亮氨酸与缬氨酸交互作用对刺参生长、体壁氨基酸组成及消化能力的影响
陆国峰
1,2
,
王际英
2

,
李宝山
2

,
刘经未
1,2,
郝甜甜
2,
孙永智
2,
黄炳山
2
1. 上海海洋大学 水产科学国家级实验教学示范中心 农业农村部鱼类营养与环境生态研究中心 水产动物遗传育种中心上海市协同创新中心 上海 201306;
2. 山东省海洋资源与环境研究院 山东省海洋生态修复重点实验室 烟台市海珍品质量控制与精深加工重点实验室 山东 烟台 264006
收稿日期:2023-12-07;收修改稿日期:2023-12-11
基金项目:山东省刺参产业技术体系(SDAIT-22-06)资助
摘要:本实验旨在研究亮氨酸和缬氨酸的交互作用对刺参(Apostichopus japonicus)生长、体壁氨基酸组成及消化能力的影响。采用双因素实验(two-way ANOVA)设计,在基础饲料中分别添加0、1.06%、2.34%、3.40%的包膜亮氨酸和0、1.74%、2.50%、3.48%的包膜缬氨酸,配制成4个亮氨酸水平(实际含量为1.00%、1.50%、2.10%和2.60%) ×4个缬氨酸水平(实际含量为0.65%、1.40%、1.70%和2.20%)的16组等氮等脂的实验饲料,饲喂初始体重(16.80±0.18) g的刺参60 d。结果表明,亮氨酸和缬氨酸的含量及其交互作用显著影响了刺参的增重率和特定生长率。当饲料中缬氨酸含量为1.40%时,刺参体壁粗脂肪含量随着亮氨酸含量的升高而升高,二者之间存在协同作用,且L1.0V1.4组(亮氨酸1.00%,缬氨酸1.40%)显著低于其他组。亮氨酸和缬氨酸的交互作用显著影响了刺参体壁异亮氨酸、蛋氨酸、苯丙氨酸和酪氨酸的含量。当饲料中亮氨酸含量为2.10%时,随着缬氨酸含量的升高,肠道脂肪酶和淀粉酶的活性均先升高后降低,二者之间表现出先协同后拮抗的作用。淀粉酶活性在L2.6V1.4(亮氨酸2.60%,缬氨酸1.40%)组活性达到最大值,且显著高于其他各组。综上所述,亮氨酸和缬氨酸的含量及其交互作用显著影响了刺参的生长性能、体壁氨基酸组成及消化酶活力,以增重率为评价指标,刺参饲料中亮氨酸和缬氨酸的比值为1.90 (亮氨酸含量2.60%,缬氨酸含量1.40%)。
关键词:刺参 亮氨酸 缬氨酸 生长 体壁氨基酸
Effects of Interaction of Leucine and Valine on Growth, Body Wall Amino Acids Composition, and Digestive Ability of Sea Cucumber Apostichopus japonicus
LU Guofeng
1,2
,
WANG Jiying
2

,
LI Baoshan
2

,
LIU Jingwei
1,2,
HAO Tiantian
2,
SUN Yongzhi
2,
HUANG Bingshan
2
1. Shanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding, Centre for Research on Environmental Ecology and Fish Nutrion of the Ministry of Agriculture and Rural Affairs, National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China;
2. Key Laboratory of Marine Ecological Restoration, Yantai Key Laboratory of Quality Control and Deep Processing of Seafood Treasures, Shandong Marine Resource and Environment Research Institute, Yantai 264006, China
Abstract: Branched chain amino acids (BCAAs) are neutral amino acids containing branched aliphatic chains on the α-carbon, including leucine (Leu), isoleucine, and valine (Val), accounting for 18%–20% of the total amino acids in animal and plant proteins. BCAAs are essential amino acids for animal growth; however, they cannot be synthesized in animals and can only be obtained from the diet. BCAAs participate in the metabolism of protein, fat, and carbohydrates; promote intestinal development and intestinal amino acid transport; and improve the immune capacity of the body. However, the imbalance of BCAAs in diets leads to poor growth and metabolic disorders in animals. BCAAs have similar chemical structures and catabolic pathways and compete for the same amino acid transporters when passing through cell membranes, resulting in antagonism. Sea cucumbers (Apostichopus japonicus) have rich nutritional value and are an important seafood source in northern China.In recent decades, the study of amino acid nutritional requirements of aquatic animals mainly focused on individual amino acid requirements, and few studies focused on the interaction between amino acids with strong correlations, particularly BCAAs. At present, the interaction of BCAAs has been studied in species such as Chinook salmon (Oncorhynchus tshawytscha), Rainbow trout (Oncorhynchus mykiss), Channel catfish (Ictalurus punctatus), Tilapia GIFT (Oreochromis niloticus), and Tiger puffer (Takifugu rubripes); however, the results have been inconsistent.The purpose of this experiment was to study the interaction effect of Leu and Val on the growth, body wall composition, and digestive ability of sea cucumbers. In this experiment, white fishmeal, algae powder, and wheat flour were used as the primary protein sources, and fish oil and soybean lecithin were used as the main lipid sources to design a basic diet with crude protein and lipid contents of 18.10% and 2.80%, respectively. In a two-way experimental design, 0%, 1.06%, 2.34%, and 3.40% coated Leu and 0%, 1.74%, 2.50%, and 3.48% coated Val were added to the basic feed. Sixteen groups of isonitrogen and isolipid diets were prepared with four Leu levels (actual content: 1.00%, 1.50%, 2.10%, and 2.60%) and four Val levels (actual content: 0.65%, 1.40%, 1.70%, and 2.20%). A total of 960 healthy sea cucumbers with an initial average weight of 16.80±0.18 g were selected and randomly assigned to 48 cylindrical circulating buckets. They were divided into 16 experimental groups, with three replicates in each group and 20 sea cucumbers in each replicate. The feeding period was 60 d. Bait was fed once a day at a fixed time (16:00). The water was changed every 2 d, and a siphon was used to withdraw the residual bait and feces from the bottom of the bucket. The amount of water changed was 50% of the water level in the bucket. During the breeding period, the water temperature was 14–17 ℃, pH was 7.4–8.2, and dissolved oxygen was at least 6 mg/L. A low-light environment was maintained indoors.The results showed that the Leu and Val contents and their interaction significantly affected the weight gain (WG) and specific growth rate of sea cucumbers. When the Val content was 1.4%, the crude lipid contents of the body wall increased with increasing dietary Leu contents, and there was a synergistic effect between Leu and Val; the L1.0V1.4 group was significantly lower than the other groups. The interaction between Leu and Val significantly affected the Val, Met, Tyr, and Phe contents in the body wall of sea cucumbers. When the Leu content was 2.1%, the intestinal lipase and amylase activities first increased and then decreased with increasing dietary Val contents; the interaction between Leu and Val showed a synergistic and then antagonistic effect. The amylase activity reached a maximum value in the L2.6V1.4 group and was significantly higher than that in other groups. In conclusion, Leu and Val contents and their interaction significantly affected the growth performance, body wall amino acid composition, and digestive ability of sea cucumbers. Taking WG as the evaluation index, the ratios of Leu and Val in sea cucumber feed were 1.90 (Leu content was 2.6%, Val content was 1.4%).
Key words:
Apostichopus japonicus Leucine Valine Growth Body wall amino acids
支链氨基酸(branched chain amino acids, BCAAs)是指α-碳上含有分支脂肪烃链的中性氨基酸,包括亮氨酸(Leu)、异亮氨酸(Ile)和缬氨酸(Val),占动物和植物蛋白总氨基酸的18%~20% (Li et al, 2009)。支链氨基酸是动物维持生长所必需的氨基酸,其不能在动物体内合成,只能从日粮中获得。研究表明,支链氨基酸在体内参与蛋白质、脂肪和糖类的代谢(Yin et al, 2019; 邵静, 2019),促进肠道发育和肠道氨基酸转运,提高机体的免疫能力(Zhang et al, 2017)。然而,日粮中支链氨基酸的失衡通常会引起动物生长不良及代谢紊乱(王莉苹等, 2015)。支链氨基酸具有相似的化学结构和分解代谢途径,通过细胞膜时,竞争相同的氨基酸转运载体,从而产生拮抗作用。在过去的几十年里,对于水产动物氨基酸营养需求的研究主要集中在对单个氨基酸需求量的研究上,很少有针对相关性较强氨基酸之间相互作用的研究,支链氨基酸更是如此。目前,支链氨基酸的相互作用在大鳞大麻哈鱼(Oncorhynchus tshawytscha) (Chance et al, 1964)、虹鳟(Oncorhynchus mykiss) (Choo et al, 1991)、斑点叉尾 (Ictalurus punctatus) (Wilson et al, 1980)、牙鲆(Paralichthysolivaceus) (王莉苹等, 2017)、吉富罗非鱼(Oreochromis niloticus) (Xu et al, 2022)及红鳍东方鲀(Takifugu rubripes) (孙志远, 2021)等物种上已有研究,但研究结果并不一致。
(Ictalurus punctatus) (Wilson et al, 1980)、牙鲆(Paralichthysolivaceus) (王莉苹等, 2017)、吉富罗非鱼(Oreochromis niloticus) (Xu et al, 2022)及红鳍东方鲀(Takifugu rubripes) (孙志远, 2021)等物种上已有研究,但研究结果并不一致。
刺参(Apostichopus japonicus)隶属于棘皮动物门(Echinodermata),海参纲(Holothuroidea),仿刺参属(Apostichopus),具有丰富的营养价值,是我国北方重要的海珍品(吴香莹等, 2020)。本研究在本实验室前期对亮氨酸与缬氨酸需求研究的基础上,通过在刺参饲料中添加不同水平的亮氨酸与缬氨酸,旨在探究2种支链氨基酸间的交互作用对刺参幼参生长、体壁营养组成、氨基酸组成及消化能力的影响,以期为支链氨基酸在刺参全价配合饲料中的应用提供参考。
1 材料与方法
1.1 实验饲料
本研究以白鱼粉、藻粉和小麦粉为主要蛋白源,以鱼油和大豆卵磷脂为主要脂肪源设计粗蛋白含量为18.10%、粗脂肪含量为2.80%的基础饲料。在基础饲料中分别添加0、1.06%、2.34%、3.40%的包膜亮氨酸和0、1.74%、2.50%、3.48%的包膜缬氨酸,配制成4个亮氨酸水平(实际含量为1.00%、1.50%、2.10%和2.60%)×4个缬氨酸水平(实际含量为0.65%、1.40%、1.70%和2.20%)的16组等氮等脂的实验饲料,分别记为L1.0V0.7、L1.5V0.7、L2.1V0.7、L2.6V0.7、L1.0V1.4、L1.5V1.4、L2.1V1.4、L2.6V1.4、L1.0V1.7、L1.5V1.7、L2.1V1.7、L2.6V1.7、L1.0V2.2、L1.5V2.2、L2.1V2.2和L2.6V2.2 (表 1)。实验亮氨酸水平与缬氨酸水平分别参考韩秀杰等(2019)和刘财礼等(2022)对刺参氨基酸需求研究而设定。
表 1(Tab.1)
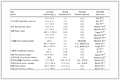
表 1 饲料配方及营养组成(风干物质)
Tab.1 Proximate composition and ingredients of the experimental diets (air dried matter)
| 项目Items |
组别Groups |
| L1.0V0.7 |
L1.5V0.7 |
L2.1V0.7 |
L2.6V0.7 |
L1.0V1.4 |
L1.5V1.4 |
L2.1V1.4 |
L2.6V1.4 |
L1.0V1.7 |
L1.5V1.7 |
L2.1V1.7 |
L2.6V1.7 |
L1.0V2.2 |
L1.5V2.2 |
L2.1V2.2 |
L2.6V2.2 |
| 原料Ingredient/% |
白鱼粉
White fishmeal |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
小麦粉
Wheat flour |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
藻粉
Algae powder |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
30.00 |
包膜亮氨酸
Coated Leu |
0.00 |
1.06 |
2.34 |
3.40 |
0.00 |
1.06 |
2.34 |
3.40 |
0.00 |
1.06 |
2.34 |
3.40 |
0.00 |
1.06 |
2.34 |
3.40 |
包膜缬氨酸
Coated Val |
0.00 |
0.00 |
0.00 |
0.00 |
1.74 |
1.74 |
1.74 |
1.74 |
2.50 |
2.50 |
2.50 |
2.50 |
3.48 |
3.48 |
3.48 |
3.48 |
包膜甘氨酸
Coated Gly |
6.40 |
5.40 |
4.20 |
3.20 |
4.80 |
3.80 |
2.60 |
1.60 |
4.10 |
3.10 |
1.90 |
0.90 |
3.20 |
2.20 |
1.00 |
0.00 |
| 海泥Sea mud |
39.50 |
39.44 |
39.36 |
39.30 |
39.36 |
39.30 |
39.22 |
39.16 |
39.30 |
39.24 |
39.16 |
39.10 |
39.22 |
39.16 |
39.08 |
39.02 |
其他成分
Other ingredients1 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
4.10 |
| 合计Total |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
| 营养组成Nutrient composition |
粗蛋白
Crude protein/% |
18.15 |
17.99 |
18.10 |
18.17 |
17.97 |
18.07 |
18.06 |
18.09 |
18.11 |
18.14 |
18.08 |
18.01 |
18.12 |
18.14 |
18.01 |
17.98 |
粗脂肪
Crude lipid/% |
2.81 |
2.81 |
2.76 |
2.87 |
2.78 |
2.80 |
2.84 |
2.80 |
2.75 |
2.84 |
2.77 |
2.84 |
2.83 |
2.82 |
2.78 |
2.77 |
粗灰分
Crude ash/% |
50.88 |
51.03 |
51.35 |
51.10 |
51.11 |
51.34 |
51.40 |
50.81 |
51.81 |
50.49 |
50.89 |
50.98 |
51.49 |
50.97 |
51.51 |
51.32 |
能量/(kJ/g)
Gross energy |
9.67 |
9.66 |
9.60 |
9.78 |
9.78 |
9.69 |
9.82 |
9.77 |
9.60 |
9.67 |
9.75 |
9.82 |
9.67 |
9.76 |
9.77 |
9.79 |
| 亮氨酸Leu/% |
1.05 |
1.45 |
2.11 |
2.60 |
1.02 |
1.42 |
2.15 |
2.66 |
1.08 |
1.47 |
2.09 |
2.64 |
1.05 |
1.43 |
2.13 |
2.66 |
| 缬氨酸Val/% |
0.63 |
0.63 |
0.67 |
0.65 |
1.38 |
1.42 |
1.41 |
1.40 |
1.70 |
1.68 |
1.74 |
1.71 |
2.21 |
2.20 |
2.17 |
2.22 |
| 亮氨酸与缬氨酸比例Leu/Val |
1.67 |
2.30 |
3.15 |
4.00 |
0.74 |
1.00 |
1.52 |
1.90 |
0.64 |
0.88 |
1.20 |
1.54 |
0.48 |
0.65 |
0.98 |
1.20 |
注:1)其他成分为1%维生素预混料,1%矿物质预混料,1%鱼油,1%大豆卵磷脂以及0.1%抗氧化剂。维生素预混料(mg/kg或IU/kg饲料):维生素A 7 500.00 IU;维生素D 1 500.00 IU;维生素E 60.00 mg;维生素K3 18.00 mg;维生素B1 12.00 mg;维生素B2 12.00 mg;维生素B12 0.10 mg;泛酸48.00 mg;烟酰胺90.00 mg;叶酸3.70 mg;D-生物素0.20 mg;吡哆醇60.00 mg;维生素C 310.00 mg;矿物质预混料(mg/kg饲料):锌35.00 mg;锰21.00 mg;铜8.30 mg;铁23.00 mg;钴1.20 mg;碘1.00 mg;硒0.30 mg。
Note: 1) The other ingredients are 1% vitamin premix, 1% mineral premix, 1% fish oil, 1% soybean lecithin, and 0.1% antioxidant. Vitamin premix (mg/kg or IU/kg diet): Vitamin A 7 500.00 IU; Vitamin D 1 500.00 IU; Vitamin E 60.00 mg; Vitamin K3 18.00 mg; Vitamin B1 12.00 mg; Vitamin B2 12.00 mg; Vitamin B12 0.10 mg; Pantothenate acid 48.00 mg; Niacin 90.00 mg; Folic acid 3.70 mg; D-biotin 0.20 mg; Pyridoxine 60.00 mg; Vitamin C 310.00 mg; Mineral premix (mg/kg diet): Zn 35.00 mg; Mn 21.00 mg; Cu 8.30 mg; Fe 23.00 mg; Co 1.20 mg; I2 1.00 mg; Se 0.30 mg. |
|
表 1 饲料配方及营养组成(风干物质)
Tab.1 Proximate composition and ingredients of the experimental diets (air dried matter)
|
实验所用亮氨酸、缬氨酸和甘氨酸购自上海麦克林生化科技有限公司(纯度≥99%),β-环状糊精购自河南省孟州市华兴生物化工有限责任公司,将氨基酸与β-环状糊精按照一定比例混合后,参考胡友军等(2002)的方法对氨基酸进行包被,包被后的有效亮氨酸、缬氨酸和甘氨酸含量分别约为48%、47%和50%。实验所用固体原料经超微粉碎后过200目标准筛,加入鱼油及适量蒸馏水,充分混匀,制成直径为7 mm的长条状饲料,60 ℃烘干,密封保存备用。
1.2 饲养管理及样品采集
养殖实验在山东省海洋资源与环境研究院东营实验基地循环水养殖系统中进行,实验时间为2022年11月9日—2023年1月7日。实验用刺参购自山东安源种业科技有限公司。正式实验前,用基础饲料驯养刺参1周,使其适应实验饲料和饲养环境。实验开始前所有刺参禁食24 h,挑选个体健壮、体质量为(16.80±0.18)的刺参960头,随机分配到48个圆柱形循环水养殖桶(ϕ 65 cm×60 cm)中。实验分为16个实验组,每组3个重复,每个重复20头刺参,每桶放2个刺参养殖筐。养殖期间,每天16:00饱食投喂1次,初始投喂量为刺参体质量的3%,每天不定时观察刺参的摄食情况,并相应地调整投喂量。每2天换1次水,换水量为水体的1/2,换水时将虹吸软管伸进桶底吸出残饵及粪便,实验进行至1个月时更换一次刺参养殖筐,并用刷子将桶壁、桶底刷洗干净。养殖期间,水温控制在14~17 ℃,溶解氧含量不低于6 mg/L,pH为7.4~8.2,室内保持弱光环境。
养殖实验结束后,禁食24 h,分别对每桶刺参称总重并记录刺参数量。取样时,从每桶随机选取8头刺参,逐一称重后进行解剖,吸取体腔液,分离肠道和体壁并称重,以计算肠壁比和肠体壁。将体壁和肠道保存于–20 ℃分别用于体成分和肠道酶活性的测定。
1.3 测定指标与方法
成活率(survival rate, SR, %)=Nt/N0×100%;
增重率(weight gain rate, WG, %)=(Wt−W0)/W0× 100%;
特定生长率(specific growth rate, SGR, %/d)= (lnWt−lnW0)/t×100%;
肠壁比(ratio of intestine weight to body wall weight, IBR, %)= Wi/Ww×100%;
肠体比(ratio of intestine weight to body weight, IWR, %)=Wi/Wt×100%。
式中,Nt为终末头数,N0为初始头数,Wt为终末体质量(final body weight, FBW) (g),W0为初始体质量(initial body weight, IBW) (g),t为养殖实验天数(d),Wi为取样肠道质量(g),Ww为取样体壁质量(g)。
实验饲料及刺参体壁水分含量采用105 ℃恒重法测定(GB/T 6435-2014),粗蛋白含量采用凯氏定氮法测定(GB/T 6432-2018),粗脂肪含量采用索氏抽提法测定(GB/T 6433-2006),粗灰分含量采用550 ℃马弗炉灼烧法测定(GB/T6438-2007),氨基酸含量采用酸水解法(GB/T18246-2019)使用全自动氨基酸测定仪(Hitachi L-8900,日本)测定,饲料能量采用燃烧法使用量热仪(IKA,C6000,德国)测定。
肠道中淀粉酶(amylase)、脂肪酶(lipase)和胰蛋白酶(trypsin)活性均使用南京建成生物工程研究所生产的试剂盒进行测定,酶活性中的蛋白含量测定采用考马斯亮蓝法,各种酶活性单位参照试剂盒说明书表示。
1.4 数据统计分析
采用SPSS Statistics 26.0软件对实验数据进行双因素方差分析(two-way ANOVA)以评价二者间的交互作用,在此基础上对所有数据进行单因素方差分析(one-way ANOVA)以确定组间的差异性,当各组间差异显著时(P<0.05),用Duncan′s检验进行多重比较分析。统计结果以平均值±标准差(Mean±SD,n=3)的形式表示。
2 结果
2.1 亮氨酸和缬氨酸交互作用对刺参生长的影响
亮氨酸和缬氨酸的交互作用对刺参的肠壁比(IBR)、肠体比(IWR)和成活率(SR)无显著影响(P > 0.05),且亮氨酸和缬氨酸含量对IBR、IWR和SR的影响均不显著(P > 0.05)。亮氨酸和缬氨酸的含量及其交互作用对刺参的增重率(WG)和特定生长率(SGR)均有显著影响(P < 0.05)。当饲料中缬氨酸含量为1.4%时,刺参的WG和SGR随亮氨酸含量的升高而升高。当饲料中亮氨酸含量为2.6%时,随着缬氨酸含量的增加,刺参的WG和SGR呈先升高后降低的趋势。L2.6V1.4组刺参的WG和SGR有最大值,且显著高于其他组(P < 0.05) (表 2)。
表 2(Tab.2)

表 2 不同饲料对刺参生长性能的影响
Tab.2 Effects of dietary diets on growth performance of sea cucumber A. japonicus
组别
Groups |
参数Paramenters |
初体重
IBW/g |
终体重
FBW/g |
增重率
WG/% |
特定生长率
SGR/(%/d) |
肠壁比
IBR/% |
肠体比
IWR/% |
成活率
SR/% |
| L1.0V0.7 |
16.75±0.09 |
21.56±0.29a |
28.74±1.25a |
0.42±0.02a |
6.14±0.43 |
3.74±0.29 |
96.67±2.89 |
| L1.5V0.7 |
16.73±0.08 |
23.03±0.37b |
37.63±1.80b |
0.53±0.02c |
6.09±0.40 |
3.75±0.29 |
98.33±2.89 |
| L2.1V0.7 |
16.80±0.09 |
23.95±0.26c |
42.56±1.22c |
0.59±0.01d |
6.12±0.31 |
3.77±0.30 |
100.00±0.00 |
| L2.6V0.7 |
16.73±0.08 |
22.03±0.43a |
31.66±2.34a |
0.46±0.03b |
6.17±0.60 |
3.71±0.28 |
93.33±5.77 |
| L1.0V1.4 |
16.78±0.15 |
22.79±0.31b |
35.81±0.66b |
0.51±0.01c |
6.18±0.48 |
3.77±0.45 |
98.33±2.89 |
| L1.5V1.4 |
16.73±0.10 |
24.33±0.28cd |
45.42±0.79cde |
0.62±0.01de |
6.19±0.63 |
3.75±0.45 |
100.00±0.00 |
| L2.1V1.4 |
16.75±0.13 |
24.66±0.31d |
47.24±1.43e |
0.64±0.02e |
6.13±0.43 |
3.69±0.46 |
95.00±5.00 |
| L2.6V1.4 |
16.80±0.13 |
25.97±0.54e |
54.55±2.10f |
0.73±0.02f |
6.21±0.64 |
3.86±0.19 |
100.00±0.00 |
| L1.0V1.7 |
16.78±0.12 |
24.55±0.21cd |
46.26±1.26de |
0.63±0.01de |
6.19±0.47 |
3.78±0.29 |
98.33±2.89 |
| L1.5V1.7 |
16.80±0.09 |
24.23±0.24cd |
44.22±1.64cde |
0.61±0.02de |
6.18±0.41 |
3.87±0.07 |
95.00±0.00 |
| L2.1V1.7 |
16.77±0.08 |
24.02±0.33cd |
43.27±2.09cd |
0.60±0.02d |
6.19±0.44 |
3.77±0.09 |
95.00±8.66 |
| L2.6V1.7 |
16.75±0.09 |
22.91±0.39b |
36.79±1.84b |
0.52±0.02c |
6.04±0.54 |
3.78±0.47 |
93.33±5.77 |
| L1.0V2.2 |
16.72±0.14 |
21.80±0.41a |
30.42±2.09a |
0.44±0.03ab |
6.14±0.25 |
3.81±0.36 |
95.00±5.00 |
| L1.5V2.2 |
16.77±0.13 |
24.33±0.43cd |
45.12±1.64cde |
0.62±0.02de |
6.14±0.43 |
3.74±0.42 |
100.00±0.00 |
| L2.1V2.2 |
16.78±0.18 |
22.70±0.40b |
35.27±3.49b |
0.50±0.04c |
6.17±0.31 |
3.78±0.27 |
98.33±2.89 |
| L2.6V2.2 |
16.75±0.13 |
21.94±0.20a |
30.96±0.52a |
0.45±0.01ab |
6.16±0.42 |
3.85±0.34 |
93.33±7.64 |
| 显著性Significance P2 |
| 交互作用F值 |
0.22 |
26.52 |
34.64 |
31.97 |
0.33 |
0.46 |
1.08 |
| Leu |
NS |
** |
** |
** |
NS |
NS |
NS |
| Val |
NS |
** |
** |
** |
NS |
NS |
NS |
| Leu×Val |
NS |
** |
** |
** |
NS |
NS |
NS |
注:同列数据不同上标字母表示差异显著(P < 0.05),NS表示没有显著性,Leu×Val表示二者的交互作用,*代表P < 0.05,**代表P < 0.01,下同。
Notes: Different superscript letters in the same column indicate significant difference (P < 0.05), NS means no significance, Leu×Val means interaction between Leu and Val, * means P < 0.05, ** means P < 0.01, the same below. |
|
表 2 不同饲料对刺参生长性能的影响
Tab.2 Effects of dietary diets on growth performance of sea cucumber A. japonicus
|
2.2 亮氨酸和缬氨酸交互作用对刺参体壁基本成分的影响
各组间刺参体壁水分、粗蛋白和粗灰分含量均无显著差异(P > 0.05)。亮氨酸和缬氨酸的含量及其交互作用对刺参体壁粗脂肪均有显著影响(P < 0.05)。当饲料中缬氨酸含量为1.4%时,随着亮氨酸含量的增加,粗脂肪含量也随之升高,且L1.0V1.4组显著低于其他组(P < 0.05)。粗脂肪含量在L2.6V1.4组达到最高,在L1.0V0.7组最低(表 3)。
表 3(Tab.3)

表 3 不同饲料对刺参体壁基本成分的影响/%
Tab.3 Effects of dietary diets on approximate composition of body wall of sea cucumber A. japonicus /%
| 组别Groups |
项目Items |
| 水分Moisture |
粗蛋白Crude protein |
粗脂肪Crude lipid |
粗灰分Crude ash |
| L1.0V0.7 |
91.37±0.18 |
50.08±0.73 |
3.60±0.14a |
29.58±0.30 |
| L1.5V0.7 |
90.95±0.12 |
49.73±0.52 |
3.94±0.04b |
29.48±0.06 |
| L2.1V0.7 |
91.24±0.09 |
49.67±0.55 |
4.48±0.09cd |
29.42±0.14 |
| L2.6V0.7 |
91.32±0.28 |
49.73±0.43 |
3.67±0.19a |
29.78±0.22 |
| L1.0V1.4 |
91.26±0.20 |
49.97±0.16 |
3.80±0.07ab |
29.76±0.16 |
| L1.5V1.4 |
90.89±0.15 |
49.58±0.48 |
4.45±0.22c |
29.55±0.07 |
| L2.1V1.4 |
91.23±0.36 |
50.23±0.92 |
4.51±0.05cd |
29.39±0.06 |
| L2.6V1.4 |
91.31±0.28 |
50.11±0.58 |
4.69±0.05d |
29.75±0.32 |
| L1.0V1.7 |
90.93±0.06 |
50.27±0.38 |
4.44±0.13c |
29.45±0.26 |
| L1.5V1.7 |
90.97±0.04 |
50.28±0.38 |
4.54±0.09cd |
29.56±0.10 |
| L2.1V1.7 |
90.97±0.11 |
50.46±0.44 |
4.35±0.05c |
29.40±0.11 |
| L2.6V1.7 |
90.98±0.09 |
49.98±0.74 |
3.70±0.05a |
29.69±0.48 |
| L1.0V2.2 |
90.99±0.44 |
50.02±0.68 |
3.64±0.04a |
29.63±0.58 |
| L1.5V2.2 |
91.22±0.45 |
49.80±0.88 |
4.33±0.26c |
29.77±0.35 |
| L2.1V2.2 |
91.08±0.23 |
49.69±0.21 |
3.82±0.14ab |
29.82±0.23 |
| L2.6V2.2 |
91.11±0.52 |
49.73±0.41 |
3.69±0.17a |
29.86±0.33 |
| 显著性Significance P2 |
| 交互作用F值 |
0.80 |
0.40 |
19.72 |
0.46 |
| Leu |
NS |
NS |
** |
NS |
| Val |
NS |
NS |
** |
NS |
| Leu×Val |
NS |
NS |
** |
NS |
注:粗蛋白、粗脂肪和粗灰分含量为干基含量。
Note: The crude protein, crude lipid and crude ash contents of body wall are based on dry basis. |
|
表 3 不同饲料对刺参体壁基本成分的影响/%
Tab.3 Effects of dietary diets on approximate composition of body wall of sea cucumber A. japonicus /%
|
2.3 亮氨酸和缬氨酸交互作用对刺参体壁氨基酸组成的影响
亮氨酸和缬氨酸的交互作用显著影响了刺参体壁蛋氨酸、苯丙氨酸、谷氨酸、酪氨酸和天冬氨酸的含量(P < 0.05),但对体壁总氨基酸含量无显著影响(P > 0.05)。亮氨酸和缬氨酸含量对体壁异亮氨酸、蛋氨酸和酪氨酸均有显著影响(P < 0.05)。当饲料中亮氨酸含量为1.5%时,随着缬氨酸含量的升高,体壁亮氨酸的含量趋于平稳,而体壁缬氨酸和异亮氨酸的含量均先升高后降低。当饲料中亮氨酸含量为2.6%时,体壁缬氨酸含量随着饲料中缬氨酸含量的升高而升高,异亮氨酸则表现为相反的趋势。当饲料中缬氨酸含量为1.4%时,随着亮氨酸含量的升高,体壁苯丙氨酸含量升高,蛋氨酸含量下降(表 4和表 5)。
表 4(Tab.4)

表 4 不同饲料对刺参体壁必需氨基酸组成的影响(%干物质)
Tab.4 Effects of dietary diets on essential amino acid composition of body wall of sea cucumber A.japonicus (% dry matter)
组别
Groups |
必需氨基酸Essential amino acid |
| 亮氨酸Leu |
缬氨酸Val |
苏氨酸Thr |
异亮氨酸Ile |
蛋氨酸Met |
苯丙氨酸Phe |
赖氨酸Lys |
组氨酸His |
精氨酸Arg |
| L1.0V0.7 |
2.63±0.02a |
2.06±0.03a |
2.55±0.04abcd |
1.62±0.05ab |
1.55±0.05efgh |
1.78±0.03d |
2.05±0.03 |
0.63±0.01ab |
3.41±0.02 |
| L1.5V0.7 |
2.69±0.02abcdef |
2.08±0.04ab |
2.55±0.03abcd |
1.64±0.06abc |
1.60±0.02h |
1.62±0.03bc |
2.07±0.02 |
0.64±0.01abc |
3.42±0.02 |
| L2.1V0.7 |
2.75±0.04ef |
2.09±0.06abc |
2.56±0.02abcd |
1.69±0.06abc |
1.50±0.06cde |
1.70±0.07bcd |
2.07±0.06 |
0.64±0.02abc |
3.46±0.02 |
| L2.6V0.7 |
2.74±0.04ef |
2.10±0.05abc |
2.54±0.02abc |
1.59±0.08a |
1.51±0.07defg |
1.63±0.09bc |
2.08±0.07 |
0.67±0.01c |
3.47±0.01 |
| L1.0V1.4 |
2.65±0.01ab |
2.09±0.01ab |
2.51±0.01a |
1.63±0.05abc |
1.58±0.02fgh |
1.51±0.06a |
2.06±0.03 |
0.62±0.02a |
3.45±0.03 |
| L1.5V1.4 |
2.66±0.05abc |
2.13±0.07abc |
2.59±0.03bcd |
1.65±0.04abc |
1.56±0.04efgh |
1.61±0.04ab |
2.10±0.06 |
0.63±0.02ab |
3.44±0.02 |
| L2.1V1.4 |
2.70±0.03bcdef |
2.14±0.06abc |
2.62±0.05d |
1.73±0.03c |
1.44±0.01bcd |
1.72±0.10bcd |
2.11±0.04 |
0.66±0.01bc |
3.45±0.02 |
| L2.6V1.4 |
2.73±0.05cdef |
2.13±0.03abc |
2.61±0.06cd |
1.72±0.04bc |
1.39±0.05b |
1.75±0.03d |
2.11±0.02 |
0.67±0.01c |
3.42±0.08 |
| L1.0V1.7 |
2.66±0.03abcd |
2.10±0.06abc |
2.61±0.05cd |
1.58±0.03a |
1.50±0.05def |
1.73±0.07cd |
2.08±0.06 |
0.64±0.01abc |
3.41±0.07 |
| L1.5V1.7 |
2.69±0.06abcdef |
2.15±0.04bc |
2.59±0.08bcd |
1.60±0.06a |
1.17±0.05a |
1.72±0.06cd |
2.09±0.06 |
0.64±0.02abc |
3.48±0.01 |
| L2.1V1.7 |
2.69±0.03abcdef |
2.12±0.05abc |
2.62±0.07d |
1.68±0.10abc |
1.44±0.04bcd |
1.70±0.03bcd |
2.12±0.02 |
0.65±0.04abc |
3.45±0.03 |
| L2.6V1.7 |
2.76±0.03f |
2.14±0.04abc |
2.56±0.01abcd |
1.66±0.09abc |
1.21±0.02a |
1.67±0.07bcd |
2.12±0.03 |
0.67±0.02c |
3.41±0.07 |
| L1.0V2.2 |
2.68±0.07abcde |
2.15±0.04bc |
2.58±0.02abcd |
1.58±0.04a |
1.55±0.05efgh |
1.72±0.04bcd |
2.08±0.05 |
0.63±0.03ab |
3.43±0.03 |
| L1.5V2.2 |
2.69±0.05abcdef |
2.14±0.03abc |
2.58±0.04abcd |
1.59±0.04a |
1.59±0.03gh |
1.73±0.05cd |
2.11±0.04 |
0.65±0.01abc |
3.41±0.02 |
| L2.1V2.2 |
2.71±0.05bcdef |
2.16±0.03bc |
2.57±0.04abcd |
1.60±0.03a |
1.53±0.06efgh |
1.70±0.08bcd |
2.10±0.08 |
0.62±0.02a |
3.47±0.02 |
| L2.6V2.2 |
2.74±0.03def |
2.17±0.02c |
2.53±0.03ab |
1.60±0.04a |
1.42±0.03bc |
1.73±0.03cd |
2.11±0.08 |
0.64±0.02abc |
3.42±0.06 |
| 显著性Significance P2 |
| 交互作用F值 |
0.95 |
0.31 |
1.84 |
0.80 |
12.60 |
5.00 |
0.93 |
1.84 |
1.62 |
| Leu |
** |
NS |
NS |
* |
** |
NS |
NS |
** |
NS |
| Val |
NS |
** |
* |
** |
** |
* |
NS |
NS |
NS |
| Leu×Val |
NS |
NS |
NS |
NS |
** |
** |
NS |
NS |
NS |
|
表 4 不同饲料对刺参体壁必需氨基酸组成的影响(%干物质)
Tab.4 Effects of dietary diets on essential amino acid composition of body wall of sea cucumber A.japonicus (% dry matter)
|
表 5(Tab.5)

表 5 不同饲料对刺参体壁非必需氨基酸组成的影响(%干物质)
Tab.5 Effects of dietary diets on non-essential amino acid composition of body wall of sea cucumber A. japonicus (% dry matter)
组别
Groups |
非必需氨基酸Non-essential amino acid |
谷氨酸
Glu |
甘氨酸
Gly |
酪氨酸
Tyr |
半胱氨酸
Cys |
丙氨酸
Ala |
天冬氨酸
Asp |
脯氨酸
Pro |
丝氨酸
Ser |
总氨基酸
TAA |
| L1.0V0.7 |
7.68±0.04abcd |
6.06±0.03ab |
1.99±0.04abc |
1.65±0.05 |
2.95±0.02cd |
4.84±0.06a |
3.04±0.03ab |
2.42±0.03ab |
48.91±0.08ab |
| L1.5V0.7 |
7.74±0.06cde |
6.01±0.05a |
2.11±0.03ef |
1.62±0.06 |
2.95±0.04cd |
4.87±0.04abc |
3.06±0.03ab |
2.46±0.07ab |
49.12±0.12ab |
| L2.1V0.7 |
7.66±0.03ab |
6.02±0.05ab |
2.06±0.02cde |
1.69±0.06 |
2.94±0.04cd |
4.94±0.04bc |
3.07±0.05ab |
2.42±0.03ab |
49.26±0.14b |
| L2.6V0.7 |
7.70±0.01bcde |
5.98±0.01a |
2.11±0.02ef |
1.68±0.08 |
2.97±0.05d |
4.86±0.04ab |
3.06±0.02ab |
2.41±0.02ab |
49.08±0.20ab |
| L1.0V1.4 |
7.75±0.02de |
6.04±0.06ab |
2.16±0.01f |
1.64±0.05 |
2.96±0.04cd |
4.95±0.03d |
3.04±0.01ab |
2.39±0.02a |
49.03±0.10ab |
| L1.5V1.4 |
7.76±0.04e |
6.01±0.09a |
1.96±0.03ab |
1.70±0.04 |
2.86±0.07ab |
4.84±0.03a |
3.08±0.02b |
2.41±0.05ab |
48.98±0.27ab |
| L2.1V1.4 |
7.65±0.01ab |
6.03±0.08ab |
2.04±0.05bcde |
1.63±0.03 |
2.90±0.02abcd |
4.86±0.03ab |
3.05±0.02ab |
2.41±0.02ab |
49.15±0.18b |
| L2.6V1.4 |
7.72±0.05bcde |
5.98±0.01a |
2.10±0.06ef |
1.70±0.04 |
2.98±0.03d |
4.78±0.04a |
3.05±0.03ab |
2.45±0.02ab |
49.26±0.22b |
| L1.0V1.7 |
7.65±0.05ab |
6.03±0.06ab |
2.01±0.05abcd |
1.66±0.03 |
2.95±0.06cd |
4.84±0.02a |
3.04±0.05ab |
2.49±0.02b |
48.98±0.03ab |
| L1.5V1.7 |
7.61±0.01a |
6.08±0.05ab |
2.09±0.08def |
1.64±0.06 |
2.88±0.05abc |
4.79±0.03a |
3.04±0.04ab |
2.46±0.04ab |
48.72±0.40a |
| L2.1V1.7 |
7.86±0.011f |
6.04±0.05ab |
1.97±0.07abc |
1.61±0.10 |
2.85±0.05a |
4.79±0.01a |
3.08±0.06b |
2.45±0.09ab |
49.12±0.41ab |
| L2.6V1.7 |
7.77±0.01e |
5.99±0.03a |
1.97±0.01abc |
1.68±0.09 |
2.98±0.01d |
4.85±0.03bcd |
3.02±0.01ab |
2.45±0.01ab |
48.90±0.19ab |
| L1.0V2.2 |
7.76±0.02de |
6.05±0.06ab |
2.11±0.02ef |
1.64±0.04 |
2.96±0.02cd |
4.86±0.03ab |
3.06±0.03ab |
2.43±0.01ab |
49.25±0.07b |
| L1.5V2.2 |
7.69±0.02abcde |
6.12±0.01b |
2.05±0.04bcde |
1.67±0.04 |
2.93±0.05bcd |
4.87±0.04abc |
3.05±0.05ab |
2.41±0.09ab |
49.26±0.19b |
| L2.1V2.2 |
7.72±0.05bcde |
6.04±0.06ab |
1.96±0.04ab |
1.69±0.03 |
2.97±0.03d |
4.83±0.14a |
3.08±0.05b |
2.48±0.03ab |
49.23±0.08b |
| L2.6V2.2 |
7.67±0.01abc |
6.05±0.05ab |
1.94±0.03a |
1.63±0.04 |
2.95±0.02cd |
4.94±0.03bc |
3.00±0.01a |
2.39±0.05a |
48.94±0.16ab |
| 显著性Significance P2 |
| 交互作用F值 |
9.16 |
0.96 |
7.16 |
1.35 |
1.87 |
4.00 |
0.91 |
1.31 |
1.60 |
| Leu |
NS |
NS |
** |
NS |
NS |
NS |
NS |
NS |
NS |
| Val |
NS |
NS |
* |
NS |
** |
* |
NS |
NS |
* |
| Leu×Val |
** |
NS |
** |
NS |
NS |
** |
NS |
NS |
NS |
|
表 5 不同饲料对刺参体壁非必需氨基酸组成的影响(%干物质)
Tab.5 Effects of dietary diets on non-essential amino acid composition of body wall of sea cucumber A. japonicus (% dry matter)
|
2.4 亮氨酸和缬氨酸交互作用对刺参肠道消化酶活性的影响
亮氨酸和缬氨酸的交互作用显著影响了刺参肠道消化酶的活性(P < 0.05)。亮氨酸和缬氨酸含量均显著影响了肠道脂肪酶和淀粉酶的活性(P < 0.05),但均对胰蛋白酶的活性无显著影响(P > 0.05)。当饲料中亮氨酸含量为2.1%时,随着缬氨酸含量的升高,肠道脂肪酶和淀粉酶的活性均呈先升高后降低的趋势。淀粉酶在L2.6V1.4组活性达到最大值,且显著高于其他各组(P < 0.05)。当饲料中亮氨酸含量为1.5%和2.6%时,各组胰蛋白酶活性无显著差异(P > 0.05) (表 6)。
表 6(Tab.6)

表 6 不同饲料对刺参肠道消化酶活力的影响
Tab.6 Effects of dietary diets on intestinal digestive enzymes of sea cucumber A. japonicus
| 组别Groups |
项目Items |
| 脂肪酶Lipase/(U/g prot) |
淀粉酶Amylase/(U/mg prot) |
胰蛋白酶Trypsin/(U/mg prot) |
| L1.0V0.7 |
21.53±0.77a |
0.86±0.03bc |
1 511.17±42.47a |
| L1.5V0.7 |
27.20±0.22d |
1.12±0.04h |
1 583.65±48.76ab |
| L2.1V0.7 |
34.78±0.76h |
1.01±0.05efg |
1 631.38±86.41b |
| L2.6V0.7 |
31.48±0.43f |
0.98±0.01def |
1 534.98±25.35ab |
| L1.0V1.4 |
31.53±0.24f |
0.97±0.09cdef |
1 551.56±59.46ab |
| L1.5V1.4 |
28.87±0.46e |
0.97±0.05cdef |
1 548.82±23.65ab |
| L2.1V1.4 |
33.50±0.71g |
1.25±0.03i |
1 559.29±39.45ab |
| L2.6V1.4 |
31.66±0.51f |
1.15±0.04h |
1 624.81±65.81b |
| L1.0V1.7 |
33.21±0.19g |
1.07±0.08fgh |
1 626.83±33.78b |
| L1.5V1.7 |
29.54±0.15e |
1.10±0.13gh |
1 592.92±36.06ab |
| L2.1V1.7 |
26.71±0.34d |
0.91±0.01bcde |
1 503.36±70.84a |
| L2.6V1.7 |
26.43±0.29d |
0.74±0.07a |
1 560.31±61.78ab |
| L1.0V2.2 |
27.01±0.38d |
0.81±0.02ab |
1 592.98±68.85ab |
| L1.5V2.2 |
25.25±0.14c |
0.91±0.06bcde |
1 586.44±14.05ab |
| L2.1V2.2 |
22.27±0.64ab |
0.82±0.05ab |
1 521.15±46.79a |
| L2.6V2.2 |
22.68±0.21b |
0.87±0.03bcd |
1 580.30±57.22ab |
| 显著性Significance P2 |
| 交互作用F值 |
238.91 |
15.49 |
2.75 |
| Leu |
** |
** |
NS |
| Val |
** |
** |
NS |
| Leu×Val |
** |
** |
* |
|
表 6 不同饲料对刺参肠道消化酶活力的影响
Tab.6 Effects of dietary diets on intestinal digestive enzymes of sea cucumber A. japonicus
|
3 讨论
3.1 亮氨酸和缬氨酸交互作用对刺参生长的影响
本研究表明,亮氨酸和缬氨酸的交互作用显著影响了刺参的WG和SGR,且亮氨酸和缬氨酸均是显著影响因子。在韩秀杰等(2019)和刘财礼等(2022)对刺参亮氨酸和缬氨酸需求量研究中发现,亮氨酸和缬氨酸均分别显著影响了刺参的WG和SGR,这与本研究结果相吻合。水产动物的生长性能与饲料中氨基酸的平衡息息相关,当饲料中氨基酸不平衡时,体内的营养物质则会通过代谢提供能量,进而影响其生长(Langar et al, 1993)。本研究中,在低缬氨酸(缬氨酸含量为1.4%)组中,饲料中添加高水平的亮氨酸(亮氨酸含量为2.6%)显著提高了刺参的WG和SGR;而在高水平缬氨酸(缬氨酸含量为2.2%)组中,饲料中添加高水平的亮氨酸(亮氨酸含量为2.6%)则显著降低了刺参的WG和SGR,表明饲料中高水平亮氨酸与高水平缬氨酸之间具有拮抗作用,抑制了刺参的生长,在吉富罗非鱼(Xu et al, 2022)、牙鲆(Han et al, 2014),虹鳟(Yamamoto et al, 2004)的研究中也得到了类似的结果。其原因可能是:一方面,过量的支链氨基酸间会竞争小肠壁处的氨基酸转运载体,影响支链氨基酸的吸收和转运,从而降低氨基酸的利用率(刘财礼等, 2022);另一方面,动物摄入过量的氨基酸会导致机体代谢紊乱并产生毒性(Abidi et al, 2007),且过量的亮氨酸会使得机体消耗一部分能量将其排出体外,影响机体生长(黄爱霞等, 2018)。Yamamoto等(2004)在对虹鳟的研究中发现,在亮氨酸过量的饲料中添加异亮氨酸和缬氨酸可以起到缓解对生长和氨基酸代谢不利影响的作用。这在本研究中也得到了证实,当饲料中亮氨酸含量为2.6%时,随着缬氨酸含量的增加,刺参的WG和SGR呈先升高后降低的趋势,且在L2.6V1.4组达到最高值,此时饲料中各氨基酸之间达到了较为平衡的状态,促进了刺参的生长。韩秀杰等(2019)在对刺参缬氨酸需求量的研究中发现,生长效果最佳组体壁亮氨酸与缬氨酸的比值为1.43。本研究中,L2.1V1.4组和L2.6V1.7组饲料亮氨酸与缬氨酸比值分别为1.52和1.54,与之相近,其中,L2.1V1.4组具有较佳的生长表现,L2.6V1.7组相反,其可能是受饲料中另一种支链氨基酸异亮氨酸含量的影响,随着饲料中亮氨酸和缬氨酸含量的升高,支链氨基酸间原有的平衡被打破,进而影响了刺参的生长表现。本研究中,L2.6V1.4组刺参WG最大,饲料中亮氨酸含量为2.6%,缬氨酸含量为1.4%,比值为1.90,与韩秀杰等(2019)和刘财礼(2021)对亮氨酸和缬氨酸需求研究中WG最高组不同,表明3种支链氨基酸之间的含量和比例均处于动态平衡中,合适的含量和比例均会促进刺参的生长。
3.2 亮氨酸和缬氨酸交互作用对刺参体壁基本成分的影响
钱晓丽(2018)研究表明,当机体摄入氨基酸不平衡的饲料,其合成蛋白质的能力受到抑制。但也有研究得出不同的结论,李燕(2010)在探究鲈鱼(Lateolabrax japonicus)和大黄鱼(Pesudosciaena crocea)支链氨基酸需求时发现,鲈鱼体蛋白含量既不受亮氨酸含量影响,也不受缬氨酸含量的影响;刘财礼(2021)研究表明,饲料中亮氨酸与缬氨酸的交互作用未对刺参体壁粗蛋白含量产生影响,这与本研究结果类似。在本研究中,亮氨酸和缬氨酸的交互作用未对刺参体壁粗蛋白的含量产生影响,其主要原因可能是当饲料中氨基酸不平衡时,刺参可以通过对自身氨基酸模式的调节来维持机体蛋白的相对恒定(刘财礼等, 2022)。此外,本研究中亮氨酸和缬氨酸的交互作用仅对体壁粗脂肪含量有影响,表明支链氨基酸对于机体的脂肪代谢具有重要的调控作用,能够显著影响机体的脂肪代谢和沉积(Cheng et al, 2010)。支链氨基酸中的亮氨酸可以调控mTOR信号通路,mTOR信号通路中的固醇调节元件结合蛋白1 (SREBP-1)起着调控脂肪合成相关酶表达,维持机体脂肪和胆固醇稳态的作用(钱晓丽, 2018)。本研究也证实了亮氨酸是体壁粗脂肪产生差异的显著影响因子。
3.3 亮氨酸和缬氨酸交互作用对刺参体壁氨基酸组成的影响
刺参的生长主要是对饲料中蛋白质利用并积累的过程,蛋白质的积累又是通过氨基酸的合成来实现的。目前,关于支链氨基酸的交互作用对鱼体氨基酸组成的影响在牙鲆上已有报道,王莉苹(2018)在研究饲料中亮氨酸与异亮氨酸的交互作用对牙鲆肌肉氨基酸组成影响的实验中发现,亮氨酸与异亮氨酸的交互作用对肌肉中总必需氨基酸的含量产生交互作用,不会对总必需氨基酸的含量产生交互作用。本研究中,亮氨酸与缬氨酸的交互作用对刺参体壁总必需氨基酸、总非必需氨基酸及总氨基酸的含量均无交互作用,但对缬氨酸、苯丙氨酸、蛋氨酸及酪氨酸产生交互作用。出现该结果的原因可能是刺参体壁的氨基酸组成相对比较稳定,相互之间的比例基本保持不变,受氨基酸这种营养素的影响比较小。在低亮氨酸组中(亮氨酸含量为1.5%),随着饲料中缬氨酸含量的升高,体壁亮氨酸的含量趋于平稳,而体壁缬氨酸和异亮氨酸的含量均先升高后降低;在高亮氨酸组中(亮氨酸含量为2.6%),随着缬氨酸含量的升高,体壁亮氨酸和缬氨酸含量也随之升高,异亮氨酸含量则呈相反的趋势,这表明刺参可以通过自我调节从而使机体支链氨基酸处于平衡状态。此外,亮氨酸与缬氨酸的交互作用对体壁非必需氨基酸中的酪氨酸产生交互作用,但并未观察到酪氨酸与这两种氨基酸之间有明显的协同或者拮抗作用,可能是受到了其他氨基酸的影响。
3.4 亮氨酸和缬氨酸交互作用对刺参肠道消化酶活性的影响
肠道是消化和吸收营养物质的主要场所,肠道酶活性决定了刺参获取和利用饲料中营养物质的能力。目前,已经有一部分研究报道了氨基酸的交互作用对水生动物肠道消化吸收能力的影响。Xu等(2022)研究表明,在HL-LV组(亮氨酸含量为2.93%,缬氨酸含量为1.04%)中,吉富罗非鱼肠道淀粉酶和脂肪酶的活性达到最高,但蛋白酶的活性却不受饲料氨基酸水平的影响。王旭等(2018)研究发现,饲料中添加适宜的异亮氨酸和缬氨酸显著提高了牙鲆肠道消化酶的活性。本研究中,当饲料中亮氨酸含量为2.1%时,随着缬氨酸含量的升高,肠道脂肪酶和淀粉酶的活性呈先升高后降低的趋势,且淀粉酶在L2.1V1.4组活性最高,表明当饲料中缬氨酸含量为1.4%、亮氨酸含量为2.1%时可以提高刺参的消化和吸收能力。这可能是因为此时饲料中支链氨基酸之间达到了最佳平衡状态,改善了刺参的肠道结构,提高了肠道功能,进而促进消化酶的分泌(Niu et al, 2021)。此外,支链氨基酸能够作为氮和碳骨架的载体,为谷氨酸以及谷氨酰胺的合成提供氮源和碳源(Bixel et al, 1997; 石亚庆等, 2014),而谷氨酰胺能够促进淀粉酶、脂肪酶等消化酶的分泌(Chen et al, 2009; 林燕, 2005)。
4 结论
综上所述,亮氨酸和缬氨酸的含量及其交互作用影响了刺参的生长、体壁氨基酸组成及消化酶活性。以增重率为评价指标,刺参饲料中亮氨酸和缬氨酸的比值为1.90 (亮氨酸含量为2.6%,缬氨酸含量为1.4%)。
参考文献
ABIDI S F, KHAN M A. Dietary leucine requirement of fingerling Indian major carp, Labeo rohita ( Hamilton). Aquaculture Research, 2007, 38(5): 478-486 DOI:10.1111/j.1365-2109.2007.01687.x |
BIXEL M G, HUTSON S M, HAMPRECHT B. Cellular distribution of branched-chain amino acid aminotransferase isoenzymes among rat brain glial cells in culture. Journal of Histochemistry and Cytochemistry, 1997, 45(5): 685-694 DOI:10.1177/002215549704500506 |
CHANCE R E, MERTZ E T, HALVER J E. Nutrition of salmonoid fishes: Ⅻ. Isoleuc ine, leucine, valine and pheny lalanine requirements of chinook salmon and interrelations between isoleucine and leucine for growth. Journal of Nutrition, 1964, 83(3): 177-185 |
CHEN L X, LI P, WANG J J, et al. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids, 2009, 37(1): 143-152 DOI:10.1007/s00726-009-0268-1 |
CHENG Y, MENG Q S, WANG C X, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes, 2010, 59(1): 17-25 DOI:10.2337/db09-0929 |
CHOO P S, SMITH T K, CHO C Y, et al. Dietary excesses of leucine influence growth and body composition of rainbow trout. Journal of Nutrition, 1991, 121(12): 1932-1939 DOI:10.1093/jn/121.12.1932 |
HAN X J, LI B S, WANG J Y, et al. Optimum dietary valine requirement of juvenile sea cucumber Apostichopus japonicus. Journal of Fisheries of China, 2019, 43(3): 628-638 [韩秀杰, 李宝山, 王际英, 等. 仿刺参幼参对缬氨酸最适需求量的研究. 水产学报, 2019, 43(3): 628-638] |
HAN Y Z, HAN R Z, KOSHIO S, et al. Interactive effects of dietary valine and leucine on two sizes of Japanese flounder Paralichthys olivaceus. Aquaculture, 2014, 432: 130-138 DOI:10.1016/j.aquaculture.2014.05.004 |
HU Y J, ZHOU A G, YANG F, et al. The study of suitable feed processing parameter for starch gelatinization. Feed Industry, 2002(12): 5-8 [胡友军, 周安国, 杨凤, 等. 饲料淀粉糊化的适宜加工工艺参数研究. 饲料工业, 2002(12): 5-8] |
HUANG A X, SUN L H, CHEN J M, et al. Effect of dietary leucine level on growth, diet utilization and body composition of juvenile grass carp (Ctenopharyngodon idella). Feed Industry, 2018, 39(2): 26-32 [黄爱霞, 孙丽慧, 陈建明, 等. 饲料亮氨酸水平对幼草鱼生长、饲料利用及体成分的影响. 饲料工业, 2018, 39(2): 26-32] |
LANGAR H, GUILLAUME J, METAILLER R, et al. Augmentation of protein synthesis and degradation by poor dietary amino acid balance in European sea bass ( Dicentrarchus labrax). Journal of Nutrition, 1993, 123(10): 1754-1761 DOI:10.1093/jn/123.10.1754 |
LI P, MAI K S, TRUSHENSKI J, et al. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids, 2009, 37(1): 43-53 DOI:10.1007/s00726-008-0171-1 |
LI Y. Optimal requirements of branch chain amino acids and histidine in diets of Japanese seabass, Lateolabrax japonicus and large yellow croaker Pesudosciaena crocea R. Doctoral Dissertation of Ocean University of China, 2010 [李燕. 鲈鱼和大黄鱼支链氨基酸与组氨酸营养生理的研究. 中国海洋大学博士研究生学位论文, 2010]
|
LIN Y. The effect of dietary glutamine supplementation on intestinal development, function and imune of juvenile Jian carp (Cyprinus carpio var. Jian). Master´s Thesis of Sichuan Agricultural University, 2005 [林燕. 谷氨酰胺对幼建鲤肠道功能和免疫力的影响. 四川农业大学硕士研究生学位论文, 2005]
|
LIU C L. Study on dietary optimun leucine requirement and interactive effect of dietary isoleucine and leucine on growth and physiological metabolism of juvenile sea cucumber Apostichopus japonicus Selenka. Master's Thesis of ShangHai Ocean University, 2021 [刘财礼. 仿刺参(Apostichopus japonicus)幼参对亮氨酸最适需求量及亮氨酸与异亮氨酸交互作用对其生长及生理代谢影响的研究. 上海海洋大学硕士研究生学位论文, 2021]
|
LIU C L, WANG J Y, LI B S, et al. Optimum dietary leucine requirement of juvenile sea cucumber Apostichopus japonicus. Journal of Fisheries of China, 2022, 46(2): 238-249 [刘财礼, 王际英, 李宝山, 等. 仿刺参幼参对亮氨酸最适需求量的研究. 水产学报, 2022, 46(2): 238-249] |
NIU X J, QIAN X L, FENG H M, et al. Growth and metabolic responses of grouper juveniles (Epinephelus coioides) fed diets containing varying levels of leucine. Aquaculture, 2021, 534 |
QIAN X L. Effects of leucine levels in diets on the growth and mTOR signaling pathway of grouper (Epinephelus coioides). Master's Thesis of Jimei University, 2018 [钱晓丽. 饲料亮氨酸水平对斜带石斑鱼生长及其mTOR信号通路的影响. 集美大学硕士研究生学位论文, 2018]
|
SHAO J. Novel roles of branched-chain amino acids and their catabolic intermediates in glucose and lipid metabolic regulation. Doctoral Dissertation of Shanghai Jiao Tong University, 2019 [邵静. 支链氨基酸及其代谢产物调控糖脂代谢的新机制. 上海交通大学博士研究生学位论文, 2019]
|
SHI Y Q, SUN Y X, LUO L, et al. Dietary leucine requirement of tilapia (GIFT Oreochromis niloticus). Journal of Fisheries of China, 2014, 38(10): 1778-1785 [石亚庆, 孙玉轩, 罗莉等. 吉富罗非鱼亮氨酸需求量研究. 水产学报, 2014, 38(10): 1778-1785] |
SUN Z Y. Nutrition physiological effects of dietary branched- chain amino acids in juvenile tiger puffer (Takifugu rubripes). Master's Thesis of Shanghai Ocean University, 2021 [孙志远. 红鳍东方鲀支链氨基酸营养生理研究. 上海海洋大学硕士研究生学位论文, 2021]
|
WANG L P. Study of interactive effect of dietary leucine and isoleucine on Japanese flounder Paralichthys olivaceus. Master's Thesis of Dalian Ocean University, 2018 [王莉苹. 牙鲆(Paralichthys olivaceus)饲料中亮氨酸与异亮氨酸交互作用的研究. 大连海洋大学硕士研究生学位论文, 2018]
|
WANG L P, CHEN F, HAN Y Z, et al. Research progress of branch chain amino acids (BCAA) and its requirement for aquatic animals. Feed Industry, 2015, 36(14): 35-40 [王莉苹, 陈飞, 韩雨哲, 等. 支链氨基酸(BCAA)及水产动物对其需求量的研究进展. 饲料工业, 2015, 36(14): 35-40] |
WANG L P, JIANG Z Q, SUN M L, et al. Interactive effect of dietary leucine and isoleucine on digestive and immune- related enzyme activities of Japanese flounder Paralichthys olivaceus. Journal of Da Lian Ocean University, 2017, 32(3): 287-293 [王莉苹, 姜志强, 孙梦蕾, 等. 饲料中亮氨酸和异亮氨酸交互作用对牙鲆消化酶及免疫相关酶活力的影响. 大连海洋大学学报, 2017, 32(3): 287-293] |
WANG X, ZHOU J, XUE X Q, et al. Interactive effect of dietary isoleucine and valine on digestive enzyme and immune- related enzyme activities of Paralichthys olivaceus. Feed Industry, 2018, 39(20): 23-28 [王旭, 周婧, 薛晓强, 等. 牙鲆饲料中异亮氨酸与缬氨酸的交互作用对消化酶和部分免疫酶的影响. 饲料工业, 2018, 39(20): 23-28] |
WILSON R P, POE W E, ROBINSON E H. Leucine, isoleucine, valine and histidine requirements of fingerling channel catfish. Joural of Nutrition, 1980, 110(4): 627-633 |
WU X Y, SHENG W B, NIE Q, et al. Effects of dietary selenium yeast on growth performance, antioxidant capacity and selenium content before and after aestivation of Apostichopus japonicus. Chinese Journal of Animal Nutrition, 2020, 32(6): 2791-2798 [吴香莹, 盛伟博, 聂琴, 等. 饲料中添加酵母硒对刺参生长、抗氧化能力及夏眠前后硒含量的影响. 动物营养学报, 2020, 32(6): 2791-2798] |
XU C, HUANG X P, GUAN J F, et al. Effects of dietary leucine and valine levels on growth performance, glycolipid metabolism and immune response in Tilapia GIFT Oreochromis niloticus. Fish and Shellfish Immunology, 2022, 121: 395-403 |
YAMAMOTO T, SHIMA T, FURUITA H. Antagonistic effects of branched-chain amino acids induced by excess protein- bound leucine in diets for rainbow trout (Oncorhynchus mykiss). Aquaculture, 2004, 232(1): 539-550 |
YIN Y L, SUN Z W. Research progress on the regulation of glucose metabolism by branched-chain amino acids. Chinese Journal of Animal Science, 2019, 55(5): 11-14 [尹玉林, 孙泽威. 支链氨基酸调控机体糖代谢的研究进展. 中国畜牧杂志, 2019, 55(5): 11-14] |
ZHANG S, ZENG X, REN M, et al. Novel metabolic and physiological functions of branched chain amino acids: A review. Journal of Animal Science and Biotechnology, 2017, 8(3): 501-512 |




