2. 江苏省海洋生物产业技术协同创新中心 江苏 连云港 222005;
3. 江苏省海洋资源开发研究院(连云港)江苏 连云港 222005;
4. 江苏省农业种质资源保护与利用平台 江苏 南京 210014;
5. 连云港赣榆佳信水产开发有限公司 江苏 连云港 222100
2. Co-Innovation Center of Jiangsu Marine Bio-industry Technology, Jiangsu Ocean University, Lianyungang 222005, China;
3. Marine Resource Development institute of Jiangsu (Lianyungang), Lianyungang 222005, China;
4. The Jiangsu Provincial Infrastructure for Conservation and Utilization of Agricultural Germplasm, Nanjing 210014, China;
5. Lianyungang Ganyu Jiaxin Aquatic Products Development Co., Ltd., Lianyungang 222100, China
脊尾白虾(Exopalaemon carinicauda)具有广温广盐、生长速度快、味道鲜美和营养丰富等优点,已成为池塘单养和混养模式比较常见的一种重要的小型经济虾类,可作为甲壳动物生物学研究的优良生物材料(马鸿梅等, 2019; Deng et al, 2021)。
水体溶解氧(dissolved oxygen, DO)是影响水生动物生存、生长、行为、繁殖、免疫和代谢的重要环境因子之一(王盼等, 2021),水体溶解氧含量为0 mg/L时称水体无氧,当含量≤2 mg/L时,就被认为低氧或缺氧状态(顾孝连等, 2009)。水生动物对缺氧胁迫表现出复杂的行为和生理反应(Wannamaker et al, 2000; Zhang et al, 2006; Dong et al, 2019)。虾池溶解氧浓度一般不能低于3 mg/L (顾孝连等, 2009),溶解氧浓度过低会引起虾类应激,导致蜕壳频率降低,影响其生长发育(王盼等, 2021),低氧严重时引起浮头甚至导致死亡。养殖规模的扩大、高密度养殖、高温以及气候变化等原因极易导致虾池出现低氧情况。
在过去已经开展了许多关于DO对甲壳动物影响的研究,主要集中在行为反应、免疫应答、能量消耗、呼吸、抗氧化及基因克隆等方面(Cheng et al, 2002; Li et al, 2009; 侯文杰等, 2014; 杨明, 2019; 邱小龙等, 2022; 曾庆辉, 2023)。甲壳动物的鳃是重要呼吸器官,具有呼吸和调节渗透压等重要功能,肝胰腺具有解毒、消化、贮存能量和免疫等重要功能。肠道细菌群落被认为是宿主的“遗忘器官”,然而它们与宿主生长、发育、生理学和病理学密切相关,肠道细菌群落组成的变化可能会影响宿主的生长和健康状况。有学者发现,在相同养殖环境下,来自同一种群的斑节对虾(Penaeus monodon)最高体重组[平均体重(36.82± 0.41) g]肠道微生物数量明显更多,肠道优势菌群主要集中在厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidota) (Uengwetwanit et al, 2020)。与正常生长组相比,大黄鱼(Pseudosciaena crocea)缓慢生长组的肠道微生物群具有更高的OTU数量、Chao1值和兼性厌氧菌(李英英等, 2017)。
近年来,已经开展了许多关于水生动物低氧胁迫的研究,然而,有关低氧胁迫对脊尾白虾组织结构及肠道菌群影响的研究鲜有报道。本研究以脊尾白虾为研究对象,开展低氧胁迫实验并观察脊尾白虾组织结构在低氧胁迫下的损伤情况,分析低氧胁迫下脊尾白虾肠道菌群的结构组成及差异变化,深入研究脊尾白虾低氧应激的生理响应过程,以期为实际生产和培育脊尾白虾耐低氧新品种(系)提供基础研究资料。
1 材料与方法 1.1 实验材料从江苏连云港佳信水产有限公司购得一批平均体长为(4.3±0.5) cm、体重为(1.47±0.24) g健康的脊尾白虾,在养殖室水箱中充气暂养1周(水温24~25 ℃、盐度25、pH 7.9±0.5,自然光照),每天换水1次(总体积的30%),07:00和18:00分别投喂一次(体重的5%),及时清理残饵,实验前1 d停止投喂。
1.2 预实验和正式实验在正式实验之前进行了预实验,准备5个养殖水箱(70 L, 0.8 m×0.5 m×0.5 m)装有水的,在第1个养殖水箱中添加无水亚硫酸钠(Na2SO3),使水体溶解氧降低至(2.5±0.2) mg/L,随后持续充氧使水体溶解氧浓度升高至(7.2±0.2) mg/L,将30尾脊尾白虾放入水箱中养殖48 h (水温24~25 ℃、盐度25、pH 7.9±0.5,自然光照),07:00和18:00分别投喂一次(体重的5%),及时清理残饵,但不换水,48 h后并未发现死虾,由此证明,使用Na2SO3降低水体溶解氧浓度的安全性。在第2个无养殖对象的水箱中添加Na2SO3,使水体溶解氧降低至(2.5±0.2) mg/L,对该水箱不进行水体充氧,每隔20 min使用便携式溶氧仪(希玛公司,中国)检测水箱的水体溶解氧浓度,在12 h后未发现该水箱的水体溶解氧发生显著变化,由此证明,使用Na2SO3降低水体溶解氧含量的稳定性。将剩余3个养殖水箱各放入30尾脊尾白虾,待虾稳定后,经过多次尝试,调整气泵(一泵三孔)控制阀至合适位置,控制充气量使水体溶解氧浓度保持在(7.2±0.2) mg/L,在接下来的12 h每隔20 min,使用便携式溶氧仪检测水箱的水体溶解氧浓度,发现水体溶解氧浓度并没有发生显著变化。验证预实验设计方案的可行性后,开始正式实验。
正式实验在温度24~25 ℃、盐度25条件下进行,将暂养稳定后的180尾虾平均分配到6个养殖水箱中(70 L,0.8 m×0.5 m×0.6 m),每个水箱30尾虾,常氧组和低氧组各3个水箱,其中,常氧组水体溶解氧浓度设置为(7.2±0.2) mg/L,低氧组包括(0~24 h)一个处理阶段。通过添加Na2SO3使低氧组的水体溶解氧浓度在40 min内降低至(2.5±0.2) mg/L时立刻开始实验,24 h期间每隔30 min使用便携式溶氧仪检测水体溶解氧含量,通过充氧和投入Na2SO3动态维持水体溶解氧浓度。
1.3 样品采集实验开始24 h后,分别从常氧组和低氧组随机各取5尾脊尾白虾的鳃和肝胰腺组织,置于4%多聚甲醛液中进行固定,用于切片观察。同时,从常氧组和低氧组各取5组脊尾白虾肠道组织,每组包括3尾脊尾白虾的肠道,常氧组编号分别为WT1、WT2、WT3、WT4和WT5,低氧组编号分别为HYPO1、HYPO2、HYPO3、HYPO4和HYPO5,经液氮速冻后转移至–80 ℃冰箱中保存,用于肠道菌群结构分析。
1.4 组织学分析将经过多聚甲醛固定24 h的低氧组和常氧组脊尾白虾鳃、肝胰腺组织放入酒精中进行梯度脱水,使用二甲苯试剂透明后放入石蜡中进行包埋,将包埋好的石蜡块放入转轮式切片机进行切片,每片切片厚约5 μm,随后进行逐级脱蜡和复水,再用苏木精–伊红试剂染色、脱水透明,最后使用中性树胶封片,使用荧光显微镜观察拍照。
1.5 基因组DNA提取及扩增产物的获取低氧组脊尾白虾在(2.5±0.2) mg/L的缺氧条件下被胁迫24 h,分别取低氧组(HYPO1、HYPO2、HYPO3、HYPO4、HYPO5)和常氧组(WT1、WT2、WT3、WT4、WT5)各5组肠道组织,每组包括3个尾脊尾白虾的完整肠道,使用CTAB提取低氧组(HYPO)和常氧组(WT)脊尾白虾肠道样品总DNA,使用琼脂糖凝胶(1%)检测DNA纯度和浓度,用无菌水稀释至1 ng/μL。用341F和806R引物(Thijs et al, 2017)扩增不同的16S rRNA基因(V3-V4),引物序列为341F (CCTAYGGGRBGCASCAG)和806R (GGACTACNN GGGTATCTAAT)。PCR包含15 µL Phusion®High-Fidelity PCR Master Mix,引物0.2 µmol/L,10 ng基因组DNA。PCR过程如下:第1次温度达到98 ℃,变性1 min。然后,在98 ℃温度下持续10 s,50 ℃温度下持续30 s,72 ℃温度下持续30 s,30个循环,72 ℃伸展5 min。采用琼脂糖凝胶电泳法检测PCR产物,使用凝胶提取试剂盒(TianGen,德国)回收。
1.6 文库构建和上机测序使用NEB Next® Ultra™ Ⅱ FS DNA PCR-free Library Prep Kit (New England Biolabs公司, 美国)进行文库构建,构建好的文库经过Qubit和Q-PCR定量,文库合格后,使用NovaSeq 6000进行PE250上机测序。
1.7 生物信息学与统计分析根据其独特的条形码对样品进行配对末端读取,使用FLASH (Version1.2.11, http://ccb.jhu.edu/software/FLASH/)合并对端reads (Magoc et al, 2011),使用fastp (Version 0.20.0)软件拼接、过滤reads,得到高质量原始Tags数据(raw tags)(Bokulich et al, 2012),统计数据量。使用QIIME2 (Version QIIME2-202006)软件中的DADA2模块进行降噪,获得最终的ASVs (amplicon sequence variants)(Wang et al, 2021)。使用QIIME2软件计算Alpha多样性,通过wilcox秩和检验分析Alpha多样性指数是否存在差异。基于主坐标分析(PCoA)估计Beta多样性。使用LEfSe软件分析各分类水平(门、纲、目、科、属HE种)上存在显著差异的物种,使用R软件进行T检验,找出各个分类水平下组间具有显著差异性的物种,显著水平为P < 0.05。
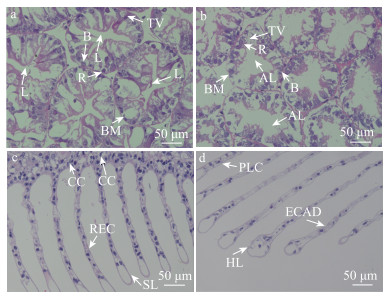
2 结果与分析 2.1 低氧胁迫对脊尾白虾组织结构的影响 2.1.1 低氧胁迫下脊尾白虾肝胰腺组织结构的变化低氧胁迫对脊尾白虾肝胰腺组织有明显的影响,常氧组(图 1a)脊尾白虾肝胰腺细胞基膜完整,形态结构正常,可以清楚地观察到B、R细胞,且B、R细胞排列整齐,转运泡大小正常,管腔呈星形。低氧胁迫24 h后(图 1b),肝胰腺出现一些组织学变化,转运空泡体积显著增大,甚至出现了破裂的现象,B、R细胞排列紊乱,管腔异常、小管扩张,星形管腔消失。
|
图 1 低氧胁迫对脊尾白虾肝胰腺、鳃组织的影响 Fig.1 Effects of hypoxic stress on gill and hepatopancreatic tissues of E. carinicauda a、b为常氧组和低氧组肝胰腺组织HE染色切片显微结构图;c、d为常氧组和低氧组鳃组织HE染色切片显微结构图。TV:转运泡;L:管腔;AL:管腔异常;B:分泌细胞;R:储存细胞;BM:基膜;CC:泌氯细胞;REC:上皮细胞;SL:次级层片;PLC:支柱细胞紊乱;HL:次级层片肥大;ECAD:上皮细胞紊乱。 a and b are microstructures of HE-stained sections of hepatopancreatic tissues in the normoxic and hypoxic groups; c and d are microstructures of HE-stained sections of gill tissues in the normoxic and hypoxic groups. TV: Transport vacuole; L: Lumen; AL: Abnormal lumen; B: Secretory cells; R: Storage cells; BM: Basement membrane; CC: Chloride cells; REC: Epithelial cells; SL: Secondary lamellae; PLC: Pillar cells arranged disorder; HL: Hypertrophy of secondary lamellae; ECAD: Epithelial cells arranged disorder. |
根据鳃组织的切片可以发现,低氧胁迫对脊尾白虾鳃组织有比较明显的损伤。常氧组(图 1c)脊尾白虾鳃组织整体形态正常,细胞排列紧密,支柱细胞和上皮细胞排列正常,次级层片大小正常。在低氧胁迫24 h后(图 1d),泌氯细胞数目显著减少,泌氯细胞由不规则的扁形变为圆形,次级层片肥大加重,部分支柱细胞与上皮细胞排列紊乱,次级层片肥大严重。这表明低氧胁迫对鳃组织具有损伤作用。
2.2 低氧胁迫对脊尾白虾肠道细菌群落多样性指数分析对肠道样本的原始序列进行过滤和降噪,得到特征序列ASVs。本研究使用Venn图统计不同组之间共有和特有的ASVs数目,2组样本共有的ASVs个数为1 000个,低氧组特有的ASVs个数为4 664个,常氧组特有的ASVs个数为2 013个(图 2)。

|
图 2 ASVs分布Veen图 Fig.2 ASVs Venn analysis |
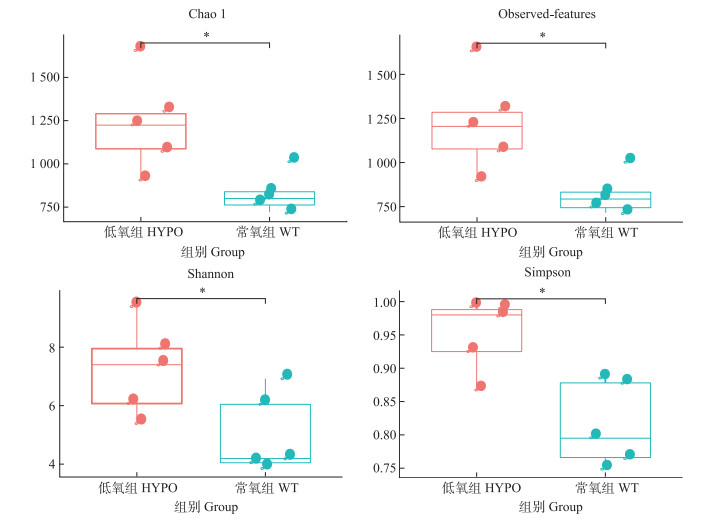
Alpha多样性是样品中微生物群落丰富度和多样性的指标,采用Alpha多样性对低氧组(HYPO)和常氧组(WT)脊尾白虾肠道微生物群落多样性进行分析,如图 3所示,低氧组Chao1、Observed-features、Shannon和Simpson 4个Alpha多样性指数均高于常氧组且有显著性差异(P < 0.05),低氧组脊尾白虾肠道微生物群落多样性和物种均匀度最高。
|
图 3 肠道细菌群落Alpha多样性指数(Chao1、Observed、Shannon和Simpson) Fig.3 Alpha-diversity indices of the gut bacterial community (Chao1, Observed, Shannon, and Simpson) *表示显著性水平P < 0.05。下同。 * indicates P < 0.05. The same below. |
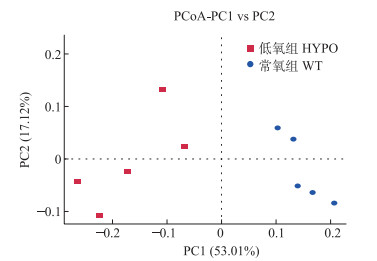
通过主坐标分析(PCoA),可以直观地显示样本群落物种组成的相似性和差异性。在本研究中,除了DO条件不同以外,其他条件(水源、水温、pH值和饲料等)尽可能保持一致,以降低环境条件对脊尾白虾肠道菌群的影响。结果如图 4所示,同组样本在细菌群落上具有更高的相似性,距离更近,不同组样本距离较远,相似性较低。
|
图 4 肠道菌群主坐标分析(PCoA) Fig.4 Principal coordinate analysis (PCoA) of the gut bacterial community |
各种肠道细菌的相对丰度在低氧胁迫24 h后发生变化。在门水平上(图 5a),变形菌门(Proteobacteria)、厚壁菌门为常氧组脊尾白虾肠道中优势菌门,变形菌门更是占总数量的81.42%以上。低氧组脊尾白虾肠道中优势菌门也为变形菌门、厚壁菌门,相比于常氧组,变形菌门在低氧组虾中的数量显著降低(P < 0.05),而拟杆菌门和放线菌门(Actinobacteriota)的数量显著升高(P < 0.05)。

|
图 5 细菌群落在门、科水平结构组成 Fig.5 Structural composition of gut bacterial communities at phylum and family level a:细菌群落在门水平相对丰度;b:细菌群落在科水平相对丰度。 a: Relative abundance of gut bacterial communities at phylum level; b: Relative abundance of gut bacterial communities at family level. |
在科水平上(图 5b),常氧组脊尾白虾肠道中有4个优势菌科,分别为红杆菌科(Rhodobacteraceae)、假单胞菌科(Pseudomonadaceae)、弧菌科(Vibrionaceae)和伯克氏菌科(Burkholderiaceae)。低氧组脊尾白虾肠道中的6个优势菌科为普氏菌科(Prevotellaceae)、鞘脂单胞菌科(Sphingomonadaceae)、硫发菌科(Thiotrichaceae)、弧菌科、毛螺菌科(Lachnospiraceae)和消化链球菌科(Streptococcaceae)。普氏菌科和毛螺菌科在低氧组中的数量显著高于常氧组(P < 0.05),而假单胞菌科和红杆菌科在低氧组中的数量显著低于常氧组(P < 0.05)。
在属水平上(表 1),低氧组脊尾白虾肠道优势菌为假单胞菌属(Pseudomonas)(占14.15%)、鞘脂单胞菌属(Sphingomonas)(占7.24%)和普氏菌属(Prevotella)(占5.24%)。常氧组的脊尾白虾肠道优势菌为假单胞菌属(占26.70%)、亚硫酸盐杆菌属(Sulfitobacter)(占23.96%)和罗尔斯通菌属(Ralstonia)(占3.44%)(表 1)。低氧组中普氏菌属、罗斯氏菌属(Rothia)的数量显著升高(P < 0.05),而假单胞菌属和亚硫酸盐杆菌属的数量显著降低(P < 0.05)。
|
|
表 1 低氧组和常氧组属水平上细菌丰度 Tab.1 Bacterial abundance at genus level in hypoxic and normoxic groups |
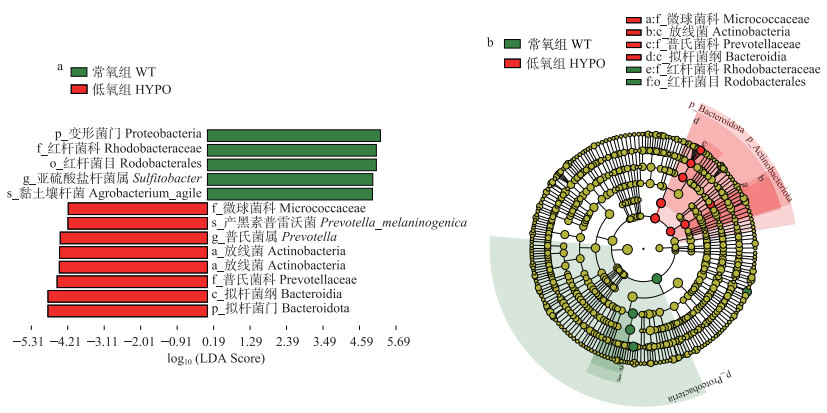
本研究以LDA > 4为阈值进行LEfSe分析,研究缺氧应激与常氧组脊尾白虾肠道样品中差异显著的菌群(图 6)。相比常氧组,低氧胁迫组个体中有13类存在显著差异,包括变形菌门、放线菌门、红杆菌科、红杆菌目(Rhodobacterales)、亚硫酸盐杆菌属和黑色素普氏菌(Prevotella_melaninogenica)、拟杆菌门、普雷沃氏菌科(Prevotellaceae)等。
|
图 6 LEfSe分析常氧组和低氧组差异肠道菌群 Fig.6 Differences in the gut bacterial communities between normoxic and hypoxic group analyzed by LEfSe a:线性判别分析(LDA);b:进化树。 a: Linear discriminant analysis Effect Size(LDA); b: Cadogram. |
目前,针对水生动物低氧胁迫的研究报道已有很多,如罗氏沼虾(Macrobrachium rosenbergii)(邱小龙等, 2022)、金头鲷(Sparus aurata)(Pérez-Jiménez et al, 2012)、中华绒螯蟹(Eriocheir sinensis)(贾慧凝等, 2022)、泥蚶(Tegillarcagranosa)(张阳等, 2023)和凡纳滨对虾(Litopenaeus vannamei) (Liu et al, 2015; Parrilla-Taylor et al, 2011)等。本研究通过人为降低水体溶解氧含量,评价低氧胁迫对脊尾白虾肠组织结构及肠道菌群的影响,发现低氧胁迫下脊尾白虾组织结构出现损伤,肠道菌群结构发生改变。
3.1 低氧胁迫对脊尾白虾鳃组织结构的影响水生动物的鳃是与外界气体交换的重要器官,与水环境直接接触,当水生动物受到胁迫时最先受到损伤,进而直接影响机体的呼吸、代谢及渗透压调节等功能(Bechmann et al, 2019)。在本研究中,低氧胁迫导致脊尾白虾鳃组织结构发生破坏,在低氧胁迫24 h后,泌氯细胞数目相比于常氧组显著减少,泌氯细胞由不规则的扁形变为圆形以增大接触面积,部分支柱细胞与上皮细胞排列紊乱,次级层片肥大,这表明低氧胁迫对鳃组织具有损伤作用,这与日本囊对虾(Marsupenaeus japonicus)和日本沼虾(Macrobrachium nipponense)在低氧胁迫下鳃组织变化情况类似。有学者结合抗氧化酶活力发现,鳃组织可以在低氧胁迫下改变形态结构来缓解低氧压力,但肌肉组织则不可以(赵思哲等, 2023; 杨明等, 2019)。在赵思哲等(2023)对日本囊对虾进行低氧复氧的实验中,进行24 h低氧胁迫之后进行12 h复氧,日本囊对虾受损的鳃组织结构有所恢复,呼吸相关的酶活力均有所恢复。在本研究中,低氧胁迫24 h后,并没有进行复氧,在复氧阶段,已经损伤的脊尾白虾鳃组织结构是否会有所恢复,仍需进一步实验探究。
3.2 低氧胁迫对脊尾白虾肝胰腺组织结构的影响肝胰腺是甲壳动物重要的功能器官,具有解毒、消化、贮存能量和免疫等重要作用,类似于脊椎动物的肝脏、胰脏的功能(杨明等, 2019; 陈信忠等, 2016)。面对低氧胁迫时,不同的组织由于其结构和功能的不同,做出的应激反应也是不同的。本研究中,在低氧胁迫24 h后脊尾白虾肝胰腺出现一些组织学改变,转运空泡体积显著增大甚至出现了破裂的现象,B、R细胞排列紊乱,管腔异常、小管扩张,星形管腔消失。低氧胁迫抑制脊尾白虾有氧代谢,机体会降低自身的代谢水平并调动体内的营养物质维持正常的机体代谢,转运空泡的增大有利于营养物质的吸收利用(赵卫红等, 2014)。在克氏原螯虾(Procambarus clarkia)和三疣梭子蟹(Portunus trituberculatus)等研究中也发现了这些现象(陶易凡等, 2016; 韩晓琳等, 2014)。长期低氧胁迫使脊尾白虾处于胁迫状态,存在组织器官受损的风险,且当胁迫超出组织承受范围时可能导致脊尾白虾出现死亡。
3.3 低氧胁迫对脊尾白虾肠道菌群的影响与鱼类等脊椎动物一样,脊尾白虾肠道内栖息着大量微生物,这些微生物在营养吸收、食物消化和宿主防御等过程中发挥着重要作用(陈贞年等, 2021)。理想状态下,肠道微生物与宿主之间相互制约、相互依赖,保持着微生物平衡,一旦肠道微生物失衡,有害微生物则会趁机入侵,引发疾病(Fabrice et al, 2009; Wong et al, 2016)。本研究对常氧组和低氧组Alpha多样性指数进行了比较,发现常氧组各指数均低于低氧组,低氧组肠道微生物多样性显著高于常氧组,这与孙永旭等(2019)探究低氧对花鲈(Lateolabrax maculatus)肠道菌群的影响结果类似。
本研究基于高通量测序技术发现,在常氧情况下,脊尾白虾肠道内变形菌门、厚壁菌门、拟杆菌门和放线菌门占细菌总量90%以上的绝对优势,这和杨明等(2019)对青虾(Macrobrachium nipponense)肠道菌群分析结果相似。经过24 h低氧胁迫后,其肠道菌群结构发生显著变化,变形菌门细菌在低氧组虾肠道中的数量显著降低,变形菌门细菌在养殖水体和虾肠道中普遍存在,其适应力强,参与水体自净和去除、转化多种污染物,有利于改善养殖水体的水质条件,其显著降低可能与低氧的养殖环境有关,这和王伟等(2023)、宫晗等(2023)的研究类似。低氧组脊尾白虾肠道内拟杆菌门和厚壁菌门细菌数量出现了一定程度的上升,原因可能是拟杆菌门和厚壁菌门细菌主要参与动物胃和肠道内残余物的分解代谢,其分解产生的小分子糖类等营养物质是肠道细胞能量代谢的重要来源(赖婷等, 2022),在24 h胁迫后,拟杆菌门和厚壁菌门数量增加,表明其在低氧胁迫下对脊尾白虾能量代谢起到了重要作用。在更长时间的低氧胁迫下,二者数量是否会持续增长并保持稳定,仍需进一步探究。
在科水平上,低氧组脊尾白虾肠道中普氏菌科和毛螺菌科的数量显著高于常氧组,而假单胞菌科和红杆菌科的数量显著降低。假单胞菌科被认为是有机污染物的降解者(Guo et al, 2023)。研究发现,红杆菌科在健康的脊尾白虾和凡纳滨对虾肠道中有较高的丰度,也普遍存在于养殖水体中,是指示对虾健康和养殖水体健康的关键菌群,是一种新型的有益菌资源,有利于降低水体化学需氧量和调控对虾肠道菌群结构,能够提高对虾抗逆防病能力(段健诚等, 2022; 高繁等, 2022; 王伟等, 2023)。弧菌科是常氧组和低氧组优势菌科之一,其数量在常氧组和低氧组中没有显著性差异,弧菌科是一类条件致病菌,24 h低氧胁迫后假单胞菌科和红杆菌科的数量显著降低,可能导致养殖水体水质恶化和脊尾白虾抵抗能力下降,从而增加了弧菌病的发病几率。
在属的水平上,低氧组和常氧组显示出了不同的差异变化,低氧组中普氏菌属和罗斯氏菌属的数量显著升高,而亚硫酸盐杆菌属的数量显著降低。普氏菌属是一个大属,包含50多个不同的种,帮助分解蛋白质等,可促进脊尾白虾在低氧环境下进行蛋白质分解,但有研究表明,普氏菌丰度的增加可与炎症性疾病联系起来(Kottrashetti et al, 2023; Xiao et al, 2023; 刘蓉蓓, 2017),低氧后普氏菌属的丰度增加到了较高的水平。罗斯氏菌属是革兰氏阳性菌,其下属龋齿罗氏菌(R. dentocariosa)、黏滑罗氏菌(R. mucilaginosa)及大气罗氏菌(R. aeria)是机会致病菌,与人类感染性心内膜炎、血流感染等疾病有关(刘青芹等, 2010; 魏莲花等, 2007; 曹敬荣等, 2016),低氧组中普氏菌属和罗斯氏菌属的数量显著升高可能造成脊尾白虾肠道炎症疾病发生的风险增加。于悦等(2022)研究发现,刺参肠道中的有益菌如亚硫酸盐杆菌属的丰度在抗生素及环境污染物残留的影响下会显著降低,低氧胁迫24 h后脊尾白虾的肠道内出现了类似的情况。上述结果表明,低氧环境会导致脊尾白虾肠道菌群结构发生显著变化,而肠道菌群结构的变化同样可能反作用于宿主的生理调节和营养代谢。
4 结论肠道微生物组成及稳定状态与宿主的健康密切相关,在本研究中,低氧胁迫造成脊尾白虾鳃和肝胰腺组织结构出现损伤,导致脊尾白虾肠道菌群发生变化,部分有益菌数量显著下降,致病菌数量显著增加,提示低氧胁迫或可干扰脊尾白虾肠道稳态而致病。在高密度养殖中,水体溶解氧成为限制脊尾白虾养殖产量的主要原因之一,本研究对提高脊尾白虾的养殖成活率具有一定的帮助作用,为脊尾白虾健康养殖和培育脊尾白虾耐低氧新品种(系)提供了基础科学研究资料。
BECHMANN K R, ARNBERG M, GOMIERO A, et al. Gill damage and delayed mortality of Northern shrimp (Pandalus borealis) after short time exposure to anti-parasitic veterinary medicine containing hydrogen peroxide. Ecotoxicology and Environmental Safety, 2019, 180: 473-482 DOI:10.1016/j.ecoenv.2019.05.045 |
BOKULICH N A, SUBRAMANIAN S, FAITH J J, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods, 2012, 10(1): 57-59 |
CAO J R, WANG P C, SHEN D X, et al. Isolation, identification and phylogenetic analysis of R. aeria from blood specimens of febrile patients. Chinese Journal of Experimental and Clinical Infectious Diseases (Electronic Edition), 2016, 10(2): 254-256 [曹敬荣, 王培昌, 沈定霞, 等. 发热患者血液标本中Rothia aeria的分离鉴定与系统发育分析. 中华实验和临床感染病杂志(电子版), 2016, 10(2): 254-256 DOI:10.3877/cma.j.issn.1674-1358.2016.02.026] |
CHEN X Z, GUO S L, GONG Y Q, et al. Progress of pathogen research on acute hepatopancreatic necrosis syndrome in shrimp. Journal of Inspection and Quarantine, 2016, 26(1): 72-76 [陈信忠, 郭书林, 龚艳清, 等. 对虾急性肝胰腺坏死综合征病原研究进展. 检验检疫学刊, 2016, 26(1): 72-76] |
CHEN Z N, WANG X Q, LUO L T, et al. High-throughput sequencing analysis of the effects of haematogenin on the intestinal flora structure of Trionyx sinensis. Progress in Fishery Sciences, 2021, 42(1): 177-185 [陈贞年, 王晓清, 罗来婷, 等. 高通量测序分析血根碱对中华鳖肠道菌群结构的影响. 渔业科学进展, 2021, 42(1): 177-185] |
CHENG W, LIU C H, HSU J P, et al. Effect of hypoxia on the immune response of giant freshwater prawn Macrobrachium rosenbergii and its susceptibility to pathogen Enterococcus. Fish and Shellfish Immunology, 2002, 13(5): 351-365 DOI:10.1006/fsim.2001.0411 |
DENG G, DUAN J, MU H, et al. Effects of dietary short chain fatty acid salts on the growth performance, digestive, antioxidant and immune enzyme activities, immune related gene expression and resistance to Vibro parahaemolytics infection in juvenile ridgetail white prawn (Exopalaemon carinicauda). Aquaculture Research, 2021, 52(12): 6716-6725 DOI:10.1111/are.15542 |
DONG Z, MAO S, CHEN Y, et al. Effects of air-exposure stress on the survival rate and physiology of Portunus trituberculatus. Aquaculture, 2019, 500: 429-434 DOI:10.1016/j.aquaculture.2018.10.049 |
DUAN J C, HU J H, SHEN Y H, et al. Effects of Enterocytozoon hepatopenaei on the gut bacterial community of Exopalaemon carinicauda. Progress in Fishery Sciences, 2022, 43(3): 75-83 [段健诚, 胡吉卉, 沈宇航, 等. 虾肝肠胞虫感染对脊尾白虾肠道菌群的影响. 渔业科学进展, 2022, 43(3): 75-83] |
FABRICE A, MIREILLE H, BERNARD V, et al. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One, 2009, 4(9): e7125 DOI:10.1371/journal.pone.0007125 |
GAO F, GAN E L, LIU W, et al. Screening of carbon enrichment sources and targeted isolation of Erythrobacteria from the intestinal tract of Litopenaeus vannamei. Journal of Microbiology, 2022, 62(5): 1805-1818 [高繁, 干恩磊, 刘巍, 等. 凡纳滨对虾肠道红杆菌科细菌富集碳源筛选及其定向分离. 微生物学报, 2022, 62(5): 1805-1818] |
GONG H, CHEN P, QIN Z, et al. Analysis of water quality indexes and microflora structure in the factory recirculation aquaculture system of Litopenaeus vannamei. Progress in Fishery Sciences, 2023, 44(1): 125-136 [宫晗, 陈萍, 秦桢, 等. 凡纳滨对虾工厂化循环水养殖系统水质指标及微生物菌群结构的分析. 渔业科学进展, 2023, 44(1): 125-136] |
GU X L, XU Z L. Effects of low oxygen environment on aquatic animals in estuarine and nearshore waters. Marine Fisheries, 2009, 31(4): 426-437 [顾孝连, 徐兆礼. 河口及近岸海域低氧环境对水生动物的影响. 海洋渔业, 2009, 31(4): 426-437] |
GUO X, QIAN Z, PAN Q, et al. Effects of florfenicol on intestinal histology, apoptosis and gut microbiota of Chinese mitten crab (Eriocheir sinensis). International Journal of Molecular Sciences, 2023, 24(5): 4412 |
HAN X L, GAO B Q, WANG H F, et al. Effects of low-salt stress on gill and hepatopancreas microstructure and family survival of Portunus trituberculatus. Progress in Fishery Sciences, 2014, 35(1): 104-110 [韩晓琳, 高保全, 王好锋, 等. 低盐胁迫对三疣梭子蟹鳃和肝胰腺显微结构及家系存活的影响. 渔业科学进展, 2014, 35(1): 104-110] |
HOU W J, ZHOU W Y, PAN G P, et al. Effects of dissolved oxygen level on oxygen consumption capacity and physiological behavior of Penaeus japonicus. Jiangsu Agricultural Science, 2014, 42(2): 190-192 [侯文杰, 周文玉, 潘桂平, 等. 溶氧水平对日本对虾耗氧能力与生理行为的影响. 江苏农业科学, 2014, 42(2): 190-192] |
JIA H N, SHI M M, BIAN Y L, et al. Effects of nanosized selenium on immunoprotection and antioxidant capacity of Eriocheir sinensis under low oxygen stress. South China Fisheries Science, 2022, 18(6): 100-109 [贾慧凝, 侍苗苗, 卞永乐, 等. 纳米硒对低氧胁迫下中华绒螯蟹免疫保护和抗氧化能力的影响. 南方水产科学, 2022, 18(6): 100-109] |
LAI T, NIE Z Y, ZHANG X Y, et al. Effects of starvation stress on physiology, biochemistry and intestinal health of Procambarus clarkii. Journal of Aquatic Biology, 2022, 46(1): 88-97 [赖婷, 聂子盈, 张小雨, 等. 饥饿胁迫对克氏原螯虾生理生化和肠道健康的影响. 水生生物学报, 2022, 46(1): 88-97] |
LI F H, LUAN W, ZHANG C S, et al. Cloning of cytoplasmic heat shock protein 90 (FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia. Cell Stress and Chaperones, 2009, 14(2): 161-172 |
LI Y Y, CHEN X, SONG T Y. Differences in intestinal flora of cultured large yellow croaker Pseudosciaena crocea with different growth rates. Journal of Dalian Ocean University, 2017, 32(5): 509-513 [李英英, 陈曦, 宋铁英. 不同生长速度的大黄鱼肠道菌群结构的差异. 大连海洋大学学报, 2017, 32(5): 509-513] |
LIU H L, YANG S P, WANG C G, et al. Effect of air exposure and resubmersion on the behavior and oxidative stress of Pacific white shrimp Litopenaeus vannamei. North American Journal of Aquaculture, 2015, 77(1): 43-49 |
LIU Q Q, LI J Z. R. dentocariosa and its clinical infection. International Journal of Laboratory Medicine, 2010, 31(9): 981-983 [刘青芹, 李金钟. 龋齿罗氏菌及其临床感染. 国际检验医学杂志, 2010, 31(9): 981-983] |
LIU R B. Study of gut bacterial community in patients with diarrhea-dominant irritable bowel syndrome. Master′s Thesis of Zhejiang University, 2017 [刘蓉蓓. 腹泻主导型肠易激综合征患者的肠道菌群研究. 浙江大学硕士研究生学位论文, 2017]
|
MA H M, WANG X Q, CAO M, et al. Research progress on the culture of Exopalaemon carinicauda. Modern Agricultural Science and Technology, 2019, 16: 171–172, 175 [马鸿梅, 王兴强, 曹梅等. 脊尾白虾养殖研究进展. 现代农业科技, 2019, 16: 171–172, 175] |
MAGOC T, SALZBERG S L. Flash: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 2011, 27(21): 2957-2963 |
PARRILLA-TAYLOR D P, ZENTENO-SAVÍN T. Antioxidant enzyme activities in Pacific white shrimp (Litopenaeus vannamei) in response to environmental hypoxia and reoxygenation. Aquaculture, 2011, 318(3/4): 379-383 |
PÉREZ-JIMÉNEZ A, PERES H, RUBIO V C, et al. The effect of hypoxia on intermediary metabolism and oxidative status in gilthead sea bream (Sparus aurata) fed on diets supplemented with methionine and white tea. Toxicology and Pharmacology, 2012, 155(3): 506-516 |
QIU X L, JIANG Y L, CAI Y S, et al. Behavioral and physiological responses of male and female Macrobrachium rosenbergii to hypoxic stress. Journal of Applied Ecology, 2022, 33(10): 2836-2844 [邱小龙, 江颖琳, 蔡雅霜, 等. 雌雄罗氏沼虾应对低氧胁迫的行为生理响应. 应用生态学报, 2022, 33(10): 2836-2844] |
SUN Y X, DONG H B, WANG W H, et al. Effects of cyclic hypoxic stress on the structure of gut bacterial community of Lateolabrax maculatus. South China Fisheries Science, 2019, 15(4): 46-52 [孙永旭, 董宏标, 王文豪, 等. 周期性缺氧应激对花鲈肠道菌群结构的影响. 南方水产科学, 2019, 15(4): 46-52] |
KOTTRASHETTI V S, BHAT K G, KUGAJI M S, et al. Simultaneous detection and evaluation of Prevotella intermedia, Prevotella nigrescens, Prevotella loescheii, and Prevotella melaninogenica in subgingival plaque samples of chronic periodontitis and healthy individuals through multiplex polymerase chain reaction. Journal of Indian Society of Periodontology, 2023, 27(3): 283-289 |
TAO Y F, QIANG J, WANG H, et al. Effects of low pH stress on gill and hepatopancreatic enzyme activity and tissue structure of Procambarus clarkii. Journal of Fishery Sciences of China, 2016, 23(6): 1279-1289 [陶易凡, 强俊, 王辉, 等. 低pH胁迫对克氏原螯虾鳃和肝胰腺酶活力及组织结构的影响. 中国水产科学, 2016, 23(6): 1279-1289] |
THIJS S, OP DE BEECK M, BECKERS B, et al. Comparative evaluation offour bacteria-specific primer pairs for 16S rRNA gene surveys. Frontiers in Microbiology, 2017, 8: 494 |
UENGWETWANIT T, UAWISETWATHANA U, ARAYAMETHAKORN S, et al. Multi-omics analysis to examine microbiota, host gene expression and metabolites in the intestine of black tiger shrimp (Penaeus monodon) with different growth performance. Peer J, 2020, 8: e9646 |
WANG P, SHI W J, WAN X H, et al. Research progress on the effects of hypoxia on shrimp. Journal of Aquatic Sciences, 2021, 34(6): 96-104 [王盼, 史文军, 万夕和, 等. 低氧对虾类影响的研究进展. 水产学杂志, 2021, 34(6): 96-104] |
WANG W, CUI Q, CAI Z Y, et al. Analysis of changes in water column and intestinal microflora in different modes of Penaeus monodon aquaculture. Journal of Xichang College (Natural Science), 2023, 37(3): 7-14 [王伟, 崔茜, 蔡章印, 等. 不同模式金刚虾养殖水体和肠道微生物菌群变化分析. 西昌学院学报(自然科学版), 2023, 37(3): 7-14] |
WANG Y Y, GUO H, GAO X G, et al. The intratumor microbiota signatures associate with subtype, tumor stage, and survival status of esophageal carcinoma. Frontiers in Oncology, 2021, 11: 754788 |
WANNAMAKER C M, RICE J A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. Journal of Experimental Marine Biology and Ecology, 2000, 249(2): 145-163 |
WEI L H, ZHANG J, ZOU F M, et al. Methodology of rapid identification and drug resistance detection of R. mucilaginosa. Journal of Clinical Laboratory, 2007(3): 218-219 [魏莲花, 张俭, 邹凤梅, 等. 黏滑口腔球菌快速鉴定的方法学探讨及耐药性检测. 临床检验杂志, 2007(3): 218-219] |
WONG N C A, VANHOVE S A, WATNICK I P. The interplay between intestinal bacteria and host metabolism in health and disease: Lessons from Drosophila melanogaster. Disease Models and Mechanisms, 2016, 9(3): 271-281 |
XIAO X L, HANG C, Q H L, et al. A case of pulmonary infection with Prevotella melanogenica having the paving stone symptom. Journal of Radiology Case Reports, 2023, 17(3): 1-7 |
YANG M, SUN S M, FU H T, et al. Effects of hypoxia and reoxygenation on antioxidant enzyme activity and tissue structure of Macrobrachium nipponense. Journal of Fishery Sciences of China, 2019, 26(3): 493-503 [杨明, 孙盛明, 傅洪拓, 等. 低氧和复氧对日本沼虾抗氧化酶活力及组织结构的影响. 中国水产科学, 2019, 26(3): 493-503] |
YANG M. Effects of low oxygen stress on enzyme activity, tissue structure and gut microorganisms of Macrobrachium nipponense. Master′s Thesis of Nanjing Agricultural University, 2019 [杨明. 低氧胁迫对青虾酶活、组织结构及肠道微生物的影响. 南京农业大学硕士研究生学位论文, 2019]
|
YU Y, REN X M, FU X D, et al. Research progress on gut bacterial community of Apostichopus japonicus. Anhui Agricultural Science, 2022, 50(7): 19-22 [于悦, 任昕淼, 付晓丹, 等. 刺参肠道菌群的研究进展. 安徽农业科学, 2022, 50(7): 19-22] |
ZENG Q H. Effects of three environmental factors on growth and immunity indexes of Procambarus clarkia. Master′s Thesis of Changjiang University, 2023 [曾庆辉. 三种环境因子对克氏原螯虾生长、免疫指标的影响. 长江大学硕士研究生学位论文, 2023]
|
ZHANG P, ZHANG X, LI J, et al. The effects of body weight, temperature, salinity, pH, light intensity and feeding condition on lethal DO levels of whiteleg shrimp, Litopenaeus vannamei. Aquaculture, 2006, 256(1/2/3/4): 579-587 |
ZHANG Y, JIN M, LIU H X, et al. Screening and analysis of hemocyte transcriptome and genes related to hypoxia tolerance in Tegillarca granosa after hypoxia stress. Marine Science, 2023, 47(1): 34-44 [张阳, 金铭, 刘宏星, 等. 泥蚶低氧胁迫后血细胞转录组及耐低氧相关基因的筛选与分析. 海洋科学, 2023, 47(1): 34-44] |
ZHAO S Z, HUA S S, LI Y G, et al. Effects of hypoxia-reoxygenation on antioxidant, respiratory metabolism and tissue structure of the Marsupenaeus japonicus. Marine Fisheries, 2023, 45(2): 150-161 [赵思哲, 花松松, 李永闯, 等. 低氧–复氧对日本囊对虾抗氧化、呼吸代谢及组织结构的影响. 海洋渔业, 2023, 45(2): 150-161] |
ZHAO W H, WANG Z S, ZHANG Y X, et al. Effects of estradiol on fatty acid content and tissue structure of hepatopancreas of Macrobrachium nipponense. Marine Fisheries, 2014, 36(6): 542-548 [赵卫红, 王资生, 张余霞, 等. 雌二醇对日本沼虾肝胰腺的脂肪酸含量及组织结构的影响. 海洋渔业, 2014, 36(6): 542-548] |



