2. 江苏省特色水产育种与绿色高效养殖技术工程研究中心 江苏 南京 210023
2. Jiangsu Province Engineering Research Center for Aquatic Animals Breeding and Green Efficient Aquacultural Technology, Nanjing 210023, China
暗纹东方鲀(Takifugu fasciatus)属鲀形目(Tetraodontiformes)、鲀科(Tetrodantidae)、东方鲀属(Takifugu),广泛分布于我国黄海、东海、渤海和通海的江河下游,是一种典型的江海洄游鱼类,因其肉质鲜嫩、营养丰富,受到广大消费者的喜爱(袁新程等, 2021)。暗纹东方鲀在洄游过程中,容易受到外界不良环境因子胁迫从而产生应激反应,导致下丘脑–脑垂体–肾间轴(HPI)的兴奋和一系列物质代谢的变化。皮质醇(cortisol)又称“氢化可的松”,是鱼类肾间细胞分泌的一种关键的皮质类固醇类激素,可参与鱼类的渗透调节与生长发育(Carbajal et al, 2019; Jerez-Cepa et al, 2019),是鱼类是否受到外界环境胁迫的主要检测指标(Ramsay et al, 2006)。研究表明,当鱼类受到外界不良环境胁迫后,其HPI轴的激活可引起血浆内皮质醇含量升高(Cockrem et al, 2019; Reichard et al, 2023)。Samaras等(2022)研究指出,真鲷(Pagrus major)血浆中皮质醇浓度与温度呈负相关。目前,皮质醇对鱼类的影响主要集中在生长发育(Vargas-Chacoff et al, 2021)、免疫应答(Vallejos-Vidal et al, 2022)和性别分化(Goikoetxea et al, 2022)方面,关于皮质醇对鱼类肠道氧化应激、细胞凋亡和脂代谢方面的影响研究鲜有报道。
当氧化应激发生时,机体会激活体内酶抗氧化系统以清除过量的活性氧(ROS),从而维持机体稳态(Foyer et al, 2011)。超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽过氧化物酶(GSH-Px)是鱼类主要的抗氧化物酶(Gauvreau et al, 2022)。过往研究表明,鱼类在受到外界刺激后,其细胞中的皮质醇含量与SOD、CAT和GSH-Px的活性呈现一定的相关性(Xie et al, 2023),暗示皮质醇会激活鱼体的抗氧化防御系统。细胞凋亡又称编程性死亡,是由基因控制的为维持机体内环境稳态而发生的细胞自主死亡(Belushkina et al, 2001)。Takagi等(2011)指出,10 nmol/L皮质醇刺激可促进青鳉(Oryzias latipes)肠上皮细胞凋亡,且反应具有剂量依赖性。鱼类生长发育过程中的细胞凋亡大多是由半胱氨酸蛋白酶(caspase)家族介导(Yamashita, 2003),Bcl-2- Associated X蛋白基因(bax)、B淋巴细胞瘤-2基因(bcl-2)和p53基因也均与细胞凋亡相关(齐志涛等, 2021; 公洁等, 2023)。
在鱼类中,皮质醇激素能够影响脂肪吸收、合成和分解的平衡,该过程涉及到许多关键基因(Leger et al, 2021)。g6pd和6gpd是产生NADPH的关键基因(Sebastian et al, 2022),acc、fas和pparγ基因也在脂肪的合成中起到了重要的作用(Kang et al, 2022)。pparα、hsl、lpl、cpt-1是参与甘油三酯(TG)水解和细胞内β氧化的重要基因(Hummasti et al, 2006; Kerner et al, 2000; 黄陈翠等, 2020),总胆固醇(TC)和游离脂肪酸(NEFA)也是检测脂代谢的关键指标(Liang et al, 2020)。
目前,研究皮质醇对鱼类生理生化的影响主要以活体动物的肝脏为研究对象(Kostyniuk et al, 2018),以鱼类肠道为模型的研究罕见。肠道作为消化系统的一部分,是鱼体与外界环境接触的重要媒介。由于机体中存在多种激素的交互作用,难以确定具体某一种激素对机体的影响(Leung et al, 2010)。研究表明,体外细胞培养可以保持组织的大部分生理功能(Battle et al, 2001)。因此,本研究以从暗纹东方鲀肠道分离的肠上皮细胞为研究对象,通过在离体培养的肠上皮细胞中加入不同浓度的皮质醇,探讨皮质醇对暗纹东方鲀肠上皮细胞氧化应激、细胞凋亡和脂代谢的影响,研究结果可为暗纹东方鲀健康养殖提供一定的参考。
1 材料与方法 1.1 实验材料实验所用健康的暗纹东方鲀(1龄)由江苏中洋集团股份有限公司提供,体长为(15.00±1.55) cm,重量为(35.00±1.65) g。将实验鱼暂养于实验室鱼类专用养殖水缸中,养殖条件:温度(25.0±0.5) ℃、盐度约为3、溶解氧浓度 > 7 mg/L、光照时间12 h昼/12 h夜、pH 7.0±0.5,使用江苏中洋集团股份有限公司提供的商业饲料(包含粗蛋白42.0%、粗脂肪8.0%、钙0.35%~ 0.5%、磷1.2%、氯化钠0.3%~2.5%)进行投喂,每天2次。正式实验前禁食24 h。
1.2 暗纹东方鲀原代肠上皮细胞的分离和培养参考骆源等(2016)的胰蛋白酶消化法,分离肠上皮细胞。将实验鱼用MS-222 (Sigma, 美国)麻醉,并用75%酒精对其腹部表面进行消毒。在无菌环境下,迅速取出肠道组织,并置于含有100 IU/mL青霉素和100 μg/mL链霉素(碧云天生物技术有限公司, 中国)的PBS缓冲液(生航生物技术有限公司, 中国)中。用高压灭菌的剪刀和镊子除去肠系膜和肠道内食物残渣,将清洗好的肠道组织放入装有0.25%胰蛋白酶(Solarbio, 中国)的离心管内,剪碎,置于28 ℃恒温金属浴中消化,30 min后加入含有20%胎牛血清(Gibco, 美国)和100 IU/mL青霉素、100 μg/mL链霉素的完全培养基停止消化反应,用100目细胞筛网将细胞悬液过滤至50 mL离心管中,4 ℃ 1 000 r/min离心5 min,去除上清液,然后加入完全培养基重悬细胞,调整细胞浓度后置于28 ℃,5% CO2培养箱中培养。
1.3 暗纹东方鲀肠上皮细胞最佳培养条件的确定分别使用0.25%胰蛋白酶和DMEM培养基(生工生物工程有限公司, 中国)(记为T+DMEM)、0.25%胰蛋白酶和1640培养基(生工生物工程有限公司, 中国) (记为T+1640)、Ⅰ型胶原酶(1 mg/mL)(Gibco, 美国)和DMEM培养基(记为C+DMEM)及Ⅰ型胶原酶(1 mg/mL)和1640培养基(记为C+1640)分离和培养细胞。培养基中均添加20%胎牛血清和100 IU/mL青霉素、100 μg/mL链霉素。将大约1×106个细胞接种于6孔细胞培养板中,每个处理设置6个生物学重复和3个技术重复共18个复孔,于28 ℃,5% CO2培养箱中培养5 d,在培养1、2、3、4和5 d后,用CCK-8法检测细胞增殖率,绘制肠上皮细胞在不同分离和培养条件下的生长曲线,并在培养24 h后显微镜下观察细胞生长状态。
1.4 不同浓度皮质醇对肠上皮细胞活力的影响采用CCK-8细胞活力检测试剂盒(诺唯赞生物科技有限公司, 中国)测定细胞活力(姚朝瑞等, 2021)。将培养24 h后的肠上皮细胞接种到96孔板中(5×104个/mL),随后,加入用完全培养基稀释好的皮质醇(Sigma, 美国)溶液,使皮质醇在细胞培养基中的终浓度分别为0、100、1 000、2 000、3 000和5 000 nmol/L,每个浓度至少设置6个复孔,于28 ℃,5% CO2培养箱中继续培养24 h后,每孔加入10 μL CCK-8溶液,28 ℃,5% CO2培养箱内孵育4 h,用酶标仪在450 nm波长下检测各孔吸光度值。以上研究结果得出后续最佳皮质醇处理浓度。
1.5 不同浓度皮质醇对肠上皮细胞超微结构的影响根据上述研究结果,皮质醇最佳处理浓度为1 000 nmol/L以内。肠上皮细胞培养24 h后,加入含有不同浓度皮质醇的完全培养基(0、10、100和1 000 nmol/L),再次培养24 h后收集细胞,加入电镜固定液(Solarbio, 中国),4 ℃固定12 h,接着进行琼脂预包埋,1%锇酸后固定1 h,随后,使用不同浓度梯度乙醇脱水,然后进行渗透包埋、聚合、超薄切片和染色,最后用H7650型透射式电子显微镜(日立, 中国)进行拍照观察。
1.6 不同浓度皮质醇对肠上皮细胞凋亡的影响采用Annexin V-FITC/PI凋亡检测试剂盒(南京建成生物工程研究所, 中国)检测细胞凋亡。肠上皮细胞培养24 h后,加入含有不同浓度皮质醇的完全培养基(0、10、100和1 000 nmol/L),每个浓度至少设置6个复孔,再次培养3、6、12和24 h后收集细胞,随后,将细胞轻轻重悬在500 μL结合液中,依次加入5 μL Annexin V-FITC和5 μL PI染色液,轻轻混匀,在室温下避光孵育10 min后,用eNib610-FL荧光显微镜(Nexcop, 中国)测定细胞凋亡百分比,定量分析细胞凋亡指数。
1.7 肠上皮细胞氧化应激、细胞凋亡和脂代谢相关基因的时序表达分析采用荧光定量PCR (qRT-PCR)检测氧化应激相关基因(sod、cat和gsh-px)、细胞凋亡相关基因(caspase-3、caspase-7、caspase-9、bax、p53和bcl-2)和脂代谢相关基因(g6pd、6gpd、pparγ、fas、acc、hsl、pparα、lpl和cpt-1)的表达情况。采用Trizol (雷根生物技术有限公司, 中国)提取肠上皮细胞总RNA,使用Hifair® Ⅲ 1st Strand cDNA Synthesis SuperMix for qPCR (翌圣生物科技有限公司, 中国)将纯化的RNA逆转录成cDNA。根据相关基因序列,运用Prime 5.0软件设计上下游引物,引物序列、扩增片段长度及扩增效率见表 1,其中,18S RNA为内参基因,引物由生工生物工程(上海)股份有限公司合成。qRT-PCR反应体系:上下游引物各0.4 μL,SYBR qPCR Master Mix 10 μL (诺唯赞生物科技有限公司, 中国),ddH2O 7.2 μL,cDNA模板2.0 μL;反应条件:预变性95 ℃ 30 s;95 ℃ 10 s,55 ℃ 30 s;95 ℃ 15 s;55 ℃ 60 s共40个循环。采用2–ΔΔCt法计算基因的相对表达量,每个样品进行3次生物学重复。
|
|
表 1 qRT-PCR引物序列 Tab.1 qRT-PCR primers for genes |
肠上皮细胞培养24 h后,加入含有不同浓度皮质醇的完全培养基(0、10、100和1 000 nmol/L),每个浓度至少设置6个复孔,再次培养24 h后收集细胞。采用试剂盒(南京建成生物工程研究所, 中国)测定肠上皮细胞内的总蛋白(TP)、甘油三酯(TG)、总胆固醇(TC)和游离脂肪酸(NEFA)含量。具体实验步骤参照试剂盒使用说明书。
1.9 数据统计分析使用SPSS 27.0 (IBM公司)软件对数据进行统计分析,实验数据均采用平均值±标准差(Means± SD)表示。采用Shapiro-Wilk´s检验数据是否符合正态分布,采用双因素方差分析(Two-way ANOVA)评价皮质醇浓度和作用时间的交互作用,采用独立样本t检验统计组间差异显著性,以P < 0.05表示差异显著。
2 结果 2.1 不同消化酶和培养基对暗纹东方鲀肠上皮细胞生长的影响为确定暗纹东方鲀肠上皮细胞体外培养的最佳条件,分析比较了0.25%胰蛋白酶和DMEM培养基(T+DMEM)、0.25%胰蛋白酶和1640培养基(T+1640)、Ⅰ型胶原酶(1 mg/mL)和DMEM培养基(C+DMEM)及Ⅰ型胶原酶(1 mg/mL)和1640培养基(C+1640) 4种培养方法在28 ℃时对原代肠上皮细胞生长的影响。结果如图 1所示,T+DMEM组的细胞增殖率在第2~5天时均高于其他处理组,所有处理组的细胞增殖率均在第3天达到峰值。双因素方差分析结果表明,培养基种类对肠上皮细胞增殖率产生了显著影响。由图 2可知,肠上皮细胞在28 ℃培养24 h后,C+1640组和T+1640组的细胞多数呈卵圆形,贴壁数量较少,C+DMEM组和T+DMEM组的细胞形态多数为成纤维样细胞,贴壁数量增加。经实验证实,暗纹东方鲀肠上皮细胞最适分离培养方法为使用0.25%胰蛋白酶和DMEM培养基分离培养。
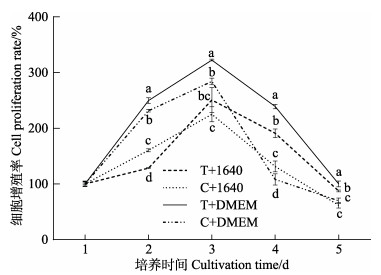
|
图 1 不同消化酶和培养基对肠上皮细胞增殖率的影响 Fig.1 Effects of different digestive enzymes and media on cell proliferation rate in intestinal epithelial cells T+1640:胰蛋白酶+1640培养基;C+1640:Ⅰ型胶原酶+1640培养基;T+DMEM:胰蛋白酶+DMEM培养基;C+DMEM:Ⅰ型胶原酶+DMEM培养基。不同小写字母表示同一时间各个处理组间存在显著性差异(P < 0.05)。 T+1640: Trypsin+RPMI 1640; C+1640: TypeⅠcollagenase+RPMI 1640; T+DMEM: Trypsin+DMEM medium; C+DMEM: TypeⅠcollagenase+DMEM medium Values with different lowercase letters are significantly different among treatments at the same time (P < 0.05). |

|
图 2 不同消化酶和培养基培养24 h后原代肠上皮细胞状态 Fig.2 Status of primary intestinal epithelial cells after 24 h incubation with different digestive enzymes and media A: TypeⅠcollagenase+RPMI 1640 (C+1640); B: Trypsin+RPMI 1640 (T+1640); C: TypeⅠcollagenase+DMEM medium (C+DMEM); D: Trypsin+DMEM medium (T+DMEM) |
如图 3所示,肠上皮细胞在经过皮质醇处理24 h后,细胞活力随皮质醇处理浓度的上升而显著性下降(P < 0.05)。1 000 nmol/L皮质醇组的细胞活力为对照组的0.80倍,且1 000与2 000 nmol/L皮质醇组的细胞活力无显著性差异(P > 0.05)。当皮质醇浓度高于2 000 nmol/L时,肠上皮细胞活力大幅下降,当浓度达到5 000 nmol/L时,肠上皮细胞活力仅为对照组的0.37倍。根据预实验得到在1 000 nmol/L皮质醇刺激下,肠上皮细胞可产生生理生化响应,且该浓度下细胞存活率较高,故选择1 000 nmol/L为皮质醇最适处理浓度。
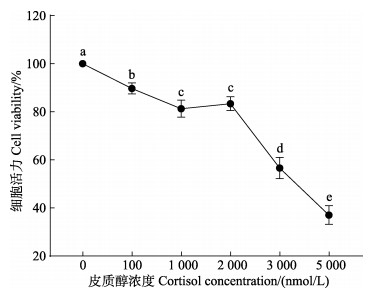
|
图 3 皮质醇对肠上皮细胞活力的影响 Fig.3 Effects of the cortisol on cell viability in intestinal epithelial cells 不同小写字母表示不同皮质醇浓度处理组间存在显著性差异(P < 0.05)。 Data with the different lowercase letters are significantly different among treatments at different cortisol concentrations (P < 0.05). |
如图 4所示,对照组肠上皮细胞在透射电镜下细胞形态结构规则,细胞核完整,线粒体呈卵圆形、外膜和内膜平滑。肠上皮细胞经皮质醇处理后出现线粒体损伤,与对照组相比,10 nmol/L和100 nmol/L皮质醇组细胞内线粒体数量增加,线粒体结构受损。1 000 nmol/L皮质醇组细胞结构弥散,线粒体数量显著上升,线粒体发生肿胀,部分线粒体甚至出现外膜破裂、内容物溢出现象。
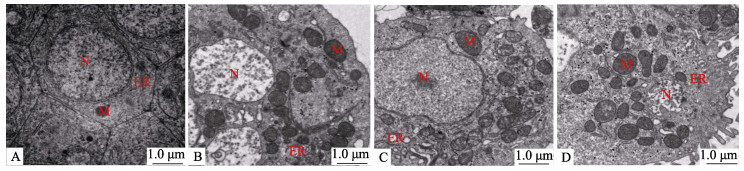
|
图 4 皮质醇对肠上皮细胞超微结构的影响 Fig.4 Effects of the cortisol on ultrastructure in intestinal epithelial cells A:对照组;B:10 nmol/L皮质醇组;C:100 nmol/L皮质醇组;D:1 000 nmol/L皮质醇组;M:线粒体;ER:内质网;N:细胞核。 A: Control group; B: 10 nmol/L cortisol group; C: 100 nmol/L cortisol group; D: 1 000 nmol/L cortisol group; M: Mitochondrion; ER: Endoplasmic reticulum; N: Nucleus. |
由图 5可知,与对照组相比,sod、cat和gsh-px基因的表达量随着皮质醇处理浓度的上升而上升,且在100 nmol/L和1 000 nmol/L皮质醇组之间无显著性差异(P > 0.05)。当皮质醇浓度为10 nmol/L时,与对照组相比,sod和cat基因的表达量显著性上升(P < 0.05),gsh-px基因的表达量无显著性差异(P > 0.05)。所有皮质醇处理组的sod、cat和gsh-px基因的表达量均随着作用时间的上升而呈上升趋势,且在24 h时达到最大值。双因素方差分析结果表明,皮质醇浓度与作用时间和二者的交互作用对肠上皮细胞sod、cat和gsh-px基因的表达量均产生了显著性影响(P < 0.05)。

|
图 5 皮质醇对肠上皮细胞氧化应激相关基因表达水平的影响 Fig.5 Effects of the cortisol on the expression levels of oxidative stress-related genes in intestinal epithelial cells 不同小写字母表示同一皮质醇浓度下各个处理组间存在显著性差异(P < 0.05),不同大写字母表示不同皮质醇浓度处理组间存在显著性差异(P < 0.05);下同。 Data with different lowercase letters are significantly different among treatments at the same cortisol concentration (P < 0.05), data with different uppercase letters are significantly different among treatments at different cortisol concentrations (P < 0.05); the same below. |
如图 6所示,随着皮质醇处理浓度的上升,细胞凋亡率逐渐上升。与对照组相比,10 nmol/L皮质醇组的凋亡率无显著性差异(P > 0.05),100 nmol/L和1 000 nmol/L皮质醇组凋亡率均显著性上升(P < 0.05)。由双因素方差分析结果可知,肠上皮细胞凋亡率受到皮质醇浓度、作用时间及这二者交互作用的影响。与皮质醇孵育3 h相比,在孵育12 h和24 h后,所有皮质醇处理组的细胞凋亡率均显著上升(P < 0.05)。
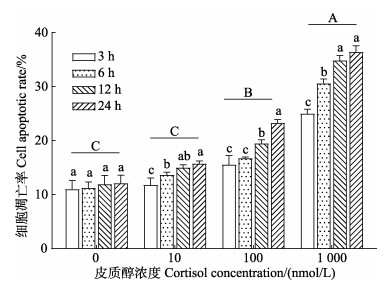
|
图 6 皮质醇对肠上皮细胞凋亡的影响 Fig.6 Effects of cortisol on the apoptosis in intestinal epithelial cells |
qRT-PCR结果如图 7所示,随着皮质醇处理浓度的上升,caspase-3、caspase-7、caspase-9、bax和p53基因的表达量与对照组相比均显著上升(P < 0.05),而bcl-2基因表达量显著下降(P < 0.05),其中caspase-7、caspase-9、bax、p53和bcl-2基因的表达量在100和1 000 nmol/L皮质醇组之间无显著性差异(P > 0.05)。随着皮质醇作用时间的延长,所有皮质醇处理组的caspase-3、bax和p53基因表达量与皮质醇处理时间呈正相关,并在24 h时达到最大值;caspase-7和caspase-9基因的表达量则呈先上升后下降的趋势,且分别在12 h和6 h时达到最大值;bcl-2基因的表达量则呈先下降后上升的趋势,并在12 h时到达最小值。双因素方差分析结果表明,皮质醇浓度、作用时间及二者的交互作用对肠上皮细胞caspase-3、caspase-7、caspase-9、bax、p53和bcl-2基因的表达量均产生了显著影响(P < 0.05)。
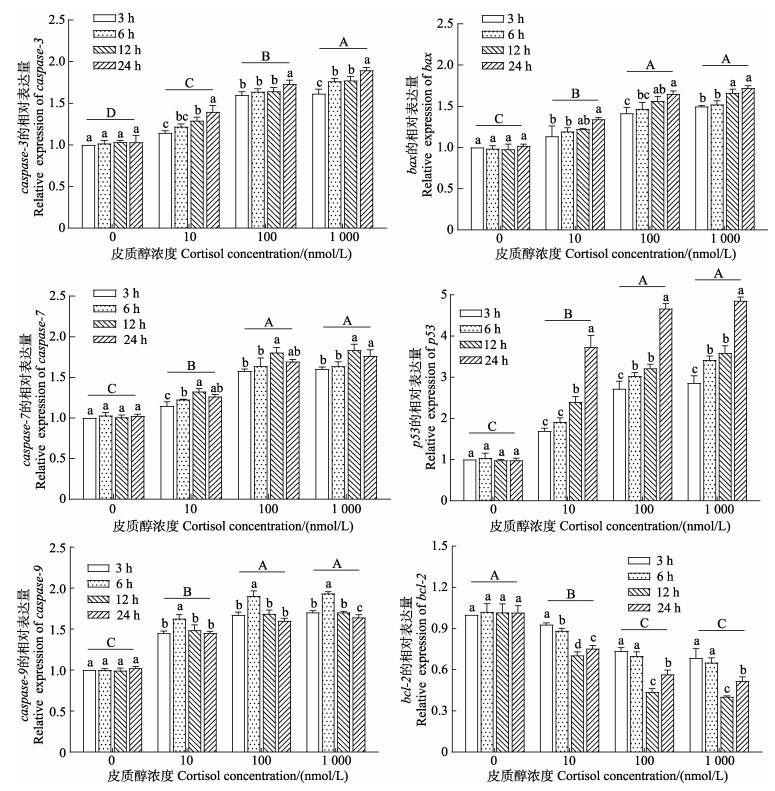
|
图 7 皮质醇对肠上皮细胞凋亡相关基因表达的影响 Fig.7 Effects of the cortisol on the expression levels of apoptosis-related genes in intestinal epithelial cells |
如图 8所示,肠上皮细胞中脂代谢相关基因表达模式受到皮质醇浓度和作用时间的影响。随着皮质醇处理浓度的上升,g6pd、6gpd、pparγ、fas和acc基因的表达量均呈下降的趋势,6gpd和pparγ基因的表达量在100 nmol/L和1 000 nmol/L皮质醇组之间无显著性差异(P > 0.05);hsl、pparα、lpl和cpt-1基因的表达量则呈上升趋势,lpl基因的表达量在100 nmol/L和1 000 nmol/L皮质醇组之间无显著性差异(P > 0.05)。随着皮质醇作用时间的延长,10 nmol/L皮质醇组的g6pd、pparγ、fas和acc基因表达量在不同时间点均无显著差异(P > 0.05),6gpd基因的表达量呈下降趋势,hsl、pparα、lpl和cpt-1基因的表达量则上升趋势。100 nmol/L和1 000 nmol/L皮质醇组的g6pd、6gpd、fas和acc基因的表达量随着处理时间的上升而显著下降(P < 0.05),hsl、lpl和cpt-1基因的表达量显著上升(P < 0.05);pparγ基因的表达量呈先下降后上升的趋势,pparα基因的表达量则呈先上升后下降的趋势,二者均在12 h时达峰值。双因素方差分析结果表明,皮质醇浓度、作用时间及二者的交互作用对肠上皮细胞g6pd、pparγ、fas、acc、hsl、pparα、lpl和cpt-1基因的表达量均产生了显著性影响(P < 0.05)。6gpd基因的表达量受到皮质醇浓度与作用时间的影响,而二者之间的交互作用对其影响效果并不显著(P > 0.05)。
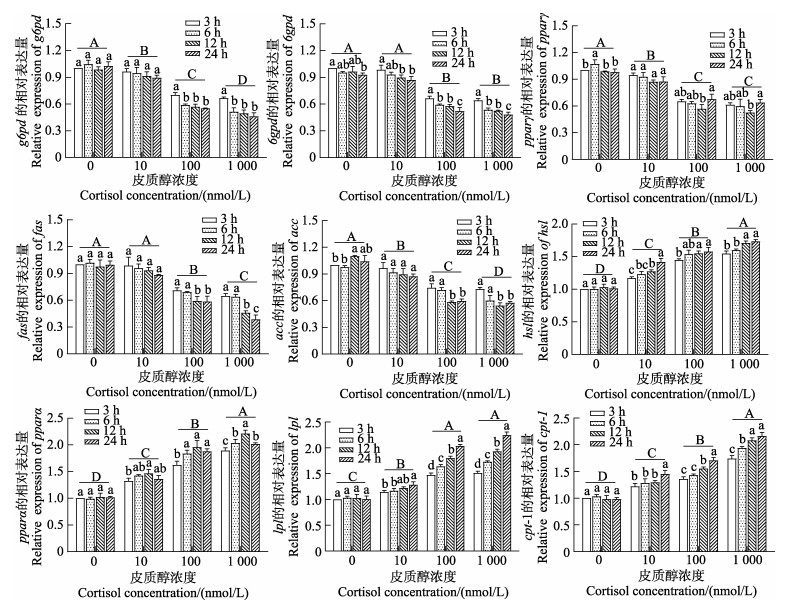
|
图 8 皮质醇对肠上皮细胞脂代谢相关基因表达的影响 Fig.8 Effects of the cortisol on the expression levels of lipid metabolism-related genes in intestinal epithelial cells |
由图 9可知,随着皮质醇处理浓度的上升,肠上皮细胞中的TG和TC含量呈显著下降的趋势,NEFA含量则呈显著上升的趋势(P < 0.05),且三者均在100 nmol/L和1 000 nmol/L皮质醇组之间无显著性差异(P > 0.05)。随着皮质醇处理时间的延长,所有皮质醇处理组TG和TC含量均呈显著下降趋势,NEFA呈显著上升趋势(P < 0.05)。双因素方差分析结果表明,皮质醇浓度、作用时间及二者的交互作用对肠上皮细胞的TG、TC和NEFA含量均产生了显著性影响(P < 0.05)。
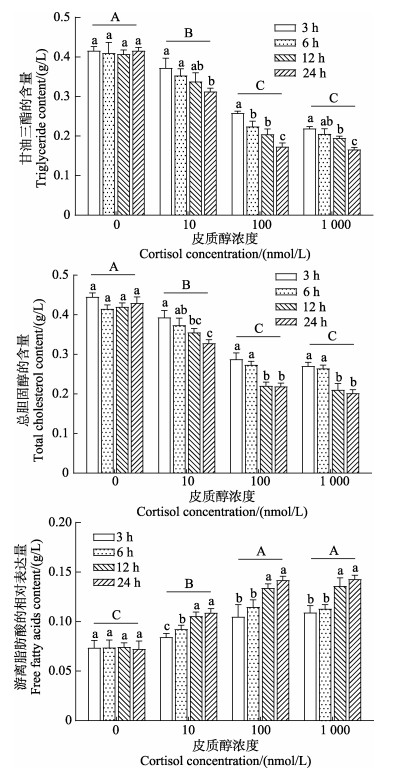
|
图 9 皮质醇对肠上皮细胞生化指标的影响 Fig.9 Effects of cortisol on biochemical indices of intestinal epithelial cells |
目前,鱼类肠细胞体外培养研究主要集中于草鱼(Ctenopharyngodon Idella)(姚仕彬等, 2013)、虹鳟(Oncorhynchus mykiss)(Langan et al, 2018)和牙鲆(Paralichthys olivaceus)(Zhang et al, 2015),有关暗纹东方鲀肠细胞体外培养的研究未见报道。本研究结果表明,经过0.25%胰蛋白酶消化,DMEM培养基培养的肠上皮细胞增殖率更高,培养24 h后贴壁生长状态良好,而使用Ⅰ型胶原酶(1 mg/mL)消化,1640培养基培养的细胞培养24 h后多处于悬浮状态。这种现象可能是因为胰蛋白酶可水解与赖氨酸或精氨酸相连接的肽键,细胞间的蛋白质发生水解使细胞离散,有利于消化上皮、胚胎等细胞间质较少的软组织(任文洁等, 2022),这与瓦氏雅罗鱼(Leuciscus waleckii)原代肠细胞的培养研究结果一致(徐悦, 2021)。DMEM培养基被广泛用于细胞原代培养,如黑鲷(Acanthopagrus schlegelii)肠上皮细胞(Pan et al, 2022)、牙鲆成纤维细胞(Nie et al, 2021)和大西洋鲟(Acipenser oxyrinchus)幼虫细胞(Grunow et al, 2011)等,这与本研究结论一致。但有些鱼类组织细胞培养采用其他培养基,如尖吻鲈(Lates calcarifer)肌肉细胞采用L-15培养基(Lai et al, 2008),塔里木裂腹鱼(Schizothorax biddulphi)尾鳍细胞采用DME/F12培养基(代金彩等, 2022),这表明鱼类细胞的最适培养基与鱼类的种类和组织有关。
3.2 皮质醇诱导暗纹东方鲀肠上皮细胞氧化应激通过CCK-8法检测细胞活力,结果显示,与对照组相比,随着皮质醇处理浓度的上升,暗纹东方鲀肠上皮细胞活力呈显著下降的趋势(P < 0.05),这可能与细胞在皮质醇处理24 h后氧化应激显著上升有关,还可能是高浓度皮质醇破坏了肠细胞的完整性进而导致其细胞活力下降。在鱼类受到胁迫时,机体内ROS含量会显著上升,其中超氧阴离子、过氧化氢和羟基自由基是导致细胞内生物大分子如蛋白质、脂质等损伤的重要物质(Alfadda et al, 2012),当机体内ROS的生成速率高于机体的清除能力时,细胞稳态遭到破坏,产生氧化应激。鱼类为防止机体内ROS的积累,会激活体内的酶抗氧化系统来清除过量的ROS,SOD、CAT和GSH-Px则是重要的抗氧化酶类,也是评价氧化应激水平的重要指标(Dasgupta et al, 2016)。其中,SOD可将超氧阴离子歧化为O2和H2O2,紧接着CAT协同GSH-Px将有毒的H2O2分解为H2O和O2,避免对机体造成氧化损伤(Liu et al, 2011)。过往研究表明,当鱼类产生氧化应激时,其机体内皮质醇水平会显著上升(Gozdowska et al, 2022),且皮质醇含量与抗氧化酶活力具有一定的关联性(Ren et al, 2020)。本研究发现,暗纹东方鲀肠上皮细胞中sod、cat和gsh-px抗氧化酶基因在皮质醇刺激下表达上调,且具有剂量和时间依赖性,说明皮质醇刺激使得暗纹东方鲀肠上皮细胞酶抗氧化系统被激活,机体通过上调抗氧化酶基因表达产生大量抗氧化酶,以清除应激时产生的过量ROS。值得注意的是,gsh-px基因的表达量在10 nmol/L皮质醇组和对照组之间无显著性差异,推测是GSH-Px和CAT之间存在互补代偿效应,导致前者转录水平被削弱(唐功, 2010)。线粒体是产生ROS的主要场所,是评判氧化应激的标志细胞器。本研究透射电镜结果显示,暗纹东方鲀肠上皮细胞在皮质醇刺激下,线粒体数量明显增加,线粒体结构完整性被破坏,这与皮质醇胁迫HT22细胞结果一致(Xu et al, 2019)。上述研究结果表明,皮质醇刺激会引起暗纹东方鲀肠上皮细胞氧化应激,细胞内sod、cat和gsh-px转录上调以减少氧化应激对机体带来的损伤。
3.3 皮质醇诱导暗纹东方鲀肠上皮细胞凋亡研究表明,皮质醇刺激不仅会引发细胞氧化应激反应,还会引发细胞凋亡(Xu et al, 2019)。本研究中,与对照组相比,皮质醇刺激显著提高了暗纹东方鲀肠上皮细胞凋亡率,且凋亡指数呈剂量依赖性增长,这与原代培养的银鲷(Sparus sarba)巨噬细胞(SSM)在皮质醇暴露后的结果相似(Deane et al, 2006)。目前,线粒体凋亡信号通路是最主要的细胞凋亡途径(Karpinich et al, 2002)。Bcl-2家族是启动细胞凋亡的重要因子,Bax是Bcl-2家族关键的促凋亡蛋白,Bcl-2是抗凋亡蛋白,在正常状态下,二者均以同源二聚体的形式存在(Yuan et al, 2016)。当细胞受到外界刺激时,线粒体外膜上会聚集大量的Bax,从而使线粒体通透性转化孔(MPTP)开放,线粒体膜电位(ΔΨm)下降,胞浆中的细胞色素C (Cyt C)因而易位到胞质中,并与凋亡蛋白酶激活因子(Apaf)结合以激活Caspase-9,活化后的Caspase-9再进一步激活Caspase-3和Caspase-7,进而诱发Caspase级联反应引发细胞凋亡。而Bcl-2则可以通过与Bax结合形成更稳定的Bcl-2/Bax异源二聚体或通过阻断Cyt C的释放来抑制细胞凋亡(Estaquier et al, 2012)。p53是重要的核转录因子,参与细胞生长和凋亡等过程,在细胞的正常生长中发挥重要作用,可通过调控Bax的表达介导线粒体凋亡信号通路(Zaib et al, 2022)。
已有报道,caspase-3和bax等凋亡相关基因在皮质醇刺激后表达出上调的模式(万学斌, 2017)。本研究中,皮质醇刺激可使暗纹东方鲀肠上皮细胞中促凋亡基因caspase-9、caspase-7和caspase-3 mRNA表达量显著上升,且分别在皮质醇处理6、12和24 h后达到最大值,推测是由于Caspase-9是凋亡的启动者,而Caspase-7和Caspase-3是凋亡的执行者,所以Caspase-9对于凋亡的响应更早。此外,bax和p53基因的转录水平也随着皮质醇处理浓度和时间的上升而显著上升,bcl-2基因的转录水平则随着皮质醇处理浓度的上升而显著下降。这些现象也发生在其他物种中,如小鼠(Mus musculus) HT22细胞在皮质醇刺激后Cleaved-Caspase9、Cleaved-Caspase3和Bax/Bcl-2等蛋白含量均上升(Xu et al, 2019)。以上结果都说明皮质醇刺激可以诱导细胞凋亡。
3.4 皮质醇诱导暗纹东方鲀肠上皮细胞脂代谢失衡鱼类机体的物质调控受基因表达量的影响。研究表明,G6PD和6GPD是磷酸戊糖途径的关键酶(Reyes et al, 2022)。PPARγ在参与和调控脂肪细胞分化和脂代谢方面发挥重要作用(Lim et al, 2016)。FAS和ACC可以催化乙酰辅酶A生成饱和脂肪酸棕榈酰酯,其基因表达量直接影响机体脂肪合成量(张雨等, 2019)。本研究中,暗纹东方鲀肠上皮细胞在受到皮质醇刺激后,细胞内参与脂肪合成的g6pd、6gpd、pparγ、fas和acc基因表达量均显著下调,说明皮质醇抑制了肠上皮细胞脂肪的合成,这与罗非鱼(Oreochromis mossambicus)肝细胞在皮质醇刺激下的研究结果相似(Sunny et al, 2002)。PPARα是调控脂代谢相关基因的上游转录因子,可通过诱导cpt-1基因的表达来增强脂肪酸β氧化(Ribet et al, 2010)。本研究表明,暗纹东方鲀肠上皮细胞在皮质醇刺激下,pparα和cpt-1基因的mRNA表达水平显著上升,说明皮质醇提高了肠细胞内脂肪酸β氧化水平。研究表明,皮质醇刺激可上调斜带石斑鱼肝细胞hsl和lpl基因的表达(骆源, 2016),HSL能促进脂肪分解为甘油和脂肪酸,在TG的水解过程中起到催化作用(Haemmerle et al, 2002),LPL能催化TG水解成NEFA (Nilsson-Ehle et al, 1980)。本研究中,随着皮质醇处理浓度的上升,hsl和lpl基因的mRNA表达水平均显著上升,验证了肠上皮细胞内TG和TC含量下降、NEFA含量上升的结果。
4 结论综上所述,本研究建立了暗纹东方鲀原代肠上皮细胞的最适分离培养方法,同时,发现肠上皮细胞活力与皮质醇浓度呈负相关。皮质醇能够促进暗纹东方鲀肠上皮细胞的氧化应激与细胞凋亡。此外,皮质醇可以促进暗纹东方鲀肠上皮细胞脂肪的分解并抑制脂肪的合成。
ALFADDA A A, SALLAM R M. Reactive oxygen species in health and disease. Journal of Biomedicine and Biotechnology, 2012, 936486 |
BATTLE T, STACEY G. Cell culture models for hepatotoxicology. Cell Biology and Toxicology, 2001, 17(4/5): 287-299 |
BELUSHKINA N N, SEVERIN S E. Molecular mechanisms of apoptosis pathology. Arkhiv Patologii, 2001, 63(1): 51-60 |
CARBAJAL A, REYES-LOPEZ F E, TALLO-PARRA O, et al. Comparative assessment of cortisol in plasma, skin mucus and scales as a measure of the hypothalamic-pituitary- interrenal axis activity in fish. Aquaculture, 2019, 506: 410-416 DOI:10.1016/j.aquaculture.2019.04.005 |
COCKREM J F, BAHRY M A, CHOWDHURY V S. Cortisol responses of goldfish (Carassius auratus) to air exposure, chasing, and increased water temperature. General and Comparative Endocrinology, 2019, 270: 18-25 DOI:10.1016/j.ygcen.2018.09.017 |
DAI J C, NIE Z L, ZHAO N H, et al. Establishment and the effect of salinity of cell lines derived from tail fin of Schizothorax biddulphi. Progress in Fishery Sciences, 2022, 43(4): 70-80 [代金彩, 聂竹兰, 赵年桦, 等. 塔里木裂腹鱼尾鳍细胞系的建立及盐碱度对其增殖的影响. 渔业科学进展, 2022, 43(4): 70-80] |
DASGUPTA S, DIGIULIO R T, DROLLETTE B D, et al. Hypoxia depresses CYP1A induction and enhances DNA damage, but has minimal effects on antioxidant responses in sheepshead minnow (Cyprinodon variegatus) larvae exposed to dispersed crude oil. Aquatic Toxicology, 2016, 177: 250-260 DOI:10.1016/j.aquatox.2016.05.022 |
DEANE E E, ZHOU L R, WOO N Y S. Cortisol can be pro- or anti-apoptotic in sea bream cells: Potential role of HSP70 induction for cytoprotection. Molecular and Cellular Endocrinology, 2006, 259(1/2): 57-64 |
ESTAQUIER J, VALLETTE F, VAYSSIERE J L, et al. The mitochondrial pathways of apoptosis. In: SCATENA R, BOTTONI P, GIARDINA B (eds). Advances in mitochondrial medicine. Advances in Experimental Medicine and Biology, 2012, 942: 157-183 |
FOYER C H, SHIGEOKA S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, 2011, 155(1): 93-100 DOI:10.1104/pp.110.166181 |
GAUVREAU N L, BRAGG L M, DHIYEBI H A, et al. Impacts on antioxidative enzymes and transcripts in darter (Etheostoma spp.) brains in the Grand River exposed to wastewater effluent. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology, 2022, 258: 109381 DOI:10.1016/j.cbpc.2022.109381 |
GOIKOETXEA A, TODD E V, MUNCASTER S, et al. Effects of cortisol on female-to-male sex change in a wrasse. PLoS One, 2022, 17(9): e0273779 DOI:10.1371/journal.pone.0273779 |
GONG J, ZHU M R, ZHAN M, et al. Cytochrome c gene in Procambarus clarkii inhibits WSSV infection by regulating the apoptosis pathway. Progress in Fishery Sciences, 2023, 44(1): 137-146 [公洁, 祝孟茹, 占铭, 等. 克氏原螯虾细胞色素c基因通过调节凋亡途径抑制WSSV感染. 渔业科学进展, 2023, 44(1): 137-146] |
GOZDOWSKA M, SOKOLOWSKA E, POMIANOWSKI K, et al. Melatonin and cortisol as components of the cutaneous stress response system in fish: Response to oxidative stress. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2022, 268: 111207 DOI:10.1016/j.cbpa.2022.111207 |
GRUNOW B, NOGLICK S, KRUSE C, et al. Isolation of cells from Atlantic sturgeon Acipenser oxyrinchus oxyrinchus and optimization of culture conditions. Aquatic Biology, 2011, 14(1): 67-75 DOI:10.3354/ab00383 |
HAEMMERLE G, ZIMMERMANN R, HAYN M, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. Journal of Biological Chemistry, 2002, 277(7): 4806-4815 DOI:10.1074/jbc.M110355200 |
HUANG C C, SUN J, JI H, et al. Effect of α-lipoic acid on lipid content and lipid metabolism related gene expression in Ctenopharyngodon idellus adipocyte. Freshwater Fisheries, 2020, 50(1): 87-92 [黄陈翠, 孙健, 吉红, 等. 硫辛酸对草鱼脂肪细胞脂质含量及脂代谢相关基因表达的影响. 淡水渔业, 2020, 50(1): 87-92] |
HUMMASTI S, TONTONOZ P. The peroxisome proliferator- activated receptor N-terminal domain controls isotype- selective gene expression and adipogenesis. Molecular Endocrinology, 2006, 20(6): 1261-1275 DOI:10.1210/me.2006-0025 |
JEREZ-CEPA I, FERNÁNDEZ-CASTRO M, DEL SANTO O'NEILL T J, et al. Transport and recovery of gilthead seabream (Sparus aurata L.) sedated with clove oil and MS-222: Effects on stress axis regulation and intermediary metabolism. Frontiers in Physiology, 2019, 10: 612 DOI:10.3389/fphys.2019.00612 |
KANG X, AMEVOR F K, ZHANG L, et al. Study on the major genes related with fat deposition in liver and abdominal fat of different breeds of chicken. Brazilian Journal of Poultry Science, 2022, 24(1): eRBCA-2020-1373 |
KARPINICH N O, TAFANI M, ROTHMAN R J, et al. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. Journal of Biological Chemistry, 2002, 277(19): 16547-16552 DOI:10.1074/jbc.M110629200 |
KERNER J, HOPPEL C. Fatty acid import into mitochondria. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids, 2000, 1486(1): 1-17 DOI:10.1016/S1388-1981(00)00044-5 |
KOSTYNIUK D J, CULBERT B M, MENNIGEN J A, et al. Social status affects lipid metabolism in rainbow trout, Oncorhynchus mykiss. American Journal of Physiology- Regulatory Integrative and Comparative Physiology, 2018, 315(2): R241-R255 DOI:10.1152/ajpregu.00402.2017 |
LAI Y S, CHIOU P P, CHEN W J, et al. Characterization of apoptosis induced by grouper iridovirus in two newly established cell lines from barramundi, Lates calcarifer (Bloch). Journal of Fish Diseases, 2008, 31(11): 825-834 DOI:10.1111/j.1365-2761.2008.00957.x |
LANGAN L M, OWEN S F, JHA A N. Establishment and long-term maintenance of primary intestinal epithelial cells cultured from the rainbow trout, Oncorhynchus mykiss. Biology Open, 2018, 7(3): bio032870 DOI:10.1242/bio.032870 |
LÉGER J A D, ATHANASIO C G, ZHERA A, et al. Hypoxic responses in Oncorhynchus mykiss involve angiogenesis, lipid, and lactate metabolism, which may be triggered by the cortisol stress response and epigenetic methylation. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2021, 39: 100860 DOI:10.1016/j.cbd.2021.100860 |
LEUNG L Y, WOO N Y S. Effects of growth hormone, insulin-like growth factor I, triiodothyronine, thyroxine, and cortisol on gene expression of carbohydrate metabolic enzymes in sea bream hepatocytes. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2010, 157(3): 272-282 DOI:10.1016/j.cbpa.2010.07.010 |
LIANG C, QIAO L Y, HAN Y L, et al. Regulatory roles of SREBF1 and SREBF2 in lipid metabolism and deposition in two Chinese representative fat-tailed sheep breeds. Animals, 2020, 10(8): 1317 DOI:10.3390/ani10081317 |
LIM S, CHOI H, PARK S S, et al. Fenoxycarb promotes adipogenesis in 3T3-L1 preadipocytes. Entomological Research, 2016, 46(1): 80-84 DOI:10.1111/1748-5967.12151 |
LIU X Q, LI K F, DU J, et al. Growth rate, catalase and superoxide dismutase activities in rock carp (Procypris rabaudi Tchang) exposed to supersaturated total dissolved gas. Journal of Zhejiang University Science B, 2011, 12(11): 909-914 DOI:10.1631/jzus.B1100071 |
LUO Y. Primary culture of hepatocytes from grouper (Epinephelus Coioides) and effect of cortisol on the metabolism of hepatocytes. Master´s Thesis of Jimei University, 2016 [骆源. 斜带石斑鱼肝细胞原代培养及皮质醇对其代谢的影响. 集美大学硕士研究生学位论文, 2016]
|
LUO Y, ZHANG C X, WANG L, et al. Study on the isolation and primary culture of hepatocytes from liver of grouper (Epinephelus coioides). Journal of Fisheries of China, 2016, 40(4): 558-565 [骆源, 张春晓, 王玲, 等. 斜带石斑鱼肝细胞分离及原代培养方法的建立. 水产学报, 2016, 40(4): 558-565] |
NIE M M, WU Z H, YOU F. Derivation and characterization of a new embryonic cell line from the olive flounder Paralichthys olivaceus. Turkish Journal of Fisheries and Aquatic Sciences, 2021, 21(4): 159-167 DOI:10.4194/1303-2712-v21_4_01 |
NILSSON-EHLE P, GARFINKEL A S, SCHOTZ M C. Lipolytic enzymes and plasma lipoprotein metabolism. Annual Review of Biochemistry, 1980, 49: 667-693 DOI:10.1146/annurev.bi.49.070180.003315 |
PAN H B, CHEN H Q, CHEN L S, et al. Establishment and characterization of a liver cell line from black porgy, Acanthopagrus schlegelii. Aquaculture Reports, 2022, 25: 101213 DOI:10.1016/j.aqrep.2022.101213 |
QI Z T, YAN D, XIONG F, et al. Progresses of Bcl-2 proteins in teleost fish and mammals. Acta Hydrobiologica Sinica, 2021, 45(4): 935-944 [齐志涛, 闫冬, 熊凡, 等. 鱼类和哺乳类Bcl-2家族蛋白的研究进展. 水生生物学报, 2021, 45(4): 935-944] |
RAMSAY J M, FEIST G W, VARGA Z M, et al. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture, 2006, 258(1/2/3/4): 565-574 |
REICHARD M, DOUDA K, BLAZEK R, et al. Increased plasma cortisol level as acute response to glochidia parasitism. Environmental Biology of Fishes, 2023, 106(1): 101-106 DOI:10.1007/s10641-022-01379-6 |
REN W J, LIN Z X. Comparison of isolation and extraction of primary mouse hepatocytes by trypsin and collagenase perfusion. Journal of Shantou University Medical College, 2022, 35(4): 204-209 [任文洁, 林哲绚. 胰蛋白酶和胶原酶灌注法分离提取小鼠原代肝细胞的比较. 汕头大学医学院学报, 2022, 35(4): 204-209] |
REN X, ZHANG J Y, WANG L, et al. Diel variation in cortisol, glucose, lactic acid and antioxidant system of black sea bass Centropristis striata under natural photoperiod. Chronobiology International, 2020, 37(2): 176-188 DOI:10.1080/07420528.2019.1675684 |
RIBET C, MONTASTIER E, VALLE C, et al. Peroxisome proliferator-activated receptor-alpha control of lipid and glucose metabolism in human white adipocytes. Endocrinology, 2010, 151(1): 123-133 DOI:10.1210/en.2009-0726 |
SAMARAS A, DIMITROGLOU A, GLENI K E, et al. Physiological responses of red seabream (Pagrus major) to stress and rearing temperature. Aquaculture Research, 2022, 53(6): 2518-2528 DOI:10.1111/are.15771 |
REYES J S, FUENTES-LEMUS E, FIGUEROA J D, et al. Implications of differential peroxyl radical-induced inactivation of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase for the pentose phosphate pathway. Scientific Reports, 2022, 12(1): 21191 DOI:10.1038/s41598-022-25474-x |
SUNNY F, LAKSHMY P S, OOMMEN O V. Rapid action of cortisol and testosterone on lipogenic enzymes in a fresh water fish Oreochromis mossambicus: Short-term in vivo and in vitro study. Comparative Biochemistry and Physiology B-Biochemistry and Molecular Biology, 2002, 131(3): 297-304 DOI:10.1016/S1096-4959(02)00023-4 |
TAKAGI C, TAKAHASHI H, KUDOSE H, et al. Dual in vitro effects of cortisol on cell turnover in the medaka esophagus via the glucocorticoid receptor. Life Sciences, 2011, 88(5/6): 239-245 |
TANG G. The discuss on the relationship among reactive oxygen species, antioxidant enzyme and antioxidant. Journal of Anhui Agricultural Sciences, 2010, 38(33): 18619-18621 [唐功. 活性氧·抗氧化酶及抗氧化剂之间关系的探讨. 安徽农业科学, 2010, 38(33): 18619-18621] |
VALLEJOS-VIDAL E, KHANSARI A R, SOLIVA-DUESO L, et al. The direct exposure of cortisol does not modulate the expression of immune-related genes on tissue explants of mucosal surfaces in rainbow trout (Oncorhynchus mykiss) nor in gilthead sea bream (Sparus aurata). Frontiers in Marine Science, 2022, 9: 828050 DOI:10.3389/fmars.2022.828050 |
VARGAS-CHACOFF L, REGISH A M, WEINSTOCK A, et al. Effects of long-term cortisol treatment on growth and osmoregulation of Atlantic salmon and brook trout. General and Comparative Endocrinology, 2021, 308: 113769 DOI:10.1016/j.ygcen.2021.113769 |
WAN X B. Analysis of the deterioration of porcine meat quality responds to increased cortisol based on transcriptome sequencing. Master´s Thesis of Huazhong Agricultural University, 2017 [万学斌. 基于转录组测序技术分析皮质醇对猪肉质的影响. 华中农业大学硕士研究生学位论文, 2017]
|
XIE T, GAO Y T, QIN H Y, et al. Physiological response of spotted knifejaw (Oplegnathus punctatus) during transportation in offshore aquaculture net pen. Aquaculture, 2023, 563: 739029 DOI:10.1016/j.aquaculture.2022.739029 |
XU B, LANG L M, LI S Z, et al. Cortisol excess-mediated mitochondrial damage induced hippocampal neuronal apoptosis in mice following cold exposure. Cells, 2019, 8(6): 612 DOI:10.3390/cells8060612 |
XU Y. Calmodulin expression mechanism of gill and intestinal tissues and primary cultured its cells of Leuciscus waleckii in the environment of alkali stress tolerance. Master´s Thesis of Shanghai Ocean University, 2021 [徐悦. 瓦氏雅罗鱼鳃、肠组织及原代细胞在碱胁迫下的钙调蛋白表达机制研究. 上海海洋大学硕士研究生学位论文, 2021]
|
YAMASHITA M. Apoptosis in zebrafish development. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2003, 136(4): 731-742 DOI:10.1016/j.cbpc.2003.08.013 |
YAO C R, MA P, LI D P, et al. Protective effect of selenium on the oxidative damage of liver cells induced by sodium nitrite in grass carp (Ctenopharyngodon idella). Acta Hydrobiologica Sinica, 2021, 45(5): 986-994 [姚朝瑞, 马聘, 李大鹏, 等. 硒对亚硝酸钠导致的草鱼肝细胞氧化损伤的保护作用. 水生生物学报, 2021, 45(5): 986-994] |
YAO S B, YE Y T, CAI C F, et al. Dissociation and primary culture of Ctenopharyngodon idellus intestinal epithelial cells. Journal of Shanghai Ocean University, 2013, 22(1): 33-41 [姚仕彬, 叶元土, 蔡春芳, 等. 草鱼肠道粘膜上皮细胞的分离与原代培养. 上海海洋大学学报, 2013, 22(1): 33-41] |
YUAN X C, LIU Y S, SHI Y H, et al. Growth performance of Takifugu obscurus and water quality variation in three aquaculture modes. Fisheries Science and Technology Information, 2021, 48(1): 14-20 [袁新程, 刘永士, 施永海, 等. 3种养殖模式下暗纹东方鲀的生长及水质变化. 水产科技情报, 2021, 48(1): 14-20] |
YUAN Z, LIU S H, YAO J K, et al. Expression of Bcl-2 genes in channel catfish after bacterial infection and hypoxia stress. Developmental and Comparative Immunology, 2016, 65: 79-90 DOI:10.1016/j.dci.2016.06.018 |
ZAIB S, HAYYAT A, ALI N, et al. Role of mitochondrial membrane potential and lactate dehydrogenase A in apoptosis. Anti-Cancer Agents in Medicinal Chemistry, 2022, 22(11): 2048-2062 DOI:10.2174/1871520621666211126090906 |
ZHANG Y J, CHEN W, MAI K S, et al. In vitro assay for evaluating the effects of three anti-nutritional factors on the primary-cultured intestinal epithelial cells isolated from Japanese flounder, Paralichthys olivaceus. Aquaculture Research, 2015, 46(1): 242-251 DOI:10.1111/are.12165 |
ZHANG Y, SHI L G, XUN W J, et al. Research progress of regulation of fat metabolism enzyme gene by energy level and hormone. Chinese Journal of Animal Nutrition, 2019, 31(8): 3505-3510 [张雨, 施力光, 荀文娟, 等. 能量水平和激素对脂肪代谢酶基因的调控研究进展. 动物营养学报, 2019, 31(8): 3505-3510] |



