2. 农业农村部水产品质量安全检测与评价重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071;
3. 青岛大学生命科学学院水生生物技术研究院 山东 青岛 266071;
4. 海水养殖生物育种与可持续产出全国重点实验室 中国水产科学研究院黄海水产研究所 山东 青岛 266071
2. Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Affairs, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China;
3. Institute of Aquatic Biotechnology, College of Life Sciences, Qingdao University, Qingdao 266071, China;
4. State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
近年来,由于人类活动和气候变化等原因导致我国近海赤潮频发,麻痹性贝类毒素(paralytic shellfish toxins,PSTs)污染问题已十分突出,基本呈现逐年加剧的趋势(梁玉波等, 2019)。欧盟、美国、加拿大等多个国家或地区对其制定了严格的限量标准(800 μg STXeq/kg)并加以监控(范礼强等, 2021; 郑旭颖等, 2022)。目前,PSTs检测方法主要包括酶联免疫吸附法(ELISA)、小鼠生物分析法(MBA)、液相色谱(HPLC)和液相色谱-串联质谱法(HPLC-MS/MS)(Wright et al, 2021)。由于食品基质的复杂性,基质效应往往会对检测方法的准确性和精密度造成一定的干扰,在分析检测过程中采用基体标准物质进行质量控制可以有效克服基质效应引起的误差(林若漫等, 2022)。目前在全球范围内,加拿大海洋研究所在售的腹泻性贝类毒素及氮杂螺环酸毒素等基体标物的制备流程最为成熟,并已获得标准物质证书。这些高质量且可靠的标准物质是分析方法开发、验证和仪器校准的宝贵工具。2013年英国的环境、渔业和水产养殖科学中心采用高密度的芬迪亚历山大藻(Alexandrium fundyense)暴露太平洋牡蛎(Magallana gigas),首次实现了8种PSTs基体标准物质的产品化。目前,我国还没有具有溯源、有证、实物标样的麻痹性贝类毒素标准物质,不利于保障检测结果数值的准确性和可溯源性(Burrell et al, 2016)。
我国近海PSTs产毒藻种类繁多且分布十分广泛,并且具有明显的区域及种间差异。我国赤潮频发的多株PSTs产毒藻中,链状亚历山大藻(A. catenella)主要分布在渤海和黄海,太平洋亚历山大藻(A. pacificum)主要分布在东海和南海(Gao et al, 2015)。链状亚历山大藻(A. catenella)和塔玛亚历山大藻(A. tamarense)主要分布在长江口及其邻近海域(柳阳, 2017)。微小亚历山大藻(A. minutum)主要分布在近岸海域(于仁成等, 2020),链状裸甲藻(Gymnodinium catenatum)主要集中在渤海、江苏连云港和福建近海(Liu et al, 2017; 梁玉波等, 2019)。此外,不同产毒藻所产PSTs毒素组成和毒性大小也存在着很大差别,从而显著影响双壳贝类中蓄积的毒素。南海区域贝类中主要毒素为膝沟藻毒素(gonyautoxin,GTX)、石房蛤毒素(saxitoxin,STX)和新石房蛤毒素(neosaxitoxin,neoSTX)((Yao et al, 2019),黄海区域毒素以N-磺酰氨甲酰基类毒素(N-sulofocarbamoyltoxins,C)和GTX为主(Wong et al, 2011),渤海区域贝类中毒素以GTX和NeoSTX为主(Liu et al, 2017),其中,赤潮较为严重的秦皇岛地区贝类中PSTs主要组成为GTX。由此可知,我国近海PSTs污染情况较为复杂,所以制备多种PSTs基体标准物质对于提升贝类质量安全监管工作的成效具有重要意义。
近年来,我国已经相继开展了PSTs基体标准物质的制备技术研究。王秋艳等(2012)以黄渤海海域自然赤潮污染PSTs的扇贝为原料,制备得到冻干粉基体标准物质,但存在毒素含量和种类不可控制且无法稳定产品化生产的不足。桂文锋(2020)用实验室培养所得的塔玛亚历山大藻投喂扇贝,制备了含有GTX1、GTX4、C1、C2的4种PSTs基体标准物质,但单一产毒藻暴露的毒素种类较少,无法覆盖国际限量目标且不符合我国多种PSTs共存的现状。兰义洋等(2023)则将链状裸甲藻养液与牡蛎均质混匀获得含有GTX1、GTX2、GTX3和GTX4的PSTs基体标准物质。综上可见,现有PSTs基体标准物质制备技术主要存在原料来源不稳定、产品毒素种类较少且无法商品化生产等缺点。基于此,我们拟通过比较我国近海常见PSTs产毒藻,筛选出产毒量高且生长较好的藻株为原料,利用混合暴露的方式,研究满足国际限量要求及我国现状的多种PSTs组分基体标准物质原料制备技术,可有望形成用于水产品质量监控、海洋环境污染风险评估等工作,并对PSTs风险形成机制等理论研究提供参考物质。
1 材料与方法 1.1 实验材料 1.1.1 试剂乙腈(质谱级),美国Merck公司;乙酸(色谱级),美国Thermo公司;甲酸(色谱级),瑞士Fluka公司;甲酸铵(色谱级),瑞士Fluka公司;超纯水(18.2 MΩ·cm),美国Millipore Simplicity公司。13种PSTs标准物质包括:石房蛤毒素(STX)、新石房蛤毒素(NEO)、脱甲酰基类毒素(dcSTX、dcNEO、dcGTX2、dcGTX3)、膝沟藻毒素(GTX1、GTX2、GTX3、GTX4)和N-磺酰氨甲酰基类毒素(GTX5、C1、C2),这些毒素均购自加拿大国家研究院海洋生物科学研究所。
1.1.2 产毒藻及贻贝链状亚历山大藻(GY-H25)、链状裸甲藻(GY-H65)和塔玛亚历山大藻(GY-H31、AT5-3)由中国科学院海洋研究所提供,太平洋亚历山大藻(TI0989)和微小亚历山大藻(GY-H46)由中国海洋大学提供。其余产毒藻购自于上海光语生物科技有限公司。
紫贻贝(Mytilus galloprovincialis)为青岛周边海域野生二龄贝,平均规格:壳高为(7.0±0.5) cm,壳长为(3.5±0.5) cm。实验室内暂养2 d,持续通气并每天更换天然海水。养殖温度为6.0~10.0 ℃,盐度为29.0±1.0。
1.1.3 主要仪器及设备光照培养箱(上海一恒科学仪器有限公司);液相色谱-四极杆/离子阱复合质谱(美国SCIEX公司, 型号: 5500 QTRAP),配有电喷雾离子源(ESI)及MultiQuant数据处理系统;高速离心机(日本Hitachi公司, 型号: Himac CR 22GⅡ);恒温水浴锅(上海亚荣生化仪器公司, 型号: B-260);旋涡混合器(上海医大仪器厂, 型号: XW-80A);超纯水仪(美国Millipore公司, 型号: Gradient A10);固相萃取装置(美国Supelco公司);超声波细胞粉碎机(新芝生物科技公司, 型号: JY92-IIN)。
1.2 实验方法 1.2.1 产毒藻三级扩大培养一级培养:产毒藻接种于经高温灭菌的100~250 mL三角烧瓶中,置于光照培养箱中培养5~7 d,细胞密度达到1×104 cell/mL。
二级培养:按照1∶3 (藻液∶培养液,V∶V) 扩培至经高温灭菌的1 L的三角瓶中,置于光照培养箱中培养8~10 d,至细胞密度达到5×105 cell/mL。
三级培养:按照1∶5 (藻液∶培养液, V∶V)扩培到灭过菌的5 L食品级PET培养罐中,置于光照培养室每3 d取用1/3藻液并补充等量培养液进行半连续培养。
光照培养箱设置条件为:光照为4 000 lx,温度为(25.0±1.0) ℃,光暗比12 h∶12 h;光照培养室设置条件为:空调控温(23.0±1.0) ℃,双侧连续光照(6 000±1 000) lx,光暗比12 h∶12 h。
通过藻细胞计数和藻毒素提取的方法检测产毒藻的生长和产毒情况。培养的藻细胞密度在饲喂贻贝前测定,取样前充分摇匀。每瓶取样3次,每次取1 mL,加入50 μL鲁格试液(Lugol's)固定,充分摇匀后在光学显微镜下进行藻细胞计数。
1.2.2 4株产毒藻单独暴露贻贝随机挑选280只贻贝平均分为4个实验组,分别放置在盛有20 L海水的4个养殖筐中进行实验。在7 d的暴露时间内,每个实验组分别单独投喂GY-H25、GY-H65、GY-H31和GY-H46等4株产毒藻。每只贝每天投喂产毒藻的数量约为1.0×105 cells,产毒藻投喂时间分别为08:00和20:00,控制2次投喂量比例为1∶2。分别于投喂产毒藻后1、2、3、4、5、6和7 d各取10只贻贝进行解剖并采集所有软组织,匀浆混匀后于–20 ℃下冷冻保存,待统一进行麻痹性贝类毒素检测。
1.2.3 4株产毒藻混合暴露贻贝随机挑选20只贻贝平均分为2个实验组,分别放置在2个盛有20 L海水的养殖筐中进行实验。在整个暴露期内,投喂不同比例的4株产毒藻GY-H25、GY-H65、GY-H31和GY-H46。低密度组实验共进行7 d,每只贝每日投喂总藻量为3.5×104 cells,投喂比例为1∶1∶1∶4。高密度组实验共进行4 d,每只贝每日投喂总藻量为2.6×105 cells,投喂比例为1∶1.6∶2.4∶8。产毒藻投喂时间分别为08:00和20:00,控制2次投喂量比例为1∶2。暴露实验结束后,收集2个实验组的所有贻贝进行解剖并采集所有软组织混匀,匀浆混匀后于冰箱内–20 ℃下冷冻保存,待统一进行麻痹性贝类毒素检测。
1.2.4 产毒藻及贻贝中PSTs测定取稳定期生长的藻细胞,用GF/F玻璃纤维滤膜抽滤并收集106 cells。将滤膜剪碎(< 0.2 cm×0.2 cm),置于15 mL离心管中并加入5 mL 1%乙酸水提取液,冰浴超声破碎(间隔2 s,破碎5 min)。静置5 min后取2 mL样液,10 000 r/min离心10 min,移取1 mL上清液过0.22 μm水系滤膜,待统一进行麻痹性贝类毒素检测。
贻贝中毒素提取和分析过程参考张海涛等(2023)检测方法。毒素提取方法简述如下:将贝类软组织样品进行匀浆处理,每个样品称取3个平行样品,重量为(5.0±0.1) g。用1%的乙酸水溶液提取,沸水浴5 min,冷却后以4 500 r/min离心10 min,移取1 mL提取液于离心管中,加入5 μL氨水,涡旋混匀,10 000 r/min离心10 min。用活化过的Supelco ENVI-Carb固相萃取柱萃取,0.22 μm滤膜过滤,滤液收集于进样小瓶中,于–20 ℃下冷冻保存供上机分析。使用高效液相色谱-质谱联用检测法进行麻痹性贝毒分析,其中藻毒素含量单位为pg/cell,贝中PSTs含量单位为μg/kg。并采用毒性等效因子(TEF)计算藻中和贝软组织PSTs总毒力(Joana et al, 2017),其单位分别为μg STX eq/cell和μg STXeq/kg (Alonso et al, 2016)。经测定贻贝中PSTs各组分均未检出后用于暴露实验。
1.2.5 PSTs蓄积转化率、平均蓄积转化率及PSTs含量(理论值)1) PSTs蓄积转化率是指贻贝对产毒藻中的PSTs蓄积转化能力。
蓄积转化率计算公式如下:
| $ \left| \!{\overline {\, {A=\left(m \times n \times X_{\text {贝 }}\right) /\left(V_{\text {藻 }} \times \rho_{\text {藻 }} \times X_{\text {藻 }}\right)} \,}} \right. $ | (1) |
式中,A为每天紫贻贝蓄积转化率(%);m为紫贻贝质量(g);n为每一养殖容器紫贻贝数量(个);
2) PSTs平均蓄积转化率是指PSTs蓄积阶段平均每天的蓄积转化率。
平均蓄积转化率计算按照式(2)进行:
| $ \left| \!{\overline {\, {\bar{A}=\frac{\sum\limits_{i=1}^t A}{t}} \,}} \right. $ | (2) |
式中,
3) PSTs含量(理论值)是指4株产毒藻混合暴露时,理论上贻贝蓄积的PSTs含量。
PSTs含量(理论值)计算公式如下:
| $ \left| \!{\overline {\, {x_T=\frac{C_{\text {贝 }} \times N_{\text {混合 }}}{N_{\text {单独 }}}} \,}} \right. $ | (3) |
式中,
实验数据采用SPSS 25.0和Excel 2019等软件进行单因素方差分析(one-way ANOVA),以P < 0.05为差异显著水平,实验结果以平均值±标准差(Mean±SD)表示。并采用Origin 2022软件作图说明。
2 结果 2.1 产毒藻生长和毒素组成分析通过逐级扩大培养6株产毒藻生长状况良好,三级培养液中平均藻细胞密度达4.0×106~4.0× 107 cells/L。其中,单藻细胞产毒量从高到低排序为GY-H46、GY-H31、AT5-3、GY-H25、GY-H65、TI0989。GY-H46产毒量可达8.3 pg/cell,而TI0989仅为0.3 pg/cell。不同藻株PSTs毒素组成具有显著差异,如图 1b所示,GY-H46仅产4种GTX类毒素,与其他5株藻产毒差异最大。GY-H31和AT5-3产生毒素种类相似,主要组分包括NEO、GTX1、GTX4、GTX5、C1和C2,但各毒素比例不同(图 1e、f)。而GY-H25和TI0989产生毒素种类相似,主要包括NEO、GTX5、C1和C2 (图 1a、d)。GY-H65的主要毒素为dcSTX、dcNEO、dcGTX3、C1和C2 (图 1c)。通过比较6株产毒藻的单细胞产毒量、藻密度和毒素组分,排除单细胞产毒量低于1.0 pg/cell的TI0989以及与GY-H31毒素类似的AT5-3,筛选GY-H46、GY-H31、GY-H25和GY-H65进行贻贝的暴露实验。该4株产毒藻所产毒素种类覆盖了我国海域常见的C和GTX毒素类型,且具有稳定的产毒性状。
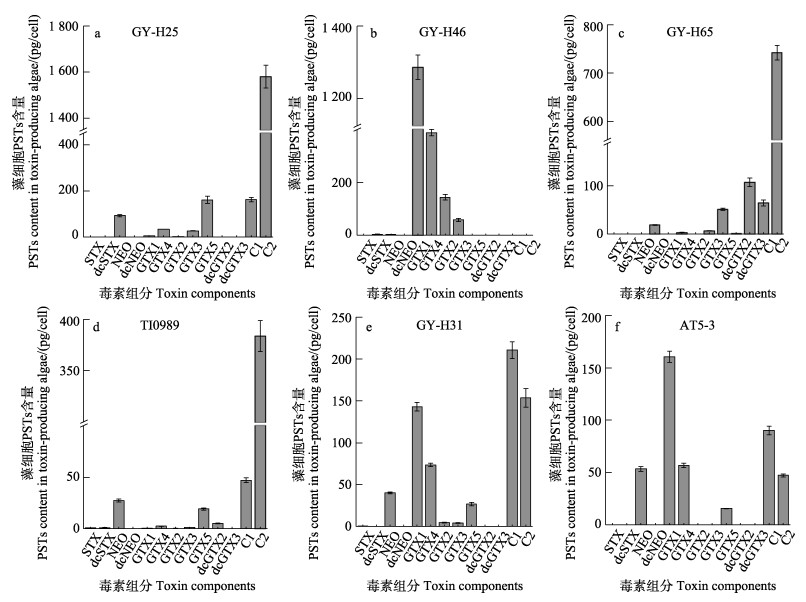
|
图 1 6株PSTs产毒藻稳定期单细胞产毒组成及含量(n=3, Mean±SD) Fig.1 PST compositions and content of six toxin-producing strains in the stationary phase(n=3, Mean±SD) |
通过4株产毒藻单一暴露实验,评估不同产毒藻中PSTs在贻贝体内的蓄积和转化差异。如图 2所示,4株产毒藻暴露贻贝后PSTs含量持续升高,至7 d达到最高,其中,GY-H46暴露的贻贝总毒力最高为(1 151.0±7.6) μg STXeq/kg,显著高于其他产毒藻(P < 0.05)。贻贝与产毒藻毒素组成高度相关,其中,GY-H25与贻贝中主要毒素(NEO、GTX5、C1和C2)组成保持一致(图 2a)。投喂GY-H31的贻贝的主要组分为GTX1、GTX5、C1和C2,与藻中的主要毒素组分相似(图 2c)。投喂GY-H46的贻贝体内主要组分与藻保持一致,其中,GTX1和GTX4占比逐渐下降,GTX2和GTX3占比逐渐上升(图 2d)。值得注意的是,GY-H65与贻贝中的毒素组成及占比具有显著差异(图 2b),且经过贻贝代谢转化出新组分dcSTX最高占比为29.6%,dcNEO在GY-H65和贝中的占比从1.9%升高至13.2%。此外,投喂GY-H25和GY-H65的贻贝体内出现了C2向C1的转化。在暴露阶段,4 d时,贻贝对产毒藻的蓄积率下降(图 2)。其中,贻贝对GY-H65的平均蓄积转化率为83.1%,显著高于其他产毒藻。GY-H46的平均蓄积转化率最低为39.7% (图 3)。
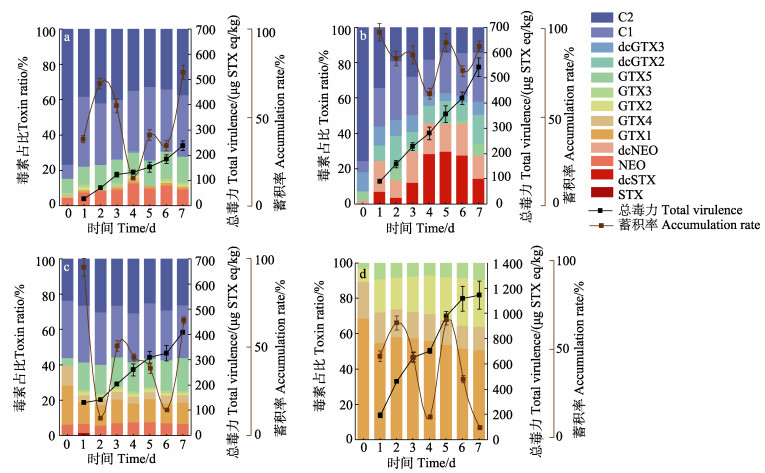
|
图 2 4株产毒藻单独暴露贻贝蓄积率、PSTs含量及各组分占比(n=3, Mean±SD) Fig.2 Accumulation rate, content of PSTs and proportion of each component in mussels exposed to four strains of toxic-producing algae alone (n=3, Mean±SD) a: GY-H25;b: GY-H65;c: GY-H31;d: GYH-46; 0 d为稳定期的产毒藻。 a: GY-H25; b: GY-H65; c: GY-H31; d: GYH-46; 0 d is the stable phase of toxic dinoflagellates. |
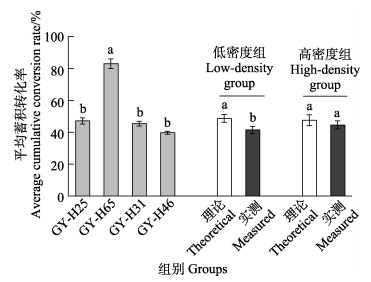
|
图 3 单一暴露和混合暴露条件下贻贝中PSTs的平均蓄积转化率 Fig.3 Mean accumulation conversion rates of PSTs in mussels under single and mixed exposure conditions 不同字母表示存在差异(P < 0.05,存在显著差异)。 Different letters indicate differences (P < 0.05, significant difference). |
比较2个混合暴露组贻贝中PSTs含量结果表明,低密度组PSTs含量(1 375.0±8.9) μg/kg高于理论值(1 093.0±9.7) μg/kg,而高密度组PSTs含量(5 456.0± 17.9) μg/kg高于理论值(4 629.0±16.3) μg/kg。经毒性因子换算后,低密度组和高密度组总毒性分别为(535.0±5.6) μg STXeq/kg和(2 636.0±12.4) μg STXeq/kg。混合暴露后2个实验组的贻贝中均蓄积了12种PSTs (图 4a、b)组分,其中,GTX1、GTX2、GTX4、GTX5、dcGTX2、C1和C2为主要毒素组分,相对含量占比均高于5%。低密度组各毒素组分代谢转化比率显著高于高密度组,其中C1和C2实测占比均升高。其中,低密度组C1和C2实测值占比高于理论值,C1的实测值和理论值分别为20.9%和13.3%,C2的实测值和理论值分别为15.4%和10.8% (图 4a)。而高密度组仅C1的占比与理论值存在明显差异,分别为17.7%和11.7% (图 4b)。与此同时,低密度组GTX1实测值和理论值分别为20.0%和29.7%。比较不同密度组的理论和实测平均蓄积转化率可知,2个混合暴露组贻贝的平均蓄积转化率基本一致,分别为41.4%和44.6%,均低于48.7%和47.5%的理论值(图 3)。
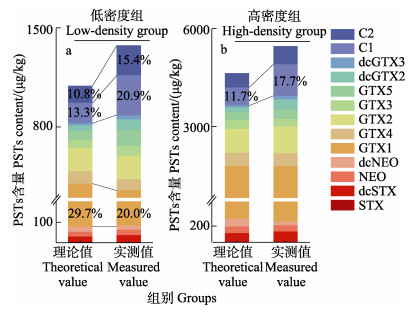
|
图 4 混合暴露下贻贝中PSTs各组分含量及占比的理论值与实测值差异比较 Fig.4 Comparison between theoretical and measured values of the content and proportion of each component of PSTs in mussels under mixed exposure |
贻贝作为我国近海重要养殖经济贝类,渤海、黄海、东海、南海四大海域贻贝的PSTs检出率和残留量都较高(范礼强等, 2021)。诸多研究表明,贻贝较其他贝类具有更高的蓄积能力(Cheng et al, 2010; Navarro et al, 2008; Carvalho et al, 2019; Qiu, 2013; 范礼强等, 2021),也是造成我国PSTs食物中毒事件的主要物种(Freitas et al, 2019)。目前,针对产毒藻暴露贝类获得PSTs基体标准物质研制已开展了相关的研究(Higman et al, 2010; 桂文锋, 2020),揭示了实验室条件制备麻痹类贝类毒素基体标准物质的可行性。本研究筛选出的4株产毒藻的单细胞产毒量和藻密度均相对较高,并且4株产毒藻的毒素组成具有一定的互补性。通过对比分析贻贝单独暴露于4株产毒藻后体内麻痹性贝毒含量和组成的变化,对4株产毒藻混合暴露贻贝的配比和毒素蓄积情况进行研究和分析。最后确定一种产毒藻混合暴露的方案,获得较为稳定的PSTs标准物质制备原料。
近年来,针对贻贝摄食不同麻痹性产毒藻后体内麻痹性贝毒的累积、转化和排出过程已开展了大量研究(Liu et al, 2017; 张海涛等, 2023; Gracia et al, 2020; Raposo et al, 2020)。研究表明,产毒藻的单细胞产毒量、暴露藻细胞密度、暴露持续时间以及PSTs的代谢转化均影响最终贻贝中PSTs的组成及含量。此外,有研究发现,滤食性贝类对水体中悬浮颗粒物的滤食存在一定选择性(刘旭平等, 2023),在3种藻相同密度混合条件下,更倾向摄食粒径较大的藻(蓝文陆等, 2018),摄食的选择性会直接影响贻贝对藻毒素的蓄积。贻贝对不同产毒藻的摄食率与藻细胞的大小具有显著相关性,而与单细胞产毒量及毒性无显著相关性(Navarro et al, 2008)。与之相似,本研究中,GY-H65为链状裸甲藻,是4种藻中个体最大的藻,因此,其蓄积转化率最高。GY-H46是4种藻中体积最小的藻,投喂GY-H46的贻贝的蓄积转化率也最低。此外,4株产毒藻联合暴露贻贝时,毒素的蓄积量由4株产毒藻的蓄积转化率共同决定。本研究发现,提高产毒藻的投喂量,总毒素的蓄积量也随之升高。赵俊梅等(2004)的研究也反映了类似的结果,投喂密度增加时贻贝的摄食率升高,PSTs蓄积量增加,这与贻贝高蓄积的特性有关。张亚亚等(2020)研究表明,自然赤潮下贻贝的PSTs毒素蓄积量最高达52 072.8 μg STXeq/kg,说明贻贝可最大化的实现PSTs的蓄积。
本研究通过4株产毒藻单一暴露发现贻贝中毒素组成与产毒藻的毒素高度相关,Lewis等(2022)用微小亚历山大藻和链状亚历山大藻露贻贝的研究发现,产毒藻中PSTs的组成影响贝类中毒素分布特征。通常贻贝体内PSTs的代谢转化率相对较低(Botelho et al, 2020),能够迅速积累毒素并在中毒后保持相对稳定的毒素分布(Lewis et al, 2022)。Botelho等(2015)研究发现,在暴露初期贻贝体内的PSTs组分与藻细胞中类似;Kim等(2015)进行了塔玛亚历山大藻中PSTs毒素组分与贻贝中的对比,发现暴露初期贻贝体内的PSTs组分比例与藻细胞中类似,均是C2和GTX4占比高于C1和GTX1。但随着暴露时间的增加贻贝中主要毒素变为C1和GTX1,贻贝与摄入的产毒藻间PSTs组成和比例有差异,这被认为是毒素被吸收后发生不同的生物转化和代谢途径的结果(Blanco et al, 2003; Yu et al, 2007)。本研究中,贻贝摄食GY-H25、GY-H31和GY-H46 3种产毒藻后,贻贝体内的毒素组分种类与摄入的产毒藻一致,但各毒素相对比例发生变化。贻贝不仅蓄积产毒藻中的PSTs组分,还可以代谢转化为其他PSTs组分(Bricelj et al, 1998),并且PSTs在贻贝体内的生物转化具有产毒藻物种特异性。本研究中,摄食GY-H65的贻贝中的毒素与藻中毒素组成和占比差异明显,并代谢转化形成dcSTX。Paulo (2010)采用产生C1、C2、C3、C4、GTX5和GTX6的链状裸甲藻暴露贻贝,主要转化形成的毒素组分包括dcGTX2、dcGTX3、dcSTX和dcNEO,与本研究的结果一致。同时,本研究发现,提高GY-H65的投喂比例,贻贝中dcSTX和dcNEO占比升高。由此可见,不同的产毒藻会在贻贝体内形成不同的毒素轮廓并且贻贝体内各毒素的占比相对稳定。
在双壳贝类中普遍存在N-磺氨甲酰基类向氨基甲酸酯类毒素的转化,如C1向GTX2,C2向GTX3,GTX5向STX的转化等(宋维佳等, 2022)。Choi等(2003)用只含有C2毒素的塔玛亚历山大藻对贻贝开展暴露实验,贻贝内检测到了GTX2和GTX3等毒素,证实了贻贝能够将低毒性的N-磺酰氨甲酰基类毒素转化成高毒性的氨基甲酸酯类毒素。本研究中没有观察到明显的C向GTX的转化,可能与暴露时间较短有关。通常双壳贝类中C11位有羟基硫酸基团的PSTs可以通过热力学平衡发生差向异构化(包振民等, 2021),毒藻中稳定的β型(GTX3、GTX4和C2)毒素在双壳贝类中逐渐转化成更稳定的α型(GTX1、GTX2和C1)毒素(Escobedo-Lozano et al, 2012)。Qiu等(2018)发现太平洋亚历山大藻中占主导的是GTX4,而在摄食该藻后的贻贝中则是GTX1占比最高。Qiu等(2013)用塔玛亚历山大藻暴露贻贝的5 d的蓄积过程中C1+C2的占比从43%增加到50%,GTX1+GTX4的占比从17%降到12%。Lewis等(2022)研究发现,微小亚历山大藻和链状亚历山大藻混合暴露贻贝的实验中发生GTX和NEO向STX的转化,暴露时间越长转化越多。本研究发现,在贻贝体内出现了C2向C1的差向异构化反应,并且混合暴露实验发现7 d时发生了明显的GTX向C的转化。总体而言,4株产毒藻混合暴露贻贝4 d的暴露期与7 d的暴露期相比毒素转化种类及比例均较低。综上,为期4 d的混合暴露的整个实验流程可控,能够实现多种麻痹性贝类毒素原料的批量制备。
4 结论本研究通过对比分析贻贝暴露于上述4株产毒藻后体内麻痹性贝毒含量和组成的变化,对贻贝蓄积转化PSTs进行了研究和分析。用不同配比和不同投喂量的4株产毒藻混合暴露贻贝,获得了2种含有12种PSTs组分的总毒性分别为(535.0±5.6) μg STXeq/kg和(2 636.0±12.4) μg STXeq/kg的基体标准物质原料。结果证实了4株产毒藻混合投喂贻贝可以迅速积累毒素,并在蓄积毒素后保持相对稳定的毒素分布。这是首次通过混合暴露制备获得麻痹性贝类毒素的基体标准物质原料,为后续研制基体标准物质提供了重要的参考方法。
ALONSO E, ALFONSO A, VIEYTES M R, et al. Evaluation of toxicity equivalent factors of paralytic shellfish poisoning toxins in seven human sodium channels types by an automated high throughput electrophysiology system. Archives of Toxicology, 2016, 90(2): 479-488 DOI:10.1007/s00204-014-1444-y |
BAO Z M, KONG L L, SHI J X, et al. Research progress on accumulation and transformation of paralytic shellfisl. Periodical of Ocean University of China, 2021, 51(10): 1-11 [包振民, 孔令玲, 史姣霞, 等. 双壳贝类积累转化麻痹性贝毒的研究进展. 中国海洋大学学报(自然科学版), 2021, 51(10): 1-11] |
BLANCO J, REYERO M I, FRANCO J, et al. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis. Toxicon, 2003, 42(7): 777-784 DOI:10.1016/j.toxicon.2003.10.007 |
BOTELHO M J, MARQUES F, FREITAS R, et al. Paralytic shellfish toxin profiles in mussel, cockle and razor shell under postbloom natural conditions: Evidence of higher biotransformation in razor shells and cockles. Marine Environmental Research, 2020, 154: 104839 DOI:10.1016/j.marenvres.2019.104839 |
BOTELHO M J, VALE C, FERREIRA J G. Profiles of paralytic shellfish toxins in bivalves of low and elevated toxicities following exposure to Gymnodinium catenatum blooms in Portuguese estuarine and coastal waters. Chemosphere, 2015, 138: 1028-1036 DOI:10.1016/j.chemosphere.2014.12.072 |
BRICELJ V M, SHUMWAY S E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Reviews in Fisheries Science, 1998, 6(4): 315-383 DOI:10.1080/10641269891314294 |
BURRELL S, CRUM S, FOLEY B, et al. Proficiency testing of laboratories for paralytic shellfish poisoning toxins in shellfish by quasimeme: A review. TrAC Trends in Analytical Chemistry, 2016, 75: 10-23 DOI:10.1016/j.trac.2015.09.004 |
CARVALHO I, PELERITO A, RIBEIRO I, et al. Paralytic shellfish poisoning due to ingestion of contaminated mussels: A 2018 case report in caparica (Portugal). Toxicon, 2019, 4: 100017 |
CHENG L M, IVANOVA O, FAN H H, et al. An integrative model of respiratory and cardiovascular control in sleep-disordered breathing. Respiratory Physiology and Neurobiology, 2010, 174(1/2): 4-28 |
CHOI M C, HSIEH D P H, LAM P K S, et al. Field depuration and biotransformation of paralytic shellfish toxins in scallop chlamys nobilis and green-lipped mussel Perna viridis. Marine Biology, 2003, 143: 927-934 DOI:10.1007/s00227-003-1148-y |
ESCOBEDO-LOZANO A Y, ESTRADA N, ASCENCIO F, et al. Accumulation, biotransformation, histopathology and paralysis in the pacific calico scallop Argopecten ventricosus by the paralyzing toxins of the dinoflagellate Gymnodinium catenatum. Marine Drugs, 2012, 10(5): 1044-1065 DOI:10.3390/md10051044 |
FAN L Q, ZHENG G C, WU H Y, et al. Research progress on the accumulation and metabolism of paralytic shellfish toxin in mussels. Marine Sciences, 2021, 45(4): 201-212 [范礼强, 郑关超, 吴海燕, 等. 贻贝对麻痹性贝类毒素的蓄积代谢研究进展. 海洋科学, 2021, 45(4): 201-212] |
FREITAS R, COPPOLA F, COSTA S, et al. The influence of temperature on the effects induced by triclosan and diclofenac in mussels. Science of The Total Environment, 2019, 663: 992-999 DOI:10.1016/j.scitotenv.2019.01.189 |
GAO Y, YU R C, CHEN J H, et al. Distribution of Alexandrium fundyense and A. pacificum (Dinophyceae) in the Yellow Sea and Bohai Sea. Marine Pollution Bulletin, 2015, 96(1/2): 210-219 |
GRACIA V L, TOBKE J L, MONTOYA N G, et al. Experimental exposure of the mussel Mytilus platensis (d'Orbigny, 1842) to the dinoflagellate Alexandrium catenella from argentine patagonia. Ecotoxicology, 2020, 29(2): 226-235 DOI:10.1007/s10646-020-02169-5 |
GUI W F. Exploration of the growth and toxic production conditions of two toxic red tide algae and preparation of basic reference materials. Master′s Thesis of Shanghai Normal University, 2020, 58–64 [桂文锋. 两种有毒赤潮藻生长产毒条件探索及基体标准物质制备. 上海师范大学硕士研究生学位论文, 2020, 58–64]
|
HIGMAN W A, TURNER A. A feasibility study into the provision of paralytic shellfish toxins laboratory reference materials by mass culture of Alexandrium and shellfish feeding experiments. Toxicon, 2010, 56(4): 497-501 DOI:10.1016/j.toxicon.2010.05.004 |
JOANA F L, MARIA L S C. Marine paralytic shellfish toxins: chemical properties, mode of action, newer analogues, and structure-toxicity relationship. Royal Society of Chemistry, 2022, 39(1): 33-57 |
KIM H Y, SHIN I S. Comparison of paralytic shellfish toxin profiles of Alexandrium tamarense and blue mussel (Mytilus edulis) in Korea. Food Science and Biotechnology, 2015, 24(2): 751-756 DOI:10.1007/s10068-015-0097-9 |
LAN Y Y, HONG L, XU B W, et al. Preparation of GTX1-4 of paralytic shellfish poisoningin oyster matrix reference material. Quality Safety Inspection and Testing, 2023, 33(1): 81-88 [兰义洋, 洪麟, 许炳雯, 等. 牡蛎基体麻痹性贝类毒素GTX1-4标准样品的研制. 质量安全与检验检测, 2023, 33(1): 81-88] |
LAN W L, FU J X, YANG B, et al. Study on feeding selectivity of Crassostrea hongkongensis feeding on three different phytoplankton. Haiyang Xuebao, 2018, 40(8): 79-88 [蓝文陆, 付家想, 杨斌, 等. 香港巨牡蛎对3种微藻的选择性摄食研究. 海洋学报, 2018, 40(8): 79-88] |
LEWIS A M, DEAN K J, HARTNELL D M, et al. The value of toxin profiles in the chemotaxonomic analysis of paralytic shellfish toxins in determining the relationship between british Alexandrium spp. and experimentally contaminated Mytilus sp.. Harmful Algae, 2022, 111: 102131 DOI:10.1016/j.hal.2021.102131 |
LIANG Y B, LI D M, YAO J Y, et al. Progresses in investigation and research on phycotoxins and toxic microalgaes in the coastal waters of China. Oceanologia et Limnologic Sinica, 2019, 50(3): 511-524 [梁玉波, 李冬梅, 姚敬元, 等. 中国近海藻毒素及有毒微藻产毒原因种调查研究进展. 海洋与湖沼, 2019, 50(3): 511-524] |
LIN R M, LIN Z H. Research progress of biotoxin matrix reference materials. China Food Safety Magazine, 2022(35): 183-187 [林若漫, 林振华. 生物毒素基体标准物质研究进展. 食品安全导刊, 2022(35): 183-187] |
LIU X P, SHAN Z C, LI Y F, et al. Research status on the selective feeding behavior of filter-feeding shellfish. Pecial Economic Animals and Plants, 2023, 26(5): 89–92, 107 [刘旭平, 单忠才, 李媛芳, 等. 滤食性贝类选择性摄食行为研究现状. 特种经济动植物, 2023, 26(5): 89–92, 107] |
LIU Y, YU R C, KONG F Z, et al. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Marine Pollution Bulletin, 2017, 115(1/2): 324-331 |
LIU Y. Studies of phycotoxin contamination in the coastal waters of China and preparation of toxin reference matreials. Doctoral Dissertation of University of Chinese Academy of Sciences (Institute of Oceanography, Chinese Academy of Sciences), 2017, 110–112 [柳阳. 我国近海藻毒素污染状况研究与毒素标准物质制备. 中国科学院大学(中国科学院海洋研究所)博士研究生学位论文, 2017, 110–112]
|
NAVARRO J M, CONTRERAS A M, CHAPARRO O R, et al. Short-term feeding response of the mussel Mytilus chilensis exposed to diets containing the toxic dinoflagellate Alexandrium catenella. Revista Chilena de Historia Natural, 2008, 81(1): 41-49 |
PAULO V. Metabolites of saxitoxin analogues in bivalves contaminated by Gymnodinium catenatum. Toxicon, 2010, 55(1): 162-165 DOI:10.1016/j.toxicon.2009.07.010 |
QIU J B, MA F F, FAN F, et al. Effects of feeding Alexandrium tamarense, a paralytic shellfish toxin producer, on antioxidant enzymes in scallops (Patinopecten yessoensis) and mussels (Mytilus galloprovincialis). Aquaculture, 2013, 396: 76-81 |
QIU J B, MENG F P, DING L, et al. Dynamics of paralytic shellfish toxins and their metabolites during timecourse exposure of scallops Chlamys farreri and mussels Mytilus galloprovincialis to Alexandrium pacificum. Aquatic Toxicology, 2018, 200: 233-240 DOI:10.1016/j.aquatox.2018.05.003 |
RAPOSO M I C, GOMES M T S R, BOTELHO M J, et al. Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxins, 2020, 12(344): 1-21 |
SONG W J, SONG X X, YU Z M, et al. Synthesis, transformation and influencing factors of paralytic shellfish toxins in the ocean. Marine Sciences, 2022, 46(9): 117-129 [宋维佳, 宋秀贤, 俞志明, 等. 海洋中麻痹性贝类毒素的合成转化及其影响因素研究进展. 海洋科学, 2022, 46(9): 117-129] |
WANG Q Y, ZHAO J H, XU Y, et al. Preparation of paralytic shellfifish poison reference material. Chemical Analysis and Meterage, 2012, 21(2): 4-6 [王秋艳, 赵景红, 徐杨, 等. 麻痹性贝类毒素标准样品的研制. 化学分析计量, 2012, 21(2): 4-6] |
WONG C K, HUNG P, NG H C C, et al. Cluster analysis of toxins profile pattern as a tool for tracing shellfish contaminated with PSP-Toxins. Environmental Research, 2011, 111(8): 90-1083 |
WRIGHT E J, MCCARRON P. A mussel tissue certified reference material for multiple phycotoxins. Part 5: Profiling by liquid chromatography-high-resolution mass spectrometry. Analytical and Bioanalytical Chemistry, 2021, 413(8): 2055-2069 DOI:10.1007/s00216-020-03133-2 |
YAO J Y, JIN W, LI D M, et al. Geographical distribution and seasonal variation in paralytic shellfish toxins in the coastal water of the South China Sea. Toxicon, 2019, 168: 67-75 DOI:10.1016/j.toxicon.2019.06.221 |
YU K N, KWONG R W M, WANG W X, et al. Biokinetics of paralytic shellfish toxins in the green-lipped mussel, Perna viridis. Marine Pollution Bulletin, 2007, 54(7): 1068-1071 DOI:10.1016/j.marpolbul.2007.02.007 |
YU R C, LV S H, QI Y Z, et al. Progress and perspectives of harmful algal bloom studies in China. Oceanologia et Limnologia Sinica, 2020, 51(4): 768-788 [于仁成, 吕颂辉, 齐雨藻, 等. 中国近海有害藻华研究现状与展望. 海洋与湖沼, 2020, 51(4): 768-788] |
ZHANG H T, WU H Y, ZHENG G C, et al. Accumulation and transformation of paralytic shellfish toxin in mussel Mytilus galloprovincialis exposed to Alexandrium catenella. Progress in Fishery Sciences, 2023, 44(1): 181-190 [张海涛, 吴海燕, 郑关超, 等. 链状亚历山大藻暴露下紫贻贝体内麻痹性贝毒蓄积转化规律. 渔业科学进展, 2023, 44(1): 181-190 DOI:10.19663/j.issn2095-9869.20210816001] |
ZHANG Y Y, YAN G W, WU H Y, et al. Establishment and application of detection methods to paralytic shellfish poisoning in water based on SPE and SPATT methods. Oceanologia et Limnologic Sinica, 2020, 51(2): 298-306 [张亚亚, 闫国旺, 吴海燕, 等. 基于SPE与SPATT的水体中麻痹性贝类毒素检测方法构建与应用. 海洋与湖沼, 2020, 51(2): 298-306] |
ZHAO J M, FANG J G, BAO Z M, et al. Filter-feeding of three bivalves on Alexandrium tamarense. Marine Fisheries Research, 2004, 25(4): 17-22 [赵俊梅, 方建光, 包振民, 等. 3种滤食性贝类对塔玛亚历山大藻的摄食研究. 海洋水产研究, 2004, 25(4): 17-22] |
ZHENG X Y, LI Z X, SUN X J, et al. Surveillance and risk assessment of diarrhetic and paralytic shellfish toxins in Tangshan shellfish culture areas of Bohai Sea, China. Progress in Fishery Sciences, 2023, 44(5): 231-241 [郑旭颖, 李兆新, 孙晓杰, 等. 渤海海域唐山贝类养殖区腹泻性和麻痹性贝类毒素的监测与风险评估. 渔业科学进展, 2023, 44(5): 231-241 DOI:10.19663/j.issn2095-9869.20220504001] |



