2. 农业部海洋渔业可持续发展重点实验室 中国水产科学研究院黄海水产研究所 青岛 266071;
3. 青岛海洋科学与技术国家实验室海洋渔业科学与食物产出过程功能实验室 青岛 266071
2. Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071
蜕皮贯穿甲壳类的整个生活史,与其生长、发育、繁殖和附肢再生等密切相关。甲壳类蜕皮发生的关键因素及其调控机制一直是研究的重点(李旭光等, 2014)。甲壳动物蜕皮一般分为以下3种类型:发育蜕皮是虾蟹类幼体进行变态发育,每一期蜕皮都会对幼体的形态和生理特点产生重要影响。生长蜕皮在胚胎经过变态发育成幼体后,要经过多次蜕皮,使体长体重逐次增加完成生长。蜕皮不仅是旧壳的更新,还包括胃、鳃、肠等的更新及断肢再生。生殖蜕皮又称青春期蜕壳或终期蜕壳,卵巢发育成熟后,在交配前雌性需要蜕壳,个体的某些部位会发生外部形态的显著变化。例如,雌蟹的腹甲可由三角形蜕变成半圆形(Hartnoll, 1974)。
蜕皮主要由互为拮抗的Y器官分泌的蜕皮激素(MH)(Skinner, 1985)和X器官窦腺复合体分泌的蜕皮抑制激素(上官步敏等, 1995)共同调节。另外,也需要多种酶的参与,如几丁质酶(Chitinase)。几丁质富含于甲壳动物围食膜及表皮中,其降解与重新合成是蜕皮过程中一个重要的生理过程,而几丁质酶在这一生理过程中起到至关重要的作用(Funke, 1989; Elyakova, 1972; Jeuniaux, 1963)。蜕皮过程会影响到许多生理功能的变化,Hose等(1992)研究表明,在蜕皮过程中虾体免疫因子会发生改变,很容易受感染。超氧化物歧化酶(SOD)、酸性磷酸酶(ACP)和碱性磷酸酶(AKP)等是体液防御系统中的重要免疫因子。酚氧化酶(PO)也是甲壳动物重要的识别和防御系统,是衡量免疫功能的重要指标(Hernández-López et al, 1996; Smith et al, 1991)。
脊尾白虾(Exopalaemon carinicauda)又名白虾、迎春虾等,系温带海水底栖虾类,在我国沿海均有分布,尤以黄渤海产量最高(李新正等, 2003)。脊尾白虾繁殖盛期一般在5~6月和8~9月;雌虾无纳精囊结构,所以产卵前需进行交配,交配前雌虾须进行生殖蜕皮。在繁殖期,由于抱卵雌虾在抱卵期间卵巢可以二次发育甚至多次成熟,因此,每尾雌虾在1年内可以多次繁殖。本研究以脊尾白虾为对象,从免疫相关的SOD、ACP、AKP、PO活力和蜕皮相关的Chitinase活力、MH浓度变化,初步探究了生长蜕皮、生殖蜕皮2种不同蜕皮分期之间的差异,为甲壳动物蜕皮调控机制的研究提供理论基础。
1 材料与方法 1.1 实验材料脊尾白虾取自山东省日照海辰水产有限公司,均为活力好、体长均匀的健康个体,其中,未性成熟个体体长为(3.22±0.21) cm,体重为(0.49±0.04) g,性成熟个体体长为(5.42±0.34) cm,体重为(1.58±0.22) g。将实验用虾暂养于200 L的PVC桶中,每桶50尾,暂养海水盐度为31,pH为8.2,水温为24℃,24 h持续充氧。每天早晚2次饲喂蛤蜊肉,吸污换水1/3,连续养殖10 d后开始实验。
1.2 实验方法 1.2.1 2种蜕皮区分未性成熟的脊尾白虾处于生长期,实验虾的蜕皮为生长蜕皮。性成熟的实验虾分为卵巢发育虾和抱卵虾,其中,卵巢发育且带有腹蓝者的蜕皮为生殖蜕皮。根据中华锯齿米虾(Neocaridina denticulata sinensis)(王战芳, 2014)和凡纳滨对虾(Litopenaeus vannamei)(Gao et al, 2015)的方法进行蜕皮分期,分为蜕皮间期(C期)、蜕皮后期(软皮期, AB期)、蜕皮前期(D期, 取自特征明显的D2亚期)。
1.2.2 样品采集及处理实验用虾暂养1周后,挑选活力好、无残缺的个体,用解剖剪剪取尾节末端,置于倒置显微镜观察分期,并拍照。将确定分期的脊尾白虾采集血淋巴:每尾虾取0.1 ml血与0.1 ml预冷的抗凝剂混合(抗凝剂配方:1.588 g柠檬酸钠,3.92 g NaCl,4.56 g葡萄糖,0.66 g EDTA-2Na,200 ml ddH2O),4℃ 5000 r/min离心10 min后,留上清液,置于‒20℃冰箱保存。每个平行取3尾,每个蜕皮分期共3个平行。
1.2.3 样品测定方法PO、Chitinase、MH测试盒购自上海酶联。应用双抗体夹心法测定标本中酶活及激素水平,测定方法参考试剂盒说明书。ACP、AKP、SOD测试盒购自南京建成生物研究所。每100 ml血清在37℃与基质作用30 min产生1 mg酚为1个ACP或AKP活力单位(U)。在反应体系中,SOD抑制率达50%时所对应的SOD量为1个SOD活力单位(U)。具体操作见说明书。
1.2.4 数据分析实验数据均表示为平均数±标准差(Mean±SD),同种蜕皮不同分期、不同种蜕皮同一分期分别利用SPSS 21.0软件进行Duncan和独立t检验,One-way ANOVA进行单因素方差分析,以P<0.05为差异显著性。
2 结果与分析 2.1 酚氧化酶(PO)活力在不同蜕皮分期的变化PO在不同蜕皮期的变化见图 1。由图 1可知,生长蜕皮各分期的PO活力差异不显著。生殖蜕皮各分期的PO活力呈先降低后升高趋势,且各分期差异显著(P<0.05)。间期时,生长蜕皮和生殖蜕皮的PO活力差异不显著;前期时,生殖蜕皮的PO活力显著低于生长蜕皮(P<0.05);后期时,生殖蜕皮的PO活力显著高于生长蜕皮(P<0.05)。
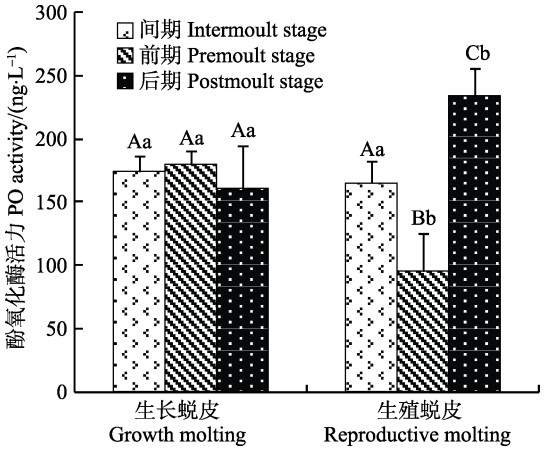
|
图 1 2种蜕皮的各分期酚氧化酶活力变化 Figure 1 Variation of the PO activity during different stages of two types of molting 同种蜕皮的不同分期用大写字母表示差异显著性,不同种蜕皮的同一分期用小写字母表示。下同 Capital letters indicated the significance of the difference during different stages of the same molting type, while lower case letters indicated the same stages of different types of molting. The same as below |
SOD活力在不同蜕皮期的变化见图 2。由图 2可知,生长蜕皮各分期SOD活力呈逐渐升高趋势,且差异显著(P<0.05)。生殖蜕皮后期的SOD活力显著低于间期和前期(P<0.05),间期和前期SOD活力差异不显著。间期时,生殖蜕皮的SOD活力显著高于生长蜕皮(P<0.05),前期二者差异不显著,后期生殖蜕皮的SOD活力显著低于生长蜕皮(P<0.05)。
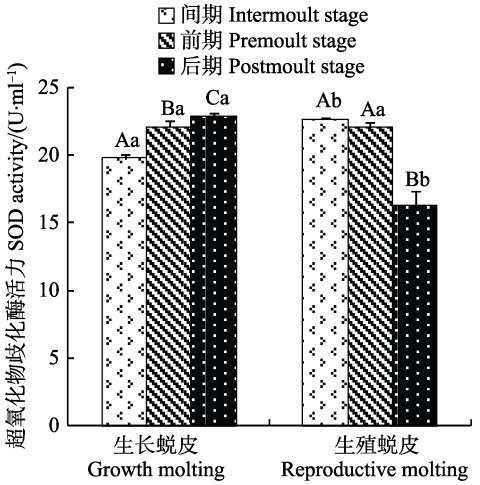
|
图 2 2种蜕皮的各分期超氧化物歧化酶活力变化 Figure 2 Variation of the SOD activity during different stages of two types of molting |
ACP活力在不同蜕皮期的变化见图 3。由图 3可知,生长蜕皮各分期ACP活力呈先升高后降低趋势,且各分期差异显著(P<0.05)。生殖蜕皮与生长蜕皮的ACP活力变化趋势相同;间期、前期、后期时,生长蜕皮的ACP活力都显著高于生殖蜕皮(P<0.05)。
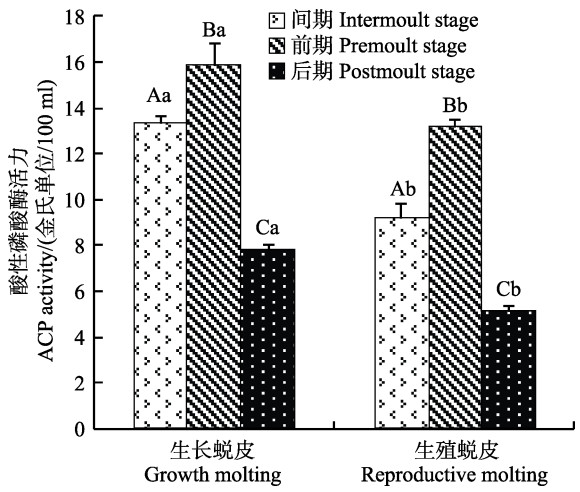
|
图 3 2种蜕皮的各分期酸性磷酸酶活力变化 Figure 3 Variation of the ACP activity during different stages of two types of molting |
AKP活力在不同蜕皮分期的变化见图 4。由图 4可知,生长蜕皮各分期AKP活力呈先升高后降低趋势,且各分期差异显著(P<0.05)。生殖蜕皮与生长蜕皮的AKP活力变化趋势相同,间期、前期、后期时,生长蜕皮的AKP活力都显著高于生殖蜕皮(P<0.05)。与ACP变化趋势相似。
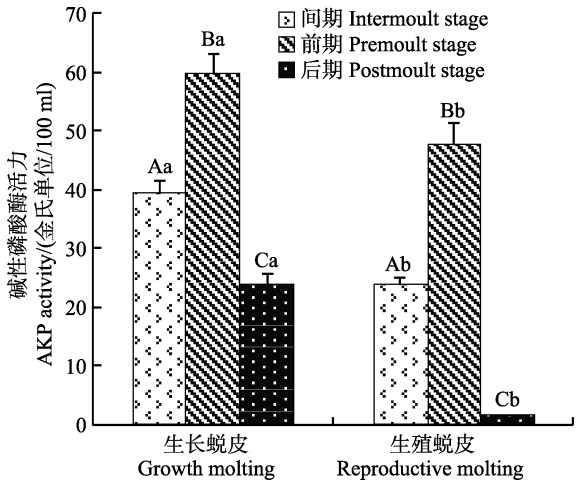
|
图 4 2种蜕皮的各分期碱性磷酸酶活力变化 Figure 4 Variation of the AKP activity during different stages of two types of molting |
Chitinase活力在不同蜕皮分期的变化见图 5。由图 5可知,生长蜕皮各分期Chitinase活力呈逐渐升高趋势,且各分期差异显著(P<0.05)。生殖蜕皮的Chitinase活力呈先降低后升高趋势,且各分期差异显著(P<0.05)。间期和前期,生长蜕皮的Chitinase活力显著低于生殖蜕皮(P<0.05);后期时,生长蜕皮的Chitinase活力显著低于生殖蜕皮(P<0.05)。
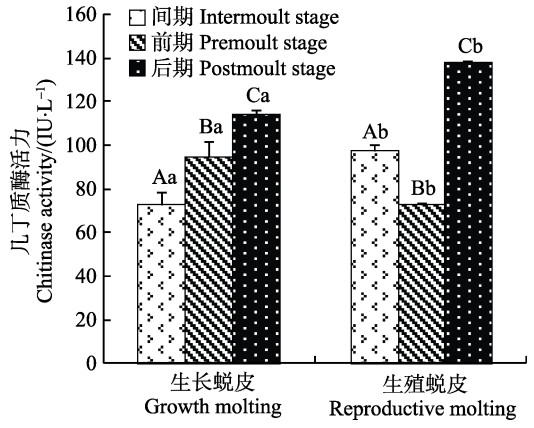
|
图 5 2种蜕皮各分期几丁质酶活力变化 Figure 5 Variation of the chitinase activity during different stages of two types of molting |
MH浓度在不同蜕皮分期的变化见图 6。由图 6可知,生长蜕皮各分期MH浓度呈逐渐升高趋势,但前期与间期和后期差异不显著,后期MH浓度显著高于间期(P<0.05)。生殖蜕皮各分期呈先降低后升高趋势,且各分期差异显著(P<0.05)。间期、前期、后期时,生长蜕皮的MH浓度都显著高于生殖蜕皮(P<0.05)。
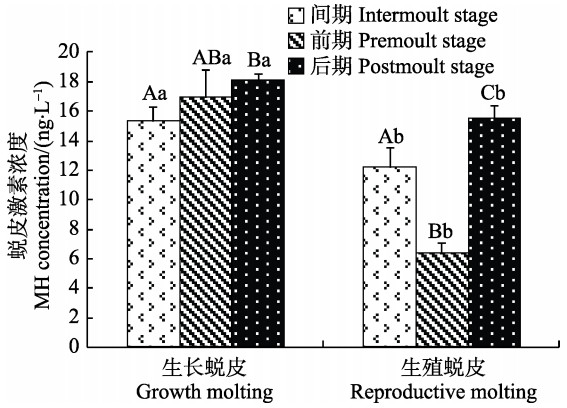
|
图 6 2种蜕皮各分期蜕皮激素浓度变化 Figure 6 Variation of MH concentration during different stages of two types of molting |
蜕皮是甲壳动物个体发育的一个标志,也是个体生长的一个必要阶段。近年来,甲壳动物蜕皮机制一直是研究的重点。在生产中,诱导蜕皮可以缩短蜕皮周期、体重增长变快、促进抱卵等(张海燕等, 1999; 崔青曼等, 2004)。抱卵虾蟹因外界环境的刺激导致蜕皮,从而使受精卵脱落的现象,严重制约了苗种的生产,给养殖业造成重大损失。脊尾白虾为抱卵繁殖虾类,同步性成熟较困难,人工控制交尾及幼体培育技术尚不完善,苗种大部分来自野采亲虾自然繁殖等问题,全人工繁育技术仍需要进一步研究。雌虾的成熟、交尾与蜕皮密切相关,因此,本文探究了生殖蜕皮与生长蜕皮过程中卵巢发育与蜕皮调控之间的相互关系,可为虾蟹类蜕皮机制的研究提供重要信息,为雌虾同步性成熟、实现全人工繁育提供理论依据。
在甲壳动物中,酚氧化酶原系统在机体对抗外物入侵过程中起着重要作用。伴随着颗粒细胞的脱颗粒和酚氧化酶原系统的激活,酚氧化酶被释放出来,起识别和调理作用。血清酚氧化酶活力是虾类的免疫指标之一(陶保华等, 2000)。在2种蜕皮过程中,生长蜕皮变化差异不大,生殖蜕皮在后期酶活性显著升高,可能与蜕皮后排卵有关,这与Alexander等(1992)的研究相似。鱼卵中存在着种类丰富的蛋白酶抑制剂成分,除在鱼卵中执行生理调节功能外,在病原生物防御中也起到了重要作用。而蛋白酶抑制剂可以调节酚氧化酶的活性、抗菌肽的合成等(Alexander, 1992; Gregorio et al, 2002)。活性氧的产生与耗氧的高低存在着一定的相关性,高的耗氧率往往导致高的活性氧水平,而活性氧再由抗氧化系统清除。SOD是唯一以活性氧为底物的抗氧化酶,催化其转化为水和氧气。蜕皮前后代谢旺盛、耗氧率高,相应的活性氧也较高,因此,生长蜕皮前期和后期有着较高的SOD活力。而生殖蜕皮在间期有着高的SOD活力,后期却显著低于生长蜕皮后期,这可能与生殖蜕皮间期卵巢发育代谢旺盛和后期排卵卵巢退化有关。作为溶酶体酶的标志酶,ACP和AKP是甲壳动物的先天免疫反应重要的参与者,同时也是甲壳动物进行酸碱调控的重要因子(Darnell et al, 1986; Jiang et al, 2015)。生长蜕皮和生殖蜕皮变化趋势相同,在前期拥有较高活力,但生殖蜕皮各期普遍低于生长蜕皮,这可能与卵巢发育有关。在蜕皮过程中,旧的几丁质外壳被几丁质酶降解。对甲壳动物几丁质酶与其蜕皮间的关系已有不少研究,Espie等(1995)发现几丁质酶的产生与蜕皮激素分泌量密切相关,几丁质酶mRNA的表达会受到蜕皮激素的调节,这与本文中Chitinase活力与MH浓度变化趋势相一致。而生长蜕皮和生殖蜕皮过程中,Chitinase活力和MH浓度变化却不相同,在生殖蜕皮前期骤降可能与性腺发育有关,这与罗荣生等(1990)的研究结果相似,该研究认为中华绒螯蟹(Eriocheir sinensis)摘除双侧眼柄后,血淋巴中20-羟蜕皮酮含量逐渐增高,在第10天后迅速下降,下降可能与性腺发育有关。综上所述,从非特异性免疫、蜕皮相关酶和蜕皮激素3项指标的变化结果可知,生长蜕皮和生殖蜕皮因为卵巢的发育而存在明显不同。卵巢发育会导致蜕皮过程中机体免疫力显著升高,而使蜕皮相关酶和激素水平显著降低。
| Alexander JB, Ingram GA. Noncellular nonspecific defence mechanisms of fish. Annual Review of Fish Diseases, 1992, 2: 249-279 DOI:10.1016/0959-8030(92)90066-7 | |
| Cui QM, Yuan CY, Wu TT. Effects of eyestalk-ablated and injecting progesterone on the Chinese mitten-handed juvenile crab ovarian development. Marine Fisheries Research, 2004, 25(6): 30-34 [崔青曼, 袁春营, 吴婷婷. 眼柄切除及注射黄体酮对中华绒螯蟹幼蟹卵巢发育的影响. 海洋水产研究, 2004, 25(6): 30-34] | |
| Darnell J, Lodish H, Baltimore D. Molecular cell biology. New York: Scientific American, 1986. | |
| Elyakova L. Distribution of cellulases and chitinases in marine invertebrates. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1972, 43(1): 67-70 DOI:10.1016/0305-0491(72)90202-7 | |
| Espie PJ, Roff JC. Characterization of chitobiase from daphnia magna and its relation to chitin flux. Physiological Zoology, 1995, 68(5): 727-748 DOI:10.1086/physzool.68.5.30163928 | |
| Funke B, Spindler KD. Characterization of chitinase from the brine shrimp Artemia. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1989, 94(4): 691-695 DOI:10.1016/0305-0491(89)90151-X | |
| Gao Y, Zhang XJ, Wei JK, et al. Whole transcriptome analysis provides insights into molecular mechanisms for molting in Litopenaeus vannamei. PLoS One, 2015, 10(12): e0144350 DOI:10.1371/journal.pone.0144350 | |
| Gregorio ED, Han SJ, Lee WJ, et al. An immune-responsive serpin regulates the melanization cascade in Drosophila. Developmental Cell, 2002, 3(4): 581-592 DOI:10.1016/S1534-5807(02)00267-8 | |
| Hartnoll RG. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda Brachyura). Crustaceana, 1974, 27(2): 131-136 DOI:10.1163/156854074X00334 | |
| Hernández-López J, Gollas-Galván T, Vargas-Albores F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1996, 113(1): 61-66 DOI:10.1016/0742-8413(95)02033-0 | |
| Hose JE, Martin GG, Tiu S. Patterns of hemocyte production and release throughout the molt cycle in the penaeid shrimp Sicyonia ingentis. Biological Bulletin, 1992, 183(2): 185-199 DOI:10.2307/1542206 | |
| Jeuniaux C. Chitine et chitinolyse: Un chapitre de la biologie moléculaire. Paris: Masson et Cie, 1963. | |
| Li XG, Zhou G, Gu XH. Review of aquatic crustaceans molting and its influencing factors. Chinese Journal of Zoology., 2014, 49(2): 294-302 [李旭光, 周刚, 谷孝鸿. 水生甲壳类蜕皮发生过程及其影响因素的研究与进展. 动物学杂志, 2014, 49(2): 294-302] | |
| Li XZ, Liu RY, Liang XQ. The zoogeography of Chinese Palaemonoidea fauna. Biodiversity Science, 2003, 11(5): 393-406 [李新正, 刘瑞玉, 梁象秋. 中国长臂虾总科的动物地理学特点. 生物多样性, 2003, 11(5): 393-406] | |
| Luo RS, Wang YL, Cao MX, et al. The role of hemolymph 20-Hydroxyecdysone in molting and oocyte development of the crab Eriocheir sinensis H. Milne-Edwards. Acta Zoologica Sinica, 1990, 36(2): 157-164 [罗荣生, 王幽兰, 曹梅讯, 等. 中华绒螯蟹血淋巴20-羟蜕皮酮诱发蜕皮和卵巢发育的作用. 动物学报, 1990, 36(2): 157-164] | |
| Shangguan BM, Li SJ. Microstructural and ultrastructural studies on the sinus gland of Scylla serrata. Acta Zoologica Sinica, 1995, 41(4): 341-346 [上官步敏, 李少菁. 锯缘青蟹窦腺显微和超微结构研究. 动物学报, 1995, 41(4): 341-346] | |
| Skinner DM. Molting and regeneration. In book: Integument, pigments, and hormonal processes, 1985: 43‒146 | |
| Smith VJ, S derh ll K. A comparison of phenoloxidase activity in the blood of marine invertebrates. Developmental & Comparative Immunology, 1991, 15(4): 251-261 | |
| Tao BH, Hu CQ, Ren CH. The immunological protection of Vibro vaccine to Penaeus monodon and Penaeus japonicus. Journal of Fisheries of China, 2000, 24(6): 564-569 [陶保华, 胡超群, 任春华. 弧菌疫苗对斑节对虾和日本对虾免疫预防的作用. 水产学报, 2000, 24(6): 564-569] | |
| Wang ZF. Study on the characteristics of reproduction and molting in Neocaridina denticulata sinensis. Masterxs Thesis of Hebei University, 2014, 22‒27[王战芳. 中华锯齿米虾繁殖及蜕皮特征的研究. 河北大学硕士研究生论文, 2014, 22‒27] | |
| Zhang HY, Chen XT, Fan YW, et al. Effects of bilateral eyestalk ablation on Macrobrachium nipponense. Journal of Shanghai Teachers University (Natural Sciences), 1999, 28(3): 84-88 [张海燕, 陈新旦, 范艳雯, 等. 切除两侧眼柄对日本沼虾的影响. 上海师范大学学报:自然科学版, 1999, 28(3): 84-88] | |
| Zhang WY, Jiang QC, Liu XQ, et al. The effects of acute ammonia exposure on the immune response of juvenile freshwater prawn, Macrobrachium nipponense. Journal of Crustacean Biology, 2015, 35(1): 76-80 DOI:10.1163/1937240X-00002292 |



