置于沸水中煮沸5 min,取出置于流水下迅速冷却至室温。4 500 r/min离心10 min,移取上清液于10 mL容量瓶中,向残渣中加入3 mL 1%乙酸水溶液,充分均匀后,4 500 r/min离心合并上清液,定容10 mL。移取上述提取液1 mL于2 mL离心管中,加入5 μL氨水,涡旋混匀,10 000 r/min离心5 min。依次用3 mL乙腈、3 mL 20%乙腈水溶液、3 mL 0.1%氨水溶液活化Supelco ENVI-Carb固相萃取柱,加入500 μL提取液,再用700 μL超纯水淋洗,正压挤干,最后用1 mL 75%乙腈水溶液洗脱混匀,定容1 mL,过0.22 μm滤膜于进样小瓶中,4 ℃下保存,供LC-MS/MS分析。
1.3.2 LC-MS/MS分析液相色谱柱为TSK- Amide-80柱(2.0 mm×15.0 cm,3 μm);进样量为5 μL,柱温40 ℃;流动相A为含2 mmol/L甲酸铵、50 mmol/L甲酸的水溶液;流动相B为含2 mmol/L甲酸铵、50 mmol/L甲酸的95%乙腈溶液。梯度洗脱条件参考张海涛等(2023):0~3.0 min,80% B;3.1~5.0 min,80%~40% B;5.1~10.0 min,40% B;10.1~11.0 min,40%~80% B;11.1~13.0 min,80% B;流速:0.35 mL/min。采用多反应监测(MRM)检测方式,离子源温度为550 ℃,喷雾电压为5 000 V、–4 500 V,气帘气压力为20 psi,雾化气压力为30 psi,辅助加热器压力为30 psi,碰撞气Medium。目标化合物质谱条件参数见表 1。
|
|
表 1 目标化合物质谱条件参数 Tab.1 Parameters of MS conditions for target compounds |
| $ \begin{array}{l} \text{PSTs} 膳食暴露量(\mathtt{μ} \mathrm{g} \text { STXeq/kg b.w./d) }= \\ \frac{\text { 贝类中PSTs浓度 }(\mathtt{μ} \mathrm{g} \text { STXeq} / \mathrm{kg}) \times \text { 贝类消费量 }(\mathrm{g} / \mathrm{d})}{\text { 体重 }(\mathrm{kg})} \times 10^{-3} \end{array} $ |
风险评估中通过替代方法进行左删失数据处理,当PSTs组分含量低于检出限时,左删失数据处理可以定量组分含量,不会产生大量删失数据,从而保证急性暴露评估信息的完整性。在下界(lower bound, LB)方案中,将所有低于检出限浓度设为0,而在上界(upper bound, UB)方案中,将低于检出限浓度设为每种毒素的1/2检出限(LOD/2)。在急性暴露评估的情况下,本研究使用获得的最大毒素水平。
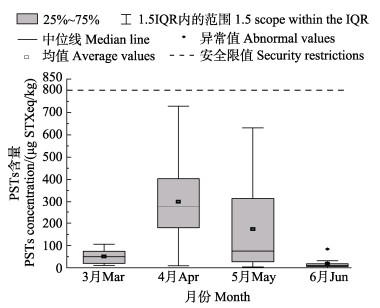
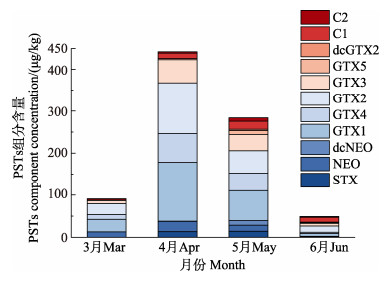
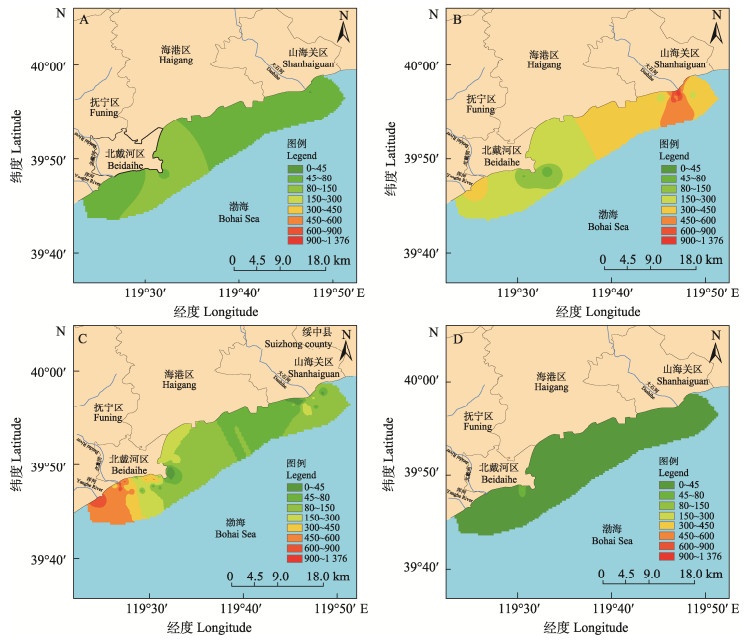
2 结果 2.1 3—6月贝类样品PSTs组分与含量分析在采集的103份贝类样品中,全部检出PSTs,检出率为100%,均未超过欧盟限量标准,3—6月份贝类样品的均值中,4月份均值含量最高(300.2 μg STXeq/kg)、其次5月、3月,6月均值含量最低,仅为18.4 μg STXeq/kg (图 1)。3—6月贝类样品检出的主要组分为GTX1&4和GTX2&3(图 2)。4月检出的含量最高,其中,GTX1含量为140.2 μg/kg、GTX4含量为68.6 μg/kg、GTX2含量为120.1 μg/kg、GTX3含量为55.8 μg/kg。除3月外,4–6月均有高毒性的STX、dcNEO检出。5–6月检出了3、4月未检出的dcGTX2。秦皇岛附近海域比唐山附近海域污染严重(唐山附近海域3–6月PSTs均值含量变化不明显,未放图),秦皇岛附近海域3—6月PSTs含量分布如图 3。
|
图 1 2022年3—6月份贝类样品中PSTs平均含量对比 Fig.1 Comparison of average PSTs concentration in shellfish samples from March to June, 2022 |
|
图 2 2022年3—6月份贝类样品中PSTs组分与含量 Fig.2 Components and concentration of PSTs in shellfish samples from March to June, 2022 |
|
图 3 2022年秦皇岛贝类样品PSTs污染分布 Fig.3 Distribution of PSTs contamination in shellfish samples from Qinhuangdao, 2022 A、B、C、D分别为河北省近岸秦皇岛附近海域3月、4月、5月和6月PSTs污染分布。 A, B, C, and D were the distribution of PSTs in the sea area near Qinhuangdao in the nearshore of Hebei Province in March, April, May and June, respectively. |
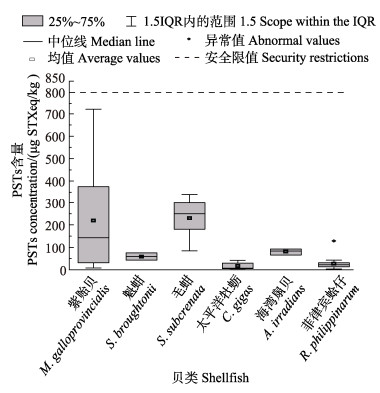
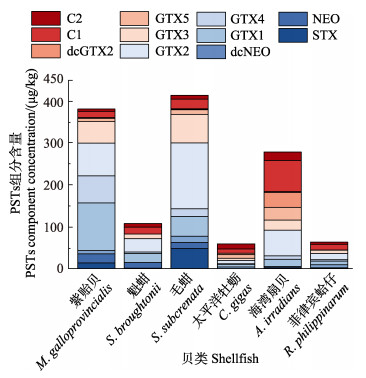
在采集的贝类样品中,不同品种的贝类样品PSTs均值含量差异明显(图 4)。紫贻贝均值含量最高为250.8 μg STXeq/kg,其次为毛蚶、海湾扇贝、魁蚶、菲律宾蛤仔和太平洋牡蛎,含量分别为229.9、80.7、59.1、27.3和17.6 μg STXeq/kg。
|
图 4 不同品种贝类样品PSTs平均含量对比 Fig.4 Comparison of average PSTs concentration in different species of shellfish samples |
6种贝类蓄积的11种PSTs组分存在较大差别(图 5),其中,GTX1-4和C1&2为6种贝类共同的检出组分,GTX1&4最高含量在紫贻贝样品检出,GTX1为114.7 μg/kg,GTX4为63.8 μg/kg;GTX2&3最高含量在毛蚶样品检出,GTX2为155.1 μg/kg,GTX3为70.5 μg/kg;C1&2最高含量在海湾扇贝样品检出,C1为73.1 μg/kg,C2为20.3 μg/kg。样品中高毒性的STX、NEO检出量较少,除紫贻贝样品和毛蚶样品中11种PSTs均有检出外,其余4种贝类样品仅检出部分组分,如魁蚶样品中未检出dcNEO、GTX5和dcGTX2,太平洋牡蛎样品中未检出STX和NEO,菲律宾蛤仔样品中未检出GTX5和dcGTX2。
|
图 5 不同品种贝类样品PSTs组分与含量 Fig.5 Components and concentration of PSTs in different species of shellfish samples |
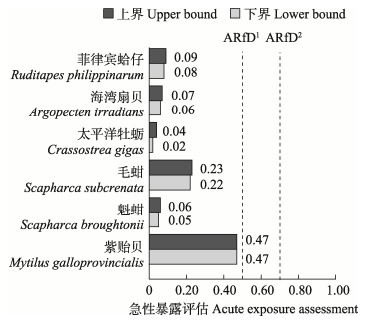
近年来,河北省近岸贝类中PSTs最高含量如表 2所示,其中,2018年为 > 4 000 μg STXeq/kg,2019年为985 μg STXeq/kg,2020年为9 147 μg STXeq/kg,2021年为1 200 μg STXeq/kg。为保护消费者健康,评估膳食暴露风险,本研究采用PSTs最大值进行急性暴露评估(Shin et al, 2018; Zhou et al, 2022)(表 3),基于PSTs的危险特征,FAO/IOC/WHO推荐最低观察水平(LOAEL)PSTs参考值为2.0 μg/kg b.w.,使用安全系数3得出急性参考剂量(ARfD) 0.7 μg/kg b.w.。欧洲食品安全局(EFSA)规定ARfD为0.5 μg/kg b.w.,观察到的LOAEL为1.5 μg/kg b.w.。两种急性参考剂量均可作为急性膳食暴露参考限值(Zhou et al, 2022)。本研究以成年人均体重60 kg、我国人均每日水产品食用量38.9 g (Guo et al, 2023)进行急性暴露评估,6种贝类急性暴露评估结果见图 6。根据贝类样品最大值的下界数据和上界数据,得出的急性暴露评估结果均未超过FAO/IOC/WHO和EFSA推荐的ARfD值,处于安全可接受状态。
|
|
表 2 不同年份河北近岸贝类样品PSTs含量 Tab.2 Concentration of PSTs in nearshore shellfish samples from Hebei in different years |
|
|
表 3 不同贝类样品PSTs含量 Tab.3 Concentration of PSTs in different shellfish samples |
|
图 6 不同品种贝类样品急性暴露评估数据
Fig.6 Acute exposure assessment data in different species of shellfish samples
ARfD1: 0.5 μg STXeq/kg b.w. (EFSA); ARfD2: 0.7 μg STXeq/kg b.w.(FAO/IOC/WHO)。 |
3—6月之间贝类样品中PSTs均值含量存在较大差异,贝类样品均值含量呈先上升、后下降趋势,4月PSTs均值含量最高,为300.2 μg STXeq/kg。Tang等(2022)研究表明,3—6月秦皇岛海域贝类样品中4月份PSTs风险最高,与本研究结果一致。温度可能是影响不同月份贝类样品PSTs含量差异的主要原因。研究表明,秦皇岛海域PSTs产毒藻主要为链状亚历山大藻(Yu et al, 2021),链状亚历山大藻最适生长温度为10~13 ℃(Tobin et al, 2019),而根据中国近海海洋气候检测月报数据,秦皇岛海域4月温度会升高至4~9.5 ℃。Natsuike等(2017)研究表明,亚历山大藻在水温接近最适生长温度即可大量繁殖,从而可能导致链状亚历山大藻细胞数量在4月达到峰值。此外,Wu等(2022)研究也表明,秦皇岛近海海域链状亚历山大藻在4月16日达到高峰期,5月藻类急剧减少,5月中旬浮游植物PSTs含量降低至0。根据中国近海海洋气候检测月报数据显示,秦皇岛海域在2022年3月温度仅为1.5~4.5 ℃,而5月秦皇岛海水温度却已达到17~18 ℃(Cao et al, 2023),不适合链状亚历山大藻生存,导致链状亚历山大藻大量减少(Miyazono et al, 2012),最终链状亚历山大藻形成休眠包囊,沉积于海水底部。
3.2 不同品种贝类样品PSTs组分含量差异双壳贝类是PSTs的主要水生动物载体,本研究发现,紫贻贝和毛蚶的PSTs均值含量远高于海湾扇贝、魁蚶、菲律宾蛤仔和太平洋牡蛎。Liu等(2017)研究表明,紫贻贝和毛蚶均值含量高于太平洋牡蛎,与本研究结论相符。首先,不同贝类蓄积速度存在差异可能是影响贝类样品PSTs含量差异的原因。Tang等(2022)研究表明,扇贝与贻贝相比,扇贝积累毒素的速度低得多,PSTs含量和毒性水平低于贻贝。此外,也有研究发现,与贻贝相比,牡蛎和菲律宾蛤仔蓄积量少(Samsur et al, 2006)。STX含量升高后,贻贝中的过氧化氢酶和铁蛋白等转录水平升高,这可能是贻贝能够快速蓄积STX的保护机制(Núñez-Acuña et al, 2013; Detree et al, 2016)。其次,不同贝类品种摄食产毒藻细胞和吸收速度可能是影响含量差异的另外一个原因,与菲律宾蛤仔相比,紫贻贝具有更快的摄食速率,并且菲律宾蛤仔吸收速率低,在摄入大量藻细胞后菲律宾蛤仔会明显产生假性粪便(Li et al, 2001; 包振民等, 2021)。最后,不同贝类PSTs的来源也会导致含量差异,毛蚶这类底栖贝类可以积累来自底栖亚历山大藻包囊的毒素,包囊中的毒素含量比营养细胞高达6倍,约有28.9%~35.2%的包囊被底栖贝类摄食(Oshima et al, 1992; Tsujino et al, 2002),这可能是其PSTs毒素含量较高的原因之一(Wang et al, 2004; Liu et al, 2017)。
贝类中蓄积的PSTs主要来源于摄入的产毒藻细胞,贝类中的毒素转化也会明显改变毒素组成(Raposo et al, 2020),这是造成不同贝类中毒素组分含量差异较大的重要原因。双壳贝类体内的酶和细菌可促进不同结构、不同种类的PSTs发生转化,从而导致贝类体内PSTs含量和组分不同(宋维佳等, 2022)。如在贝类体内可发生C1→GTX2,C2→GTX3,使低毒性的N-磺酰氨甲酰基类毒素转化为高毒性的氨基甲酸酯类毒素(Ding et al, 2017)。贝类中产生的C1&2是由摄入产N-磺基氨甲酰基毒素的亚历山大藻属造成的(Gao et al, 2015)。Kim等(2015)研究表明,随着紫贻贝在塔玛亚历山大藻(Alexandrium tamarense)中暴露时间的延长,通过酶促紫贻贝中的C1和GTX1,逐渐转化为C2和GTX4。与扇贝和牡蛎相比,贻贝的PSTs代谢转化率低(Botelho et al, 2020),从而使紫贻贝与扇贝、牡蛎中的毒素组分含量出现差异。林卓如等(2022)研究表明,毛蚶体内缺乏磺胺甲酰基类水解酶和氨甲酰基水解酶,无法进行相应转化反应,同时,在毛蚶体内没有明显的β型毒素通过差向异构化转化为α型毒素,从而导致毛蚶与其他贝类之间在组分含量上存在差异。
3.3 贝类食用安全评估随着河北省入海河流全流域系统治理、入海排污口分类治理和强力推动海洋垃圾污染防治等措施的落实,河北省海洋生态环境逐渐变好(刘明等, 2018)。自2020年后,河北近岸PSTs最高含量呈降低趋势,本研究2022年贝类样品PSTs最高含量未超过安全限量标准。根据不同品种的贝类样品PSTs含量,对河北近岸居民进行膳食暴露评估,6种贝类未超过ARfD,处于安全可接受状态,其中,紫贻贝膳食暴露风险最高,存在部分残留,大量摄入紫贻贝后可能会出现头晕、恶心等PSTs中毒症状。对于紫贻贝、毛蚶等膳食暴露评估数值较大的贝类,应加强关注。考虑到PSTs对不同人群的不同影响,应更多关注水产养殖户和海岛居民,他们的水产品膳食摄入量要高于普通人群,存在更高的食用风险(Wu et al, 2014)。
4 结论本研究通过对河北省近岸贝类中PSTs调查,结果发现3—6月贝类样品均有PSTs检出,4月均值含量最高,其次为5月和3月,6月份均值含量最低;另外贝类样品检出的主要组分为GTX1&4和GTX2&3。对不同品种贝类PSTs含量的研究显示,紫贻贝均值含量最高,其次为毛蚶、海湾扇贝、魁蚶、菲律宾蛤仔和太平洋牡蛎;6种贝类蓄积的PSTs组分中,GTX1~4、C1&2为共同的检出组分。对河北近岸居民进行膳食暴露评估,结果显示毒素风险均处于安全可接受状态。但由于对河北省近岸不同城市以及多种不同贝类样品的调查较少,后续还需持续进行调查研究。
BAO Z M, KONG L L, SHI J X, et al. Research progress on accumulation and transformation of paralytic shellfish toxins in bivalves. Periodical of Ocean University of China, 2021, 51(10): 1-11 [包振民, 孔令玲, 史姣霞, 等. 双壳贝类积累转化麻痹性贝毒的研究进展. 中国海洋大学学报(自然科学版), 2021, 51(10): 1-11] |
BOTELHO M J, MARQUES F, FREITAS R, et al. Paralytic shellfish toxin profiles in mussel, cockle and razor shell under post-bloom natural conditions: Evidence of higher biotransformation in razor shells and cockles. Marine Environmental Research, 2020, 154: 104839 DOI:10.1016/j.marenvres.2019.104839 |
BROWN A R, LILLEY M, SHUTLER J, et al. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Reviews in Aquaculture, 2019, 12(3): 1663-1688 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2023. Beijing: China Agriculture Press, 2023 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2023中国渔业统计年鉴. 北京: 中国农业出版社, 2023]
|
CAO Y D, QIU J B, LI A F, et al. Occurrence and spatial distribution of paralytic shellfish toxins in seawater and marine organisms in the coastal waters of Qinhuangdao, China. Chemosphere, 2023, 315: 137746 DOI:10.1016/j.chemosphere.2023.137746 |
DETREE C, NÚÑEZ-ACUÑA G, ROBERTS S, et al. Uncovering the complex transcriptome response of Mytilus chilensis against saxitoxin: Implications of harmful algal blooms on mussel populations. PLoS One, 2016, 11(10): e165231 |
DING L, QIU J B, LI A F. Proposed biotransformation pathways for new metabolites of paralytic shellfish toxins based on field and experimental mussel samples. Journal of Agricultural and Food Chemistry, 2017, 65(27): 5494-5502 DOI:10.1021/acs.jafc.7b02101 |
FAN L Q, ZHENG G C, WU H Y, et al. Research progress on the accumulation and metabolism of paralytic shellfish toxin in mussels. Marine Sciences, 2021, 45(4): 201-212 [贻贝对麻痹性贝类毒素的蓄积代谢研究进展. 海洋科学, 2021, 45(4): 201-212] |
GAO Y, YU R C, CHEN J H, et al. Distribution of Alexandrium fundyense and A. pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea. Marine Pollution Bulletin, 2015, 96(1/2): 210-219 |
GUO M M, WU F, GENG Q Q, et al. Perfluoroalkyl substances (PFASs) in aquatic products from the Yellow-Bohai Sea coasts, China: Concentrations and profiles across species and regions. Environmental Pollution, 2023, 327: 121514 DOI:10.1016/j.envpol.2023.121514 |
KIM H Y, SHIN I S. Comparison of paralytic shellfish toxin profiles of Alexandrium tamarense and blue mussel (Mytilus edulis) in Korea. Food Science and Biotechnology, 2015, 24(2): 751-756 DOI:10.1007/s10068-015-0097-9 |
LI S C, WANG W X, Hsieh D P H. Feeding and absorption of the toxic dinoflagellate Alexandrium tamarense by two marine bivalves from the South China Sea. Marine Biology, 2001, 139(4): 617-624 DOI:10.1007/s002270100613 |
LIANG Y B, LI D M, YAO J Y, et al. Progresses in investigation and research on phycotoxins and toxic microalgaes in the coastal waters of China. Oceanologia et Limnologia Sinica, 2019, 50(3): 511-524 [中国近海藻毒素及有毒微藻产毒原因种调查研究进展. 海洋与湖沼, 2019, 50(3): 511-524] |
LIN Z R, GENG H X, TANG W J, et al. Biotransformation of paralytic shellfish toxins in blood clam Scapharca subcrenata. Oceanologia et Limnologia Sinica, 2022, 53(5): 1131-1142 [麻痹性贝毒在毛蚶体内的转化过程研究. 海洋与湖沼, 2022, 53(5): 1131-1142] |
LIU M, YIN F, WANG X L. Consideration on strategic positioning of marine economy development in Qinhuangdao. Journal of Environmental Management College of China, 2018, 28(5): 55-58 [秦皇岛市海洋经济发展战略定位的思考. 中国环境管理干部学院学报, 2018, 28(5): 55-58] |
LIU Y, YU R C, KONG F Z, et al. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Marine Pollution Bulletin, 2017, 115(1/2): 324-331 |
MIYAZONO A, NAGAI S, KUDO I, et al. Viability of Alexandrium tamarense cysts in the sediment of Funka Bay, Hokkaido, Japan: Over a hundred year survival times for cysts. Harmful Algae, 2012, 16: 81-88 DOI:10.1016/j.hal.2012.02.001 |
NATSUIKE M, YOKOYAMA K, NISHITANI G Y, et al. Germination fluctuation of toxic Alexandrium fundyense and A. pacificum cysts and the relationship with bloom occurrences in Kesennuma Bay, Japan. Harmful Algae, 2017, 62: 52-59 DOI:10.1016/j.hal.2016.11.018 |
NÚÑEZ-ACUÑA G, ABALLAY A E, HÉGARET H, et al. Transcriptional responses of Mytilus chilensis exposed in vivo to saxitoxin (STX). Journal of Molluscan Studies, 2013, 79(4): 323-331 DOI:10.1093/mollus/eyt030 |
OSHIMA Y, BOLCH C J, HALLEGRAEFF G M. Toxin composition of resting cysts of Alexandrium tamarense (Dinophyceae). Toxicon, 1992, 30(12): 1539-1544 DOI:10.1016/0041-0101(92)90025-Z |
QIU J B, MENG F P, DING L, et al. Dynamics of paralytic shellfish toxins and their metabolites during timecourse exposure of scallops Chlamys farreri and mussels Mytilus galloprovincialis to Alexandrium pacificum. Aquatic Toxicology, 2018, 200: 233-240 DOI:10.1016/j.aquatox.2018.05.003 |
RAPOSO M I C, GOMES M T S R, BOTELHO M J, et al. Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxicon, 2020, 12(5): 344 |
SAMSUR M, YAMAGUCHI Y, SAGARA T, et al. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella. Toxicon, 2006, 48: 323-330 DOI:10.1016/j.toxicon.2006.06.002 |
SHIN C, JO H, KIM S, et al. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Research International, 2018, 108: 274-279 DOI:10.1016/j.foodres.2018.03.061 |
SONG W J, SONG X X, YU Z M, et al. Research progress on the biosynthesis, transformation, and factors affecting paralytic shellfish toxins in the ocean. Marine Sciences, 2022, 46(9): 117-129 [海洋中麻痹性贝类毒素的合成转化及其影响因素研究进展. 海洋科学, 2022, 46(9): 117-129] |
TANG W J, LIN Z R, ZHANG Q C, et al. An investigation on bloom dynamics of Alexandrium catenella and A. pacificum and toxin accumulation in shellfish along the coast of Qinhuangdao, China. Marine Pollution Bulletin, 2022, 183: 114058 DOI:10.1016/j.marpolbul.2022.114058 |
TOBIN E D, WALLACE C L, CRUMPTON C, et al. Environmental drivers of paralytic shellfish toxin producing Alexandrium catenella blooms in a fjord system of northern Southeast Alaska. Harmful Algae, 2019, 88: 101659 DOI:10.1016/j.hal.2019.101659 |
TSUJINO M, KAMIYAMA T, UCHIDA T, et al. Abundance and germination capability of resting cysts of Alexandrium spp. (Dinophyceae) from faecal pellets of macrobenthic organisms. Journal of Experimental Marine Biology and Ecology, 2002, 271(1): 1-7 |
WANG D Z, ZHANG S F, ZHANG Y, et al. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. Journal of Proteomics, 2016, 135: 132-140 DOI:10.1016/j.jprot.2015.08.008 |
WANG Z H, MATSUOKA K, QI Y Z, et al. Dinoflagellate cysts in recent sediments from Chinese coastal waters. Marine Ecology, 2004, 25(4): 289-311 DOI:10.1111/j.1439-0485.2004.00035.x |
WU H Y, DONG C F, ZHENG G C, et al. Formation mechanism and environmental drivers of Alexandrium catenella bloom events in the coastal waters of Qinhuangdao, China. Environmental Pollution, 2022, 313: 120241 DOI:10.1016/j.envpol.2022.120241 |
WU H Y, ZHANG F, DONG C F, et al. Variations in the toxicity and condition index of five bivalve species throughout a red tide event caused by Alexandrium catenella: A field study. Environmental Research, 2022, 215: 114327 DOI:10.1016/j.envres.2022.114327 |
WU X, GAO M, WANG L, et al. The arsenic content in marketed seafood and associated health risks for the residents of Shandong, China. Ecotoxicology and Environmental Safety, 2014, 102: 168-173 DOI:10.1016/j.ecoenv.2014.01.028 |
YU R C, ZHANG Q C, LIU Y, et al. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China. Harmful Algae, 2021, 103: 101980 DOI:10.1016/j.hal.2021.101980 |
ZHANG H T. Studies on the source apportionment and damage mechanism of paralytic shellfish toxins in Mytilus galloprovincialis from the Bohai Sea. Master's Thesis of Ocean University of Jiangsu, 2022 [张海涛. 渤海贻贝中麻痹性贝类毒素来源解析、危害形成机制研究. 江苏海洋大学硕士研究生学位论文, 2022]
|
ZHANG H T, WU H Y, ZHENG G C, et al. Accumulation and transformation of paralytic shellfish toxin in mussel Mytilus galloprovincialis exposed to Alexandrium catenella. Progress in Fishery Sciences, 2023, 44(1): 181-190 [链状亚历山大藻暴露下紫贻贝体内麻痹性贝毒蓄积转化规律. 渔业科学进展, 2023, 44(1): 181-190 DOI:10.19663/j.issn2095-9869.20210816001] |
ZHANG Y Y, YAN G W, WU H Y, et al. Establishment and application of detection methods to paralytic shellfish poisoning in water based on SPE and SPATT methods. Oceanologia et Limnologia Sinica, 2020, 51(2): 298-306 [基于SPE与SPATT的水体中麻痹性贝类毒素检测方法构建与应用. 海洋与湖沼, 2020, 51(2): 298-306] |
ZHENG X Y, LI Z X, SUN X J, et al. Surveillance and risk assessment of diarrhetic and paralytic shellfish toxins in Tangshan shellfish culture areas of Bohai Sea, China. Progress in Fishery Sciences, 2023, 44(5): 231-241 [渤海海域唐山贝类养殖区腹泻性和麻痹性贝类毒素的监测与风险评估. 渔业科学进展, 2023, 44(5): 231-241 DOI:10.19663/j.issn2095-9869.20220504001] |
ZHOU Y, LI S P, ZHANG J Y, et al. Dietary exposure assessment of paralytic shellfish toxins through shellfish consumption in Shenzhen population, China. Environmental Science and Pollution Research, 2022, 29: 10222-10234 DOI:10.1007/s11356-021-16249-4 |



