2. 水产科学国家级实验教学示范中心 上海海洋大学 上海 201306
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China
病毒性偷死病(covert mortality disease, VCMD)是偷死野田村病毒(covert mortality nodavirus, CMNV)引起的养殖对虾病毒性疾病(Zhang et al, 2014)。患病对虾主要在养殖池塘底部深水区陆续死亡,较难观察到,早期被养殖户称为“偷死病”。发病池塘中,每天都会有少量患病对虾死亡,这种死亡会一直持续到收获期,因此,该病在东南亚也被养殖者称作持续性死亡综合征(running mortality syndromes, RMS)。对虾感染CMNV后,通常在水温高(28 ℃以上)和环境急剧变化时发病,病虾多表现出甲壳变软,肝胰腺颜色变浅、萎缩,空肠空胃,生长缓慢等症状,急性感染期还可见病虾腹部肌肉不透明或局部发白(Zhang et al, 2014、2017)。但上述症状在CMNV低剂量感染时往往不十分明显(Liu et al, 2022)。
CMNV在分类上属于野田村病毒科(Nodaviridae) α野田村病毒属(Alphanodavirus),病毒粒子直径约为32 nm,外形为球形(二十面体状),无囊膜,基因组由2条单链正义RNA组成。CMNV宿主范围极广,除感染海水养殖的凡纳对虾(Penaeus vannamei)、斑节对虾(P. monodon)、中国对虾(P. chinensis)和脊尾白虾(Exopalaemon carinicauda)外,还可感染淡水养殖的罗氏沼虾(Macrobrachium rosenbergii)和克氏原螯虾(Procambarus clarkia)等(Zhang et al, 2017; Xia et al, 2022)。最近研究显示,该病毒还能跨物种感染牙鲆(Parallichthys olivaceus)、鲻虾虎鱼(Mugilogobius abei)、鲫鱼(Carassius auratus)、斑马鱼(Danio rerio)、小黄鱼(Larimichthys polyactis)和大黄鱼(Larimichthys crocea)等养殖以及野生鱼类(Zhang et al, 2018; Wang et al, 2019、2021; 王崇等, 2019; Xu et al, 2021、2022; Xia et al, 2022; 桑松文等, 2021)。CMNV感染鱼类可引起鱼类多组织尤其是视网膜和神经系统损伤,与β野田村病毒属(Betanodavirus)病毒感染鱼类引起的坏死症状类似(Wang et al, 2021; Xu et al, 2021、2022)。
研究发现,CMNV在对虾养殖池塘中的贮存宿主或媒介载体众多,对虾池塘养殖系统中包括轮虫(Rotifera)、卤虫(Artemia sinica)、桡足类(Copepoda)、枝角类(Cladocera)、蜾蠃蜚(Corophium sinense Zhang)、寄居蟹(Diogenes edwardsii)、招潮蟹(Tubuca arcuate)、沙蟹(Ocypode cordimundus)和野生鱼类等均可作为CMNV传播的媒介生物感染或携带CMNV,这些生物具有导致CMNV传播扩散的潜在风险(Liu et al, 2018; 王崇等, 2019; Wang C et al, 2022)。CMNV在养殖池塘中可以通过水平方式传播,而Liu等(2018)研究表明,CMNV在脊尾白虾中还可以经卵或精细胞垂直传播,CMNV宿主范围广,媒介载体多,传播模式多样,导致该病毒在甲壳类养殖区长期广泛流行。2016年和2017年作者所在实验室分别从河北、河南、山东、江苏、上海、浙江、福建、广东和海南等地采集254份和387份虾类样品,随后,基于RT-nPCR、RT-LAMP和TaqMan RT-qPCR三种方法对样品进行检测,发现2016和2017年样品中CMNV总阳性检出率分别为26.8% (68/254)和16.3% (63/387) (李小平等, 2019)。
本研究于2021—2022年间在全国主要虾类养殖地区开展CMNV流行病学调查和样品采集工作,并利用分子生物学、组织病理学和电镜分析等方法对所采集的样品进行系统分析,从而更深入地揭示CMNV在我国养殖虾类中的流行及传播情况。
1 材料与方法 1.1 样品采集在养殖现场将虾类头胸部用一次性解剖刀沿着中心均分切成2份,从其中一半头胸部切取1 mm3长条状的肝胰腺保存于2.5%中性戊二醛固定液中,用于后续透射电子显微镜分析;另一半则保存在4%多聚甲醛溶液(4% PFA)中,用于后续组织病理学分析。剩余的样品部分切碎混匀保存于RNAstore固定液(中国北京,天根生物)中,用于分子生物学检测。
1.2 RNA提取和纯化用镊子或牙签取出保存于RNAstore中的样品组织,放入1.5 mL的离心管中用RNase-free H2O进行清洗,用吸水纸吸去多余水分,然后,用RNAiso plus (货号:D9108A,TaKaRa,中国)法抽提对虾总RNA;最后,通过NanoDrop 2000 (Thermo Fisher Scientific,美国)测量所制备RNA的浓度和纯度。
1.3 利用TaqMan RT-qPCR方法检测样品中CMNV采用Luna® Universal Probe One-Step RT-qPCR Kit Protocol (货号:E3006,NEB,美国)、表 1中的引物和探针(根据GenBank: MZ643944设计) (Wang W et al, 2022)进行TaqMan RT-qPCR,检测样品中的CMNV。反应体系为20 μL,各组分及用量如下:Luna Universal Probe One-Step Reaction Mix (2×) 8 μL,LunaWarmStart® RT Enzyme Mix (20×) 0.8 μL,引物CMNV-CP-TaqIDT-R (10 μmol/L) 0.8 μL、CMNV-CP- TaqIDT-F (10 μmol/L) 0.8 μL,探针CMNV-CP-TaqIDT-P (10 μmol/L) 0.4 μL,RNA-free H2O 8.2 μL,核酸模板1 μL。在冰上配制反应体系。利用Bio-Rad实时荧光定量PCR仪进行TaqMan RT-qPCR扩增,反应程序:59.4 ℃保温15 min进行反转录;95 ℃预变性1 min;95 ℃变性10 s,54.9 ℃退火延伸25 s,共40个循环。
|
|
表 1 CMNV TaqMan RT-qPCR实验所用引物和探针序列 Tab.1 Primer and probe sequences of CMNV TaqMan RT-qPCR |
不同年份不同地区样品中CMNV阳性检出率计算公式:
不同年份不同种类样品中CMNV阳性检出率计算公式:
选取TaqMan RT-qPCR检测呈阳性的样品进行组织切片和病理学观察。具体步骤:将保存在4% PFA固定液中的样品(已固定12~24 h)转移至70%乙醇中,依照组织样本处理标准流程进行脱水、包埋和切片处理,制备组织病理切片(Bell et al, 1988)。根据常规组织切片染色方法(Zhang et al, 2005)对切片进行苏木精–伊红(HE)染色,随后,利用Nikon Eclipse E80i显微镜(Nikon, 德国)对组织切片进行观察和拍照。
1.6 原位杂交分析根据本实验室先前报道的方法制备CMNV的RNA探针,并用其与制备好的组织切片进行原位杂交(ISH) (Liu et al, 2017)和显色,对原位杂交显色后的切片进行核固红复染和封片,然后,在Nikon Eclipse E80i显微镜(Nikon, 德国)下进行观察。
1.7 透射电镜分析将固定于戊二醛中的样品,参照之前报道的方法(Liu et al, 2018)进行固定、包埋、切片和染色处理,并用树脂酒精包埋液(比例为3∶1)处理60 min,随后,用纯树脂包埋3 d,并制作包埋样品的超薄切片(切片厚度为50 nm)。用醋酸铀和柠檬酸铅对切片进行染色,染色后,利用透射电子显微镜(JEOL JEM- 1200,日本电子)观察并拍照。
2 结果 2.1 流行病学调查与样品采集情况2021年从山东、新疆、江苏、湖北、天津和海南等地共采集687份虾类样品,包括凡纳对虾、罗氏沼虾和日本对虾(P. japonicus);从上述虾类的养殖池塘中共采集41份共生生物样品,包括卤虫、单环刺螠(Urechis unicinctus)、轮虫、桡足类、克氏原螯虾、鼠妇(Porcellio)和沙蚕。
2022年从山东、新疆、江苏、湖北、天津和海南等地共采集612份虾类样品,包括凡纳对虾、罗氏沼虾、中国对虾和日本对虾;从上述虾类养殖池塘中共采集78份共生生物样品,包括卤虫和枪乌贼(Loligo)。2021—2022年所采集样品的信息见表 2、表 3和表 4。
|
|
表 2 样品采集省市及数量信息 Tab.2 The distribution of sampling sites and amount of samples |
|
|
表 3 样品种类及数量信息 Tab.3 The information of sampling species and amount of samples |
|
|
表 4 养虾池塘共生生物种类及数量信息 Tab.4 Species and quantity information of commensal organisms in shrimp ponds |
在流行病学调查过程中发现,室外池塘养殖对虾中CMNV感染会引起一定程度的累计死亡,而室内养殖对虾中CMNV感染主要引起对虾甲壳软化和生长缓慢,通常不导致感染对虾的大量死亡。
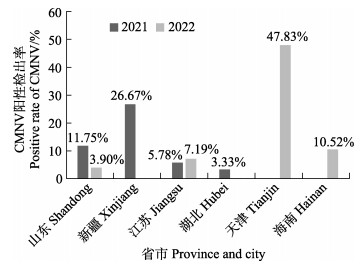
2.2 CMNV的流行区域分析对流行病学调查中所采集的样品进行TaqMan RT-qPCR检测,并将样品检测结果按照采样年份和区域进行分析,结果如图 1所示。2021年不同省市样品中CMNV阳性检出率如下:山东为11.75% (49/417),新疆为26.67% (8/30),江苏为5.78% (7/121),湖北为3.33% (1/30);2022年不同省市样品中CMNV阳性检出率如下:山东为3.90% (6/154),江苏为7.19% (12/167),天津为47.83% (33/69),海南为10.52% (8/76)。
|
图 1 基于TaqMan RT-qPCR检测方法分析2021—2022年不同省市样品中CMNV的阳性检出率 Fig.1 CMNV positive rate in samples collected from different provinces and cities in 2021 and 2022 based on TaqMan RT-qPCR detecting method |
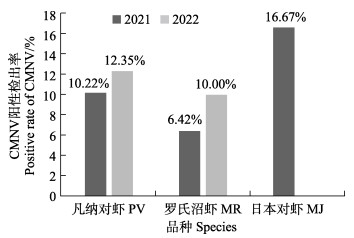
采用TaqMan RT-qPCR方法对2021年和2022年流行病学调查中所采集的不同虾类样品进行检测分析,结果如图 2所示。2021年凡纳对虾、罗氏沼虾和日本对虾样品中可检测到CMNV,CMNV的阳性检出率分别为10.22% (52/509)、6.42% (7/109)和16.67% (2/12);2022年凡纳对虾和罗氏沼虾样品中可检测到CMNV,CMNV的阳性检出率分别为12.35% (41/332)和10.00% (16/160)。
|
图 2 基于TaqMan RT-qPCR方法分析2021—2022年不同虾类样品中CMNV的阳性检出率 Fig.2 CMNV positive rates in different shrimp samples collected in 2021 and 2022 based on TaqMan RT-qPCR |
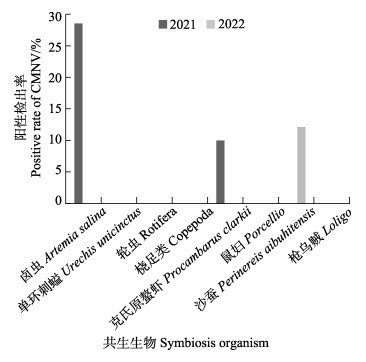
采用TaqMan RT-qPCR方法对2021—2022年在不同虾类养殖池塘中采集到的共生生物样品进行检测分析(图 3),结果显示,2021年卤虫样品中CMNV的阳性检出率为28.57% (2/7),克氏原螯虾样品中的阳性检出率为10% (1/10),其他样品中未检出;2022年沙蚕样品中阳性检出率为12.16% (9/74)。
|
图 3 基于TaqMan RT-qPCR方法分析2021—2022年虾类池塘中共生生物的CMNV阳性检出率 Fig.3 CMNV positive rates in the organisms living in shrimp ponds collected in 2021 and 2022 based on TaqMan RT-qPCR detecting method |
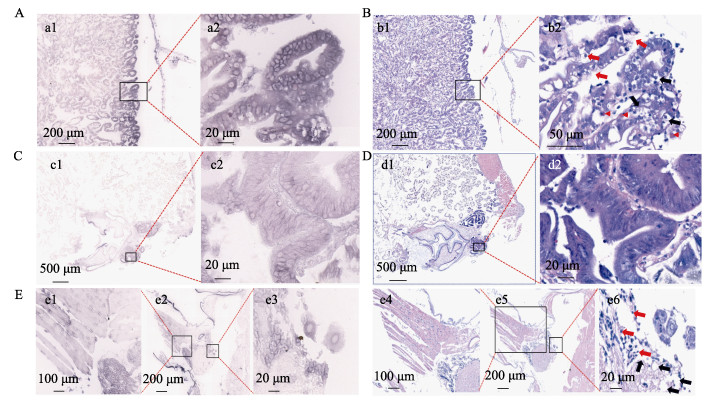
对在TaqMan RT-qPCR检测中呈CMNV阳性的虾类样品进行原位杂交和组织病理分析。原位杂交结果显示,在患病凡纳对虾肝胰腺(图 4A)、前中肠盲囊组织(图 4C)和附肢神经组织(图 4E)中均可观察到大量明显的蓝紫色CMNV探针杂交信号。在观察到CMNV杂交信号的地方,也能观察到典型的病理变化。HE染色结果显示,患病凡纳对虾肝胰腺(图 4B)部分上皮细胞内略呈空泡化(黑色箭头),细胞质内存在嗜酸性病毒包涵体(红色无尾箭头)。前中肠盲囊组织(图 4D)上皮细胞的细胞膜受损而变得边界不清晰,其细胞质内也存在嗜酸性包涵体(红色无尾箭头)。此外,附肢神经细胞(图 4Ee6)内可见空泡化(黑色箭头),并伴有少量核固缩现象(红色箭头)。
|
图 4 凡纳对虾肝胰腺、前中肠盲囊和附肢的组织病理及原位杂交图 Fig.4 Histopathology and in situ hybridization pictures of hepatopancreas, anterior midgut caecum and appendages of P. vannamei A和B为采集自山东日照养殖凡纳对虾(样品编号20210630021)肝胰腺的原位杂交及组织病理切片结果。a1和a2的原位杂交显示,病虾肝胰腺上皮细胞内可见大量浅紫色CMNV探针杂交信号。b1和b2的组织病理图片显示,病虾肝胰腺上皮细胞出现空泡化(黑色箭头)及坏死(红色箭头),胞质内可见嗜酸性包涵体(红色无尾箭头)。C和D为凡纳对虾前中肠盲囊的原位杂交及组织病理切片结果。c1和c2的原位杂交显示,在前中肠盲囊上皮组织中出现大量浅紫色CMNV探针杂交信号。d1和d2的组织病理图片显示,前中肠上皮细胞的细胞膜受损而变得边界不清晰。E为凡纳对虾附肢的原位杂交及组织病理切片结果。e1、e2和e3的原位杂交显示,在病虾附肢的神经组织内可见大量浅紫色CMNV探针杂交信号。e4、e5和e6的病理组织切片显示,附肢肌纤维断裂,肌细胞出现轻微的溶解样坏死,伴有核固缩现象;附肢神经细胞出现空泡化(黑色箭头)和核固缩现象(红色箭头)。 A, B: Micrographs of haematoxylin and eosin (HE) staining and in situ hybridization (ISH) of hepatopancreas of P. vannamei collected in Rizhao City, Shandong Province (sample No. 20210630021). a1, a2: CMNV-positive hybridization signals (colored purple) were detected in the tubular epithelium of hepatopancreas by ISH. b1, b2: The histopathological images revealed vacuolization (black arrows) and necrosis (red arrows) of the hepatopancreatic epithelial cells of the diseased shrimp. Additionally, eosinophilic inclusion bodies (red arrows without tail) were visible in the cytoplasm. C and D: Micrographs of ISH and HE of the midgut diverticulum of P. vannamei. c1, c2: Micrographs of ISH revealed numerous light purple hybridization signals of the CMNV probe in the epithelial tissue of the midgut diverticulum. d1, d2: The cell membranes of the anterior midgut epithelial cells were damaged and poorly defined in HE. E: Micrographs of ISH and HE of the appendages of P. vannamei. e1, e2, e3: Micrographs of ISH showed that a large number of light purple CMNV probe hybridization signals were visible within the neural tissue of the diseased shrimp appendage. e4, e5, e6: Micrographs of HE showed broken muscle fibres of the appendages and slight lysis-like necrosis of the myocytes with karyopyknosis. Additionally, the appendage nerve exhibited vacuolization (black arrows) and karyopyknosis (red arrows). |
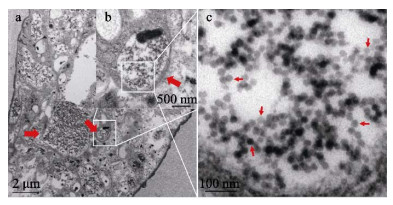
对在TaqMan RT-qPCR检测中呈CMNV阳性的虾类样品进行组织超薄切片和电镜观测,如图 5所示,罗氏沼虾鳃组织上皮细胞内出现明显的空泡化病变,且存在大量病毒包涵体,包涵体内可见密集的CMNV样病毒颗粒,病毒颗粒直径约为32 nm。
|
图 5 透射电镜观测患病罗氏沼虾鳃组织的超薄切片 Fig.5 Transmission electron micrographs of ultrathin section of the gills of M. rosenbergii a为鳃上皮细胞的超微组织结构,图中可见上皮细胞胞质局部空泡化以及大量大小不均一的包涵体(红色粗箭头)。b为a图中单个病毒包涵体区域(白色框)的放大图,图中可见散布的病毒颗粒。c为b图内白色框内病毒包涵体的局部放大图,图中可见大量清晰、直径约为32 nm的CMNV样病毒颗粒(红色箭头)。 a: Shows the ultrastructure of gill epithelial cells, with localised vacuolization of the epithelial cell cytoplasm and a large number of unevenly sized inclusion bodies (thick red arrows); b: Magnified micrograph of the area in the white frame in Fig.a. Note that scattered viral particles can be observed; c: Magnified micrograph of viral inclusions in the area in the white frame in Fig.b. Note that a large number of clear, approximately 32 nm diameter CMNV-like particles can be observed. |
在过去的十多年里,养殖虾类新疫病不断出现,如2003—2004年前后,在东南亚和我国出现的VCMD/RMS;2010年,在越南和中国最早发生的急性肝胰腺坏死病(acute hepatopancreatic necrosis disease, AHPND);2013—2014年前后,在我国出现的十足目虹彩病毒病(infection with Decapod iridescent virus 1, iDIV1);2019—2020年,在我国出现的玻璃苗病害(transparent post-larva disease, TPD)。这些新发疫病给对虾养殖业造成了严重影响(Thitamadee et al, 2016; Qiu et al, 2017; Zou et al, 2020)。
研究显示,2013—2020年间,CMNV在我国沿海和内陆16个省市的海水和淡水养殖甲壳类中持续流行,检出CMNV流行的沿海11个省市包括辽宁、河北、天津、山东、江苏、上海、浙江、福建、广东、广西和海南(Zhang et al, 2017; 李小平等, 2019; Xia et al, 2022);内陆省份中,新疆、湖北、湖南和河南等地养殖的凡纳对虾、克氏原螯虾和罗氏沼虾以及西藏等地内陆养殖的鱼类中也检测到CMNV阳性(Zhang, 2019c; Xia et al, 2022; 张庆利, 2022)。此外,东南亚国家如泰国、越南、马来西亚、印度尼西亚、文莱和菲律宾等地养殖的凡纳对虾和海水鱼类中也有CMNV阳性检出(Zhang, 2019a、b、c)。
本研究于2021—2022年针对我国主要虾类养殖地区的CMNV流行病学调查结果表明,在山东、江苏、海南、新疆、广西和天津等省市采集的样品均可检出CMNV阳性,检出CMNV阳性的主要养殖种类包括凡纳对虾、日本对虾和罗氏沼虾;2021和2022年所采集的虾类样品中CMNV阳性检出率分别为10.04% (69/687)和11.44% (70/612),该结果表明,CMNV仍在我国主要虾类养殖地区和主养种类中持续流行。流行病学调查还发现,室外池塘养殖对虾中CMNV感染会引起一定程度的累计死亡,在养殖环境条件急剧变化(如暴雨、高温、溶氧等条件)时,患病虾群体会发生明显死亡;室内工厂化养殖条件下,CMNV感染主要引起对虾软化和生长缓慢,通常不会导致染病对虾的大量死亡。
2020年底至2021年中,作者实验室在开展VCMD典型病例流行病学研究中还发现,山东省东营和潍坊室内养殖凡纳对虾出现较高比例的软壳个体,检测发现,其CMNV的载量较高,而无高拷贝的其他常见虾类病原检出。在针对一典型VCMD病例研究中,作者实验室发现,携带高拷贝(4.2×107拷贝/μg对虾组织总RNA) CMNV的卤虫被养殖者作为饵料投喂工厂化养殖凡纳对虾是导致当年山东潍坊和东营一些养殖企业发生CMNV感染的主要原因(Yao et al, 2022)。本研究于2021年采集的卤虫(鲜活饵料)、2022年采集的沙蚕(池塘共生生物)等甲壳类养殖池塘(土池)共生生物中也可检测到CMNV阳性,且病毒载量较高;而把卤虫作为鲜活饵料的工厂化对虾养殖场,以及池塘内存在CMNV阳性沙蚕的对虾养殖场,其养殖凡纳对虾中均存在较高的CMNV感染率,这说明无论是室内工厂化养殖模式还是传统的土池养殖模式,卤虫和沙蚕等媒介生物可能均存在传播CMNV的较高风险,并威胁养殖系统内的养殖虾类健康。卤虫有较强的适口性、较高的营养价值,可提高养殖生物的存活率,是传统对虾养殖模式中使用的开口饵料(吕光俊等, 2004);沙蚕因体内具有优质蛋白、活性物质等营养物质,被广泛用作对虾亲体的促成熟饵料。但鉴于卤虫和沙蚕可传播CMNV,对虾养殖过程中应避免使用携带或感染CMNV的卤虫或沙蚕作为饵料,以降低该病毒引入养殖系统导致对虾感染和发病的风险。
最近,有关CMNV致病机理的研究表明,该病毒主要感染虾类肝胰腺、淋巴器官、附肢、鳃、眼柄和神经等器官内的上皮组织、神经组织和结缔组织等(Zhang et al, 2014; Liu et al, 2022),引起肝胰腺萎缩坏死以及肝胰腺小管上皮细胞坏死、淋巴器官细胞核固缩严重,并伴随出现嗜酸性细胞质包涵体、神经组织空泡化以及神经细胞坏死;上述组织病理变化仅在患病严重的对虾中出现,在感染初期以及慢性感染期并不明显(NACA, 2012; Liu et al, 2022),本研究对TaqMan RT-qPCR检测呈现阳性的样品进行了组织病理和原位杂交分析,在肝胰腺和前中肠组织观测到嗜酸性病毒包涵体,在患病对虾附肢神经可见空泡化病理损伤,且这些组织中均有明显CMNV RNA探针阳性杂交信号,这些研究结果进一步证实了本研究中有关荧光定量PCR检测虾类样品中CMNV的阳性检测结果。
本研究表明,与往年相比,我国多地养殖虾类以及虾类养殖池塘共生生物中仍存在较高的CMNV阳性检出率,该病毒的流行危害风险仍然较高;在对虾养殖池塘共生生物和饵料生物中也检测到多批次CMNV阳性,这些结果提示渔业主管部门以及虾类养殖业利益相关者应进一步加强对CMNV的监测与预警,防止虾类种苗或虾类鲜活饵料带毒传播,以降低其流行危害风险,助力虾类养殖业绿色健康发展。
BELL T A, LIGHTNER D V. A handbook of normal penaeid shrimp histology. Baton Rouge: World Aquaculture Society, 1988
|
LI X P, WANG X Y, ZHANG Q L, et al. Molecular epidemiological survey of covert mortality nodavirus (CMNV) in cultured crustaceans in China in 2016~2017. Progress in Fishery Sciences, 2019, 40(2): 65-73 [李小平, 万晓媛, 张庆利, 等. 2016–2017年中国沿海省市虾类偷死野田村病毒(CMNV)分子流行病学调查. 渔业科学进展, 2019, 40(2): 65-73 DOI:10.19663/j.issn2095-9869.20180420003] |
LIU S, LI J T, TIAN Y, et al. Experimental vertical transmission of covert mortality nodavirus in Exopalaemon carinicauda. Journal of General Virology, 2017, 98(4): 652-661 DOI:10.1099/jgv.0.000731 |
LIU S, WANG X H, XU T T, et al. Vectors and reservoir hosts of covert mortality nodavirus (CMNV) in shrimp ponds. Journal of Invertebrate Pathology, 2018, 154: 29-36 DOI:10.1016/j.jip.2018.03.011 |
LIU S, XIA J T, TIAN Y, et al. Investigation of pathogenic mechanism of covert mortality nodavirus infection in Penaeus vannamei. Frontiers in Microbiology, 2022, 13: 904358 DOI:10.3389/fmicb.2022.904358 |
LÜ G J, ZHAO W, JIA Q X. Effect of artemia stock density on its growth. Journal of Henan University of Science and Technology (Agricultural Science), 2004, 24(1): 26-29 [吕光俊, 赵文, 贾沁贤. 不同的卤虫放养密度对其生长的影响. 河南科技大学学报(农学版), 2004, 24(1): 26-29] |
Network of Aquaculture Centres in Asia-Pacific (NACA). Asia Pacific Emergency regional consultationon the emerging shrimp disease: Early mortality syndrome (EMS)/Acute hepatopancreatic necrosis syndrome (AHPNS). Final Report, Bangkok, Thailand, 2012
|
QIU L, CHEN M M, WAN X Y, et al. Characterization of a new member of Iridoviridae, shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Scientific Reports, 2017, 7(1): 11834 DOI:10.1038/s41598-017-10738-8 |
SANG S W, LI X P, ZHANG Q L. Establishment and application of the TaqMan RT-PCR detection method for the shrimp movement disorder nodavirus (MDNV). Progress in Fishery Sciences, 2021, 42(5): 77-85 [桑松文, 李小平, 张庆利. 虾类行动障碍野田村病毒(MDNV) TaqMan RT-PCR检测方法的建立与应用. 渔业科学进展, 2021, 42(5): 77-85 DOI:10.19663/j.issn2095-9869.20200331001] |
THITAMADEE S, PRACHUMWAT A, SRISALA J, et al. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture, 2016, 452: 69-87 DOI:10.1016/j.aquaculture.2015.10.028 |
WANG C, LIU S, LI X P, et al. Infection of covert mortality nodavirus in Japanese flounder reveals host jump of the emerging alphanodavirus. Journal of General Virology, 2019, 100(2): 166-175 DOI:10.1099/jgv.0.001177 |
WANG C, LIU S, TANG K F J, et al. Natural infection of covert mortality nodavirus affects zebrafish (Danio rerio). Journal of Fish Disease, 2021, 44(9): 1315-1324 DOI:10.1111/jfd.13390 |
WANG C, WANG X H, LIU S, et al. Preliminary study on the natural infection of Carassius auratus with covert mortality nodavirus (CMNV). Progress in Fishery Sciences, 2019, 40(2): 25-32 [王崇, 王秀华, 刘爽, 等. 鲫鱼自然感染对虾偷死野田村病毒(CMNV)的初步研究. 渔业科学进展, 2019, 40(2): 25-32 DOI:10.19663/j.issn2095-9869.20180420006] |
WANG C, LIU S, XU T T, et al. Pathogenicity study of covert mortality nodavirus (CMNV) infection in zebrafish model. Aquaculture, 2022, 546: 737378 DOI:10.1016/j.aquaculture.2021.737378 |
WANG W, LIU S, YAO L, et al. Development of a novel RT-qPCR detecting method of covert mortality nodavirus (CMNV) for the national proficiency test in molecular detection. Viruses, 2022, 14(7): 1475 DOI:10.3390/v14071475 |
XIA J T, WANG C, YAO L, et al. Investigation on natural infection of covert mortality nodavirus in farmed giant freshwater prawn (Macrobrachium rosenbergii). Animals, 2022, 12(11): 1370 DOI:10.3390/ani12111370 |
XU T T, FAN Y D, JIA T C, et al. Investigation on natural infection of covert mortality nodavirus in large yellow croaker, Larimichthys crocea. Frontiers in Marine Science, 2022, 9: 789128 |
XU T T, LI Y X, SHAN X J, et al. Natural infection of covert mortality nodavirus in small yellow croaker in coastal water. Frontiers in Marine Science, 2021, 8: 670831 |
YAO L, WANG C, WANG W, et al. Cases report of covert mortality nodavirus infection in indoor farming Penaeus vannamei. Aquaculture Reports, 2022, 25: 101238 |
ZHANG Q L, LIU Q, LIU S, et al. A new nodavirus is associated with covert mortality disease of shrimp. Journal of General Virology, 2014, 95(12): 2700-2709 |
ZHANG Q L, LIU S, LI J, et al. Evidence for cross-species transmission of covert mortality nodavirus to new host of Mugilogobius abei. Frontiers in Microbiology, 2018, 9: 1447 |
ZHANG Q L, XU T T, WAN X Y, et al. Prevalence and distribution of covert mortality nodavirus (CMNV) in cultured crustacean. Virus Research, 2017, 233: 113-119 |
ZHANG Q L. Aquatic disease prevention and control system: Biosecurity and Biosafety. The 11th China-Japan-Korea Meeting of Heads of Aquatic Research Institutions. November 19. 2019a, Busan, South Korea
|
ZHANG Q L. Ecological risk of covert mortality nodavirus: From ponds to wild sea. SG-IMCE PICES, 2019b, Victoria, Canada
|
ZHANG Q L. Evidences for cross-species infection in fish of covert mortality nodavirus (CMNV). 12th Asian Fisheries and Aquaculture Forum, April 8–12 2019c, Iloilo Convention Center, Iloilo City, Pilipinas
|
ZHANG Q L. Pathogenic mechanism and pathogen ecology of covert mortality nodavirus in shrimp, 16th Symposium of Crustacean Zoology Society, Baoding, Hebei, 2022, 11, 12–13 [张庆利. 虾类偷死野田村病毒致病机制与病原生态学. 甲壳动物学分会第十六次学术研讨会, 河北保定, 2022, 11, 12–13]
|
ZHANG X W, YAP Y L, DANCHIN A. Testing the hypothesis of a recombinant origin of the SARS-associated coronavirus. Archives of Virology, 2005, 150(1): 1-20 |
ZOU Y, XIE G S, JIA T C, et al. Determination of the infectious agent of translucent post-larva disease (TPD) in Penaeus vannamei. Pathogens, 2020, 9(9): E741 |



