2. 宁波大学海洋学院 浙江 宁波 315832
2. School of Marine Science, Ningbo University, Ningbo 315832, China
细胞转染是将外源分子如RNA、DNA导入细胞的专门技术,现已成为制备转基因生物和基因功能研究的常规手段(柴百惠等, 2018)。细胞转染常用方法有电穿孔法、病毒感染法和脂质体转染法。电穿孔法利用瞬时高脉冲的电压击穿细胞膜,适用范围广,但操作较为繁琐,转染后致死率较高,且所需的细胞和DNA量较大,一般很少在鱼类细胞转染的过程中使用(Rambabu et al, 2005)。病毒感染法通过病毒携带DNA侵染细胞,能够实现细胞系的稳定转染,但适用范围窄,对鱼类细胞转染效率较低,一时间很难筛选到适合鱼类细胞转染的病毒,且成本较高、实验流程复杂(Ruiz et al, 2009)。脂质体法的原理是通过带正电的脂质体与细胞膜上带负电的磷酸基团相互作用,形成复合体,引导外源基因导入细胞内。脂质体转染具有适用范围广、转染效率高且操作简单等优点(Jiang et al, 2004)。然而,市面上常见的脂质体转染试剂大都以哺乳动物细胞为研究对象设计开发。海水硬骨鱼细胞的细胞膜组分、温度适应范围、需氧量等参数与哺乳动物细胞差距较大(Logue et al, 2000; Zabelinskii et al, 1995)。因此,利用常见的脂质体转染试剂转染硬骨鱼类细胞的效率相对较低,进而很难获得稳转的牙鲆(Paralichthys olivaceus)细胞。此外,传统瞬时表达载体经脂质体转染进入牙鲆细胞后,随着筛选时间的延长,大部分质粒会随着细胞分裂而逐渐丢失,少部分保留质粒的细胞也很难正确地整合到基因组上,这导致很难筛选到稳转细胞系(武标等, 2015)。受限于上述原因,长期以来,牙鲆的基因功能研究一直采用脂质体瞬时转染的方式获得转基因细胞,很难高效制备稳定转染外源基因的牙鲆细胞,这使得研究牙鲆基因的长效调控机制受到极大的限制(司瑜等, 2020)。同时,如何快速高效地制备稳转细胞已成为牙鲆细胞生物和分子生物学研究的难题。
Tol2是在青鳉(Oryzias latipes)中发现的自主型天然转座子,是histone acetyltransferase (hAT)家族成员之一,其末端反向重复序列(terminal inverted repeats, TIRs)和亚末端重复序列(subterminal repeats, SRs)对于成功转座十分重要(汤泽源等, 2011)。基因工程研究中使用Tol2转座子系统转化外源基因时,一般通过“识别–剪切–整合”的方式将外源基因整合到宿主基因组中(陶然等, 2014)。整个转座过程可概括为以下3个关键步骤:第一步,外源转化的Tol2 DNA或mRNA翻译形成转座酶;第二步,Tol2转座酶识别关键序列,形成转座复合体切割供体质粒的特定序列;第三步,转座子与宿主基因组重新整合。目前,Tol2转座子系统已成功应用于爪蟾(Xenopus tropicalis) (Hamlet et al, 2006)、鸡(Gallus gallus) (Sato et al, 2007)、小鼠(Mus musculus)(Sumiyama et al, 2010)、斑马鱼(Danio rerio) (张运生, 2020; Suster et al, 2009)、罗非鱼(Oreochromis niloticus) (Fujimura et al, 2011)、慈鲷鱼(Astatotilapia burtoni)(Juntti et al, 2013)等生物的转基因实验。此外,利用Tol2转座子系统也可以实现外源基因在中国仓鼠(Cricetulus griseus)卵巢细胞(CHO) (Yamaguchi et al, 2023)、人类(Homo sapiens)原代T细胞(Huang et al, 2010)、鸡原始生殖细胞(MacDonald et al, 2012)等细胞系的稳定表达。Tol2转座子系统是基因工程研究中构建转基因脊椎动物的基本手段,也是细胞工程研究中构建稳转外源基因细胞系的优秀工具。但到目前为止,利用Tol2转座子介导海水硬骨鱼类稳转细胞构建的研究尚未见报道。本研究探究利用脂质体转染Tol2转座子重组载体的方式迅速获得牙鲆稳定转染细胞的方法。其中,重组Tol2转座子载体是在pminiTol2质粒(Balciunas et al, 2006)的基础上改造而来,保留了Tol2转座子的功能,以实现将外源基因高效地插入到牙鲆细胞的基因组DNA中;设计增加新霉素抗性筛选标记,用来实现细胞的快速筛选;同时,融合增强绿色荧光蛋白(enhanced green fluorescent protein, EGFP)表达系统,用于示踪外源基因在细胞的表达和亚定位情况。
1 材料与方法 1.1 材料实验用牙鲆饲养在山东烟台海阳黄海水产有限公司的循环水系统中,养殖海水取自海阳地区地下水。细胞转染采用LipofectamineTM 2000转染试剂(Thermo Fisher Scientific, 美国)。细胞培养及传代采用U形斜颈细胞培养瓶,细胞转染采用12孔细胞小室培养板(NEST Biotechnology, 中国)。实验所用pminiTol2质粒由Stephen Ekker馈赠(Addgene plasmid #31829; http://n2t.net/addgene:31829; RRID: Addgene_31829)。pCS-TP、pEGFP-C1和pEGFP-N1质粒为本实验室保存。实验过程中用到的限制性核酸内切酶、无缝克隆试剂盒、DNA片段纯化试剂盒均购自于大连宝生物有限公司(TaKaRa)。
1.2 方法 1.2.1 牙鲆卵巢颗粒细胞的培养牙鲆卵巢原代细胞采用组织块直接培养法获得。先将1.5年龄健康的雌性牙鲆置于30 mg/mL MS-222中麻醉,断椎后迅速取出卵巢。将取出的卵巢组织浸泡于磷酸盐缓冲液(PBS)中5~10 min (PBS配方见表 1),并在生物安全柜中用3~4倍体积的PBS清洗5~10次,其中,PBS中添加了2×青霉素–链霉素(Gibco, 美国)。清洗后,将牙鲆卵巢组织浸泡于DMEM-F12 (BI, Bet-Haemek, 以色列)培养基中,用手术剪剔除覆膜和杂质,并在安瓿瓶中迅速剪成边长约为0.25 cm的小块。将剪好的组织块转移至15 mL的离心管中,加入适量DMEM-F12混匀,800 g离心1~2 min后弃上清液,去除组织碎片。取适量组织铺在T25细胞培养瓶中,24 ℃培养箱中倒置培养4~6 h,之后加入5 mL的DMEM-F12培养基培养,同时添加10%胎牛血清FBS、100 μmol/L非必需氨基酸和1%青霉素–链霉素(Gibco, 美国)(王宣刚等, 2021)。后续培养的过程中每4~5 d更换一次培养基,直至细胞迁出80%左右后传代培养。细胞操作培养详细规程参考Yu等(2020)。
|
|
表 1 海水硬骨鱼PBS缓冲液配方 Tab.1 The formulation of marine teleost phosphate buffer saline (PBS) |
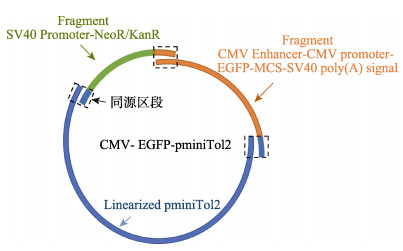
CMV-EGFP-pminiTol2重组载体的构建是以pminiTol2质粒为模板,通过同源重组插入CMV Enhancer-CMV Promoter-EGFP-MCS-SV40 poly(A) signal和SV40 Promoter-NeoR/KanR序列获得。CMV- EGFP-pminiTol2重组载体的构建思路见图 1,具体操作步骤如下:(1)用BglⅡ限制内切酶切割pminiTol2载体,在Tol2 ITR(L)和Tol2 ITR(R)转座元件之间线性化pminiTol2质粒。(2)利用DNA高保真酶(PFU DNA Polymerase),通过PCR方式将pEGFP-C1质粒的多克隆位点(MCS)突变成只含有XhoⅠ和XmaⅠ酶切位点的序列。PCR突变引物为Primer-del。(3)以突变后的pEGFP-C1质粒为模板,通过DNA高保真酶分别扩增得到SV40 Promoter-NeoR/KanR区段和CMV Enhancer-CMV Promoter-EGFP-MCS-SV40 poly(A) signal区段,并在该扩增引物两端添加18 bp同源序列用于无缝克隆构建载体。PCR扩增引物为SV40-NK-FW、SV40-NK-RV、CMV-EGFP-FW和CMV-EGFP-RV。(4)利用DNA片段纯化试剂盒纯化上一步骤PCR扩增得到的DNA片段。利用无缝克隆试剂盒,将线性化的pminiTol2载体、SV40 Promoter- NeoR/KanR区段和CMV Enhancer-CMV Promoter- EGFP-MCS-SV40 poly(A) signal区段连接成一个完整的载体。(5)把构建好的载体转化至大肠杆菌(Escherichia coli) DH5α,经过菌落PCR和Sanger测序验证序列的正确性。CMV-EGFP-pminiTol2重组载体构建过程中的PCR引物序列详见表 2。
|
图 1 CMV-EGFP-pminiTol2重组载体的构建思路 Fig.1 Construction of CMV-EGFP-pminiTol2 recombinant vector |
|
|
表 2 实验中用到的引物序列 Tab.2 Primer sequences used in this study |
牙鲆卵巢颗粒细胞的转染采用Lipofectamine™ 2000转染试剂在12孔细胞小室培养板中进行,实验设计见表 3。具体操作步骤如下:(1)将生长状态良好的牙鲆卵巢颗粒细胞用胰酶消化后传代至12孔板,待其生长至90%~95%融合,转染前一天更换不含抗生素和血清的DMEM-F12培养基。(2)用50 μL DMEM-F12培养基分别稀释实验组和对照组的质粒DNA (2 000 ng/孔),用50 μL DMEM-F12培养基稀释LipofectamineTM 2000转染试剂(2 μL/孔),室温孵育5 min。(3)将步骤2中稀释的质粒和转染试剂轻轻混匀,室温放置20 min,然后加入至12孔细胞小室培养板中,每孔100 μL。(4)转染5 h后更换DMEM-F12培养基,同时添加10% FBS、1%非必需氨基酸、1%青霉素–链霉素双抗。(5)转染24 h后加入800 μg/mL的G418筛选,筛选时长2~3周,期间每2~3天换一次培养液。筛选2周后再稳定培养2~3周,期间不加G418筛选。(6)在倒置荧光显微镜中观察细胞的EGFP绿色荧光,确认细胞的转染效率。
|
|
表 3 细胞转染及稳转筛选实验设计 Tab.3 Experimental design of cell transfection and stable transfection screening |
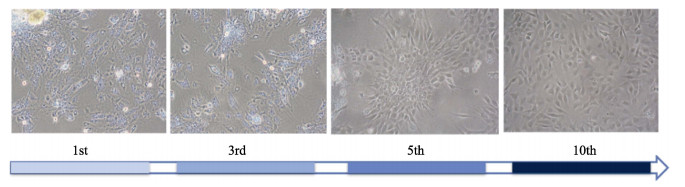
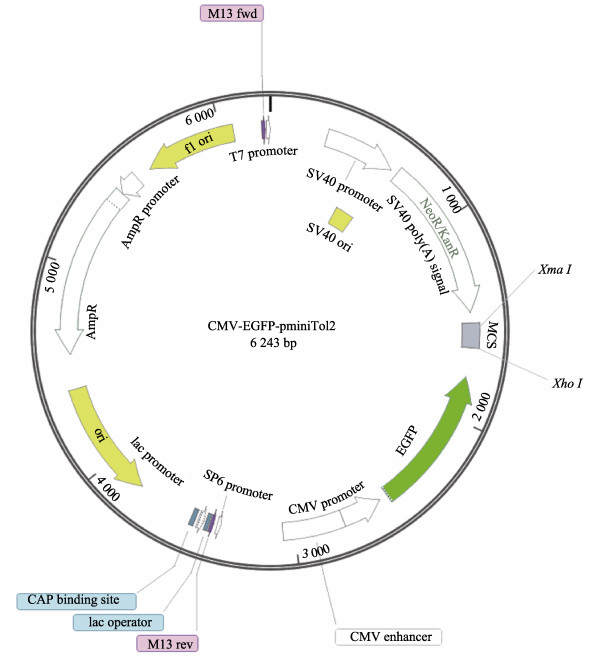
由于卵母细胞是终端分化细胞,大部分卵母细胞会在细胞培养过程中逐渐死亡(朱丹丹等, 2019)。因此,本研究最终培养获得的细胞大部分是牙鲆卵巢颗粒细胞。牙鲆卵巢颗粒细胞在培养初期1~3代时细胞生长较慢,且在细胞中会出现大量的小空泡室(图 2)。分析可能原因是环境变化导致细胞内质网的应激反应。经10代传代培养后生长稳定,细胞结构清晰,细胞呈梭形或菱形。构建好的CMV-EGFP-pminiTol2质粒载体经Sanger测序序列准确无误,质粒图谱信息见图 3。经过改造后CMV-EGFP-pminiTol2质粒更加适合牙鲆细胞的稳转筛选,主要体现在以下4个方面:(1) CMV-EGFP-pminiTol2质粒上携带自主性的转座子元件,结合编码合成转座酶的质粒pCS-TP共转染能够显著提高牙鲆鱼细胞整合外源基因的能力。(2) CMV-EGFP-pminiTol2质粒上携带EGFP绿色荧光蛋白,可通过荧光显微镜直接观察细胞转染及筛选的效果,还可以与目标基因形成融合表达蛋白示踪基因的表达位置。(3) CMV-EGFP-pminiTol2质粒上还携带新霉素抗性筛选标记,可以通过G418快速筛选转染成功的细胞。(4) CMV-EGFP-pminiTol2质粒上MCS区域经位点突变仅保留XhoⅠ和XmaⅠ酶切位点,且XhoⅠ和XmaⅠ酶切位点在整个质粒上是唯一的,可通过这2个位点线性化质粒,然后利用同源重组酶以无缝克隆的方式插入外源基因。
|
图 2 牙鲆卵巢颗粒细胞第1、3、5和10代原代细胞 Fig.2 Primary flounder ovary granulosa cell lines of the 1st, 3rd, 5th and 10th generations |
|
图 3 CMV-EGFP-pminiTol2质粒图谱信息 Fig.3 CMV-EGFP-pminiTol2 plasmid map information |
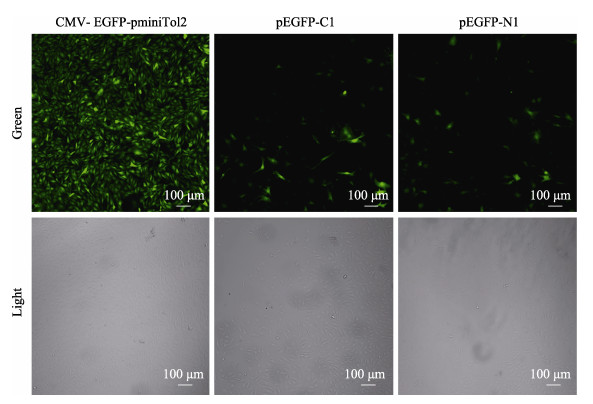
选取第20代牙鲆卵巢颗粒细胞用于细胞转染实验。CMV-EGFP-pminiTol2和pCS-TP质粒经脂质体包裹后共转染至牙鲆卵巢颗粒细胞,利用G418筛选2~3周,恢复培养2~3周,激光共聚焦显微镜观察转染效果。结果显示,实验组中CMV-EGFP-pminiTol2质粒转染组经G418筛选后全部的细胞都能成功表达EGFP绿色荧光蛋白,而对照组中pEGFP-C1和pEGFP-N1质粒转染组经G418筛选后仅有少部分的细胞(≤ 10%)能持续表达EGFP绿色荧光蛋白(图 4)。结果表明,相较于传统真核表达载体pEGFP-C1和pEGFP-N1,本实验改造得到的CMV-EGFP-pminiTol2载体更适合牙鲆稳转细胞的制备,具有筛选时间短、外源基因表达稳定、操作简单、成本较低等优势。因此,研究人员可以在CMV-EGFP-pminiTol2载体上插入目标基因,以脂质体转化的方式构建牙鲆稳转细胞,用于探究基因功能、亚细胞定位、长效调控等科学问题。还可以以CMV-EGFP-pminiTol2载体为蓝本,针对不同鱼类细胞特点进行特异改造,使之更适用于基因功能分析。例如,将CMV-EGFP-pminiTol2载体中的CMV启动子更换为某些细胞的特异的启动子序列,则会使转基因功能探究更加趋近基因调控的本质。
|
图 4 筛选后CMV-EGFP-pminiTol2转染牙鲆卵巢颗粒细胞效果 Fig.4 Effects of CMV-EGFP-pminiTol2 transfected ovarian granulosa cells of flounder after screening |
本研究设计构建了CMV-EGFP-pminiTol2重组载体,并通过脂质体转染后筛选的方式迅速获得了稳定转染的牙鲆卵巢颗粒细胞。相较于传统的真核细胞表达载体,本研究提供了一种能够在牙鲆细胞中高效整合并稳定表达的载体CMV-EGFP-pminiTol2。并且,
CMV-EGFP-pminiTol2载体的转化仅需借助脂质体转染,操作简单、成本较低,在一次牙鲆细胞转染中实现了基因转化和稳定表达两个功效,极大地提高了制备牙鲆稳转细胞的效率。本研究旨在提供牙鲆稳转细胞构建及筛选的新思路,并为其他硬骨鱼类细胞的构建及稳转细胞的筛选提供参考。
BALCIUNAS D, WANGENSTEEN K J, WILBER A, et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genetics, 2006, 2(11): e169 DOI:10.1371/journal.pgen.0020169 |
CHAI B H, SHENG X Z, TANG X Q, et al. Improvement and optimization of liposome-mediated tramsfection of madin-darby canine kidney cells. Periodical of Ocean University of China (Natural Science), 2018, 48(S2): 9-16 [柴百惠, 绳秀珍, 唐小千, 等. 脂质体介导转染犬肾上皮细胞的方法改进与优化. 中国海洋大学学报(自然科学版), 2018, 48(S2): 9-16] |
FUJIMURA K, KOCHER T D. Tol2-mediated transgenesis in tilapia (Oreochromis niloticus). Aquaculture, 2011, 319(3/4): 342-346 |
HAMLET M R J, YERGEAU D A, KULIYEV E, et al. Tol2 transposon-mediated transgenesis in Xenopus tropicalis. Genesis, 2006, 44(9): 438-445 DOI:10.1002/dvg.20234 |
HUANG X, GUO H, TAMMANA S, et al. Gene transfer efficiency and genome-wide integration profiling of sleeping beauty, Tol2, and piggyBac transposons in human primary T cells. Molecular Therapy, 2010, 18(10): 1803-1813 DOI:10.1038/mt.2010.141 |
JIANG M, DENG L, CHEN G. High Ca2+-phosphate transfection efficiency enables single neuron gene analysis. Gene Therapy, 2004, 11(17): 1303-1311 DOI:10.1038/sj.gt.3302305 |
JUNTTI S A, HU C K, FERNALD R D. Tol2-mediated generation of a transgenic haplochromine cichlid, Astatotilapia burtoni. PLoS One, 2013, 8(10): e77647 DOI:10.1371/journal.pone.0077647 |
LOGUE J A, DE VRIES A L, FODOR E, et al. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. Journal of Experimental Biology, 2000, 203(14): 2105-2115 DOI:10.1242/jeb.203.14.2105 |
MACDONALD J, TAYLOR L, SHERMAN A, et al. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(23): E1466-E1472 |
RAMBABU K M, RAO S H N, RAO N M. Efficient expression of transgenes in adult zebrafish by electroporation. BMC Biotechnology, 2005, 5: 1-6 |
RUIZ S, SCHYTH B D, ENCINAS P, et al. New tools to study RNA interference to fish viruses: Fish cell lines permanently expressing siRNAs targeting the viral polymerase of viral hemorrhagic septicemia virus. Antiviral Research, 2009, 82(3): 148-156 DOI:10.1016/j.antiviral.2009.02.200 |
SATO Y, KASAI T, NAKAGAWA S, et al. Stable integration and conditional expression of electroporated transgenes in chicken embryos. Developmental Biology, 2007, 305(2): 616-624 DOI:10.1016/j.ydbio.2007.01.043 |
SI Y, WANG X G, WANG M Y, et al. Expression analysis and functional characterization of JNK2 in Japanese flounder (Paralichthys olivaceus). Progress in Fishery Sciences, 2020, 41(4): 12-22 [司瑜, 王宣刚, 王梦娅, 等. 牙鲆JNK2基因的表达分析及免疫功能探究. 渔业科学进展, 2020, 41(4): 12-22] |
SUMIYAMA K, KAWAKAMI K, YAGITA K. A simple and highly efficient transgenesis method in mice with the Tol2 transposon system and cytoplasmic microinjection. Genomics, 2010, 95(5): 306-311 DOI:10.1016/j.ygeno.2010.02.006 |
SUSTER M L, KIKUTA H, URASAKI A, et al. Transgenesis in zebrafish with the tol2 transposon system. Methods of Molecular Biology, 2009, 561: 41-63 |
TANG Z Y, XUE L Y. Application of Tol2 transposon system in transgenes. Biotechnology Bulletin, 2011(10): 43-48 [汤泽源, 薛良义. Tol2转座子系统在转基因动物中的应用. 生物技术通报, 2011(10): 43-48] |
TAO R, CHANG Y M, LI S G, et al. Characteristics and application of Tol2 transposon in transgenic fish. Chinese Journal of Fisheries, 2014, 27(1): 60-64 [陶然, 常玉梅, 李世国, 等. Tol2转座子的特性及其在转基因鱼类中的应用. 水产学杂志, 2014, 27(1): 60-64 DOI:10.3969/j.issn.1005-3832.2014.01.015] |
WANG X G, KONG X F, WANG X T, et al. Isolation, culture, and characterization of macrophages from the head kidney of Japanese flounder (Paralichthys olivaceus). Progress in Fishery Sciences, 2021, 42(5): 55-61 [王宣刚, 孔祥福, 王欣桐, 等. 牙鲆头肾巨噬细胞的分离培养与鉴定. 渔业科学进展, 2021, 42(5): 55-61] |
WU B, XU L L, WANG Y, et al. Establishment of a stable SW620 cell strain expressing prohibitin 2 protein. Jiangsu Medicine Journal, 2015, 41(20): 2373-2376 [武标, 徐丽丽, 王妍, 等. 稳定表达Prohibitin 2蛋白SW620细胞株的建立. 江苏医药, 2015, 41(20): 2373-2376] |
YAMAGUCHI K, OGAWA R, TSUKAHARA M, et al. Efficient production of recombinant proteins in suspension CHO cells culture using the Tol2 transposon system coupled with cycloheximide resistance selection. Scientific Reports, 2023, 13(1): 7628 DOI:10.1038/s41598-023-34636-4 |
YU H, WANG Y, WANG M, et al. Growth differentiation factor 9 (gdf9) and bone morphogenetic protein 15 (bmp15) are potential intraovarian regulators of steroidogenesis in Japanese flounder (Paralichthys olivaceus). General and Comparative Endocrinology, 2020, 297: 113547 DOI:10.1016/j.ygcen.2020.113547 |
ZABELINSKII S A, BROVTSYNA N B, CHEBOTAREVA M A, et al. Comparative investigation of lipid and fatty acid composition of fish gills and mammalian lungs: A model of the membrane lipid component areas. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 1995, 111(1): 127-140 DOI:10.1016/0305-0491(94)00210-L |
ZHANG Y S. A method for establishing pure lines of transgenic fish mediated by Tol2 transposon. Hunan Province: CN111218478A, 2020-06-02 [张运生. 一种Tol2转座子介导的转基因鱼纯系建立方法. 湖南省: CN111218478A, 2020-06-02]
|
ZHU D D, JIANG S G, HUANG J H, et al. Optimization of tissue and primary cell culture of tiger shrimp Penaeus monodon. Fisheries Science, 2019, 38(2): 163-172 [朱丹丹, 江世贵, 黄建华, 等. 斑节对虾性腺组织、细胞的原代培养条件优化. 水产科学, 2019, 38(2): 163-172] |



