环境内分泌干扰物(EDCs)是一类存在于自然环境中的具有类激素效应的化学物质,对生物体内源激素的合成、分泌、转运和降解过程具有干扰作用,进而损伤生物体的生殖发育、免疫、内分泌和神经系统,甚至导致其后代的内分泌代谢失衡(Cabana et al, 2007; Tri et al, 2016)。当前,EDCs主要来源于工农业生产和民众日常生活产生的废气、废水和废渣,常见的包括多氯联苯(PCBs)、多环芳烃(PAHs)、二噁英、有机氯杀虫剂(OCPs)、雌二醇和双酚A(BPA)以及Cd、Pb、Hg等重金属等。大量文献表明,当前全球主要水体中均已含有多种EDCs。由于水体的流动性较强,EDCs可以随水体流动进行长距离的扩散污染,因此,EDCs对水生动物的暴露毒理效应已经成为一个受到广泛关注的全球性环境问题(Huang et al, 2012)。相应地,EDCs对水生动物的生态和生理毒性及其生物学机制也成为近年来学界的研究热点。目前,EDCs对水生动物的毒理效应研究主要集中于EDCs对水生动物生长发育和生殖性能的损伤机制研究。已知水环境中的雌激素能够诱发鱼类性腺在发育的过程中产生畸形(Bernet et al, 2008),降低鱼类的生殖能力(Milestone et al, 2012),诱导雄鱼性逆转成为雌雄同体甚至是雌鱼(Baroiller et al, 1999; Kidd et al, 2007)。而PCBs可以诱发鱼类肾细胞形状生长异常,肾小球毛细血管扩张,肾小球间质水肿,进而降低鱼体的免疫功能(Koponen et al, 2001)。水体沉积物中PAHs的浓度为0.5~10 µg/g dw (干重)时,可显著降低水体中鱼类胚胎的孵化率和成活率,降低幼鱼的生长速率,并且造成DNA的损伤和破坏(Le Bihanic et al, 2014)。然而,与生长发育和生殖过程相比,人们对EDCs如何影响水生动物代谢过程却知之甚少。
在当前的水产养殖中,鱼类的代谢性疾病十分普遍,尤其体现在鱼体的脂肪过度沉积,包括脂肪肝和腹腔脂肪严重沉积等(杜震宇, 2014)。大量研究表明,鱼体脂肪过度沉积,不仅对鱼体自身的生长发育产生不利的影响,造成养殖鱼类抗应激能力下降,同时也降低水产品品质,增加食品安全的风险(黄春红等, 2014)。目前,一般认为造成鱼类脂肪严重沉积的主要因素包括:能量摄入过度、营养素摄入不均衡、鱼体的生理和物种差异、遗传和基因突变以及养殖环境中污染物的代谢干扰(杜震宇, 2014)。其中,环境污染物影响鱼类脂质代谢的研究相对较少。然而,近年来,随着环境水体的污染日益严重,养殖水体中的环境污染物对养殖鱼脂肪沉积的影响也引起了研究者的重视。当前,研究者已经在持久性有机污染物(POPs)、重金属和环境雌激素对鱼类脂质代谢的影响方面取得了诸多研究进展。但是,至今尚无人对这一领域的研究进展进行综述。为了总结该领域的研究进展,并进一步推动相关研究,本文回顾并总结了主要的环境污染物影响鱼类脂质代谢的生理表型与可能机制,并对本领域今后的研究方向提出了建议。
1 持久性有机污染物在鱼体中的沉积及其对鱼类脂质代谢的影响持久性有机污染物(POPs)包括PCBs、OCPs、二噁英、呋喃和PAHs等,主要来源于工业生产和农业杀虫剂的大规模使用。POPs在生物体内难以被降解代谢,是一类对动物体和环境产生严重危害的天然或者人工合成的有机污染物。POPs可以通过大气和海洋的流动进行全球性的远距离迁移,并通过食物链产生生物放大和生物富集效应(Larsson et al, 1996; Wania, 2003)。目前,全球各主要水域都已检出浓度不等的POPs残留。研究表明,中国东部沿海PCB和滴滴涕的浓度范围分别为1.23~16.6 ng/L和0.28~13.4 ng/L (Lin et al, 2012; Liu et al, 2015; Tang et al, 2013; Zhang et al, 2011)。此外,中国内陆水域中的POPs残留浓度为0.395~5290 ng/L (曹秀芹等, 2016; Liu et al, 2012; 刘艳霖, 2008; Wang et al, 2010)。尽管当前大部分国家已经开始针对某些POPs采取了强制性的限用、禁用和消除措施,但由于POPs的难降解性、生物蓄积性和高毒性,残留在水体中的POPs依然对水生动物造成持久而严重的危害(Trudeau et al, 2007)。
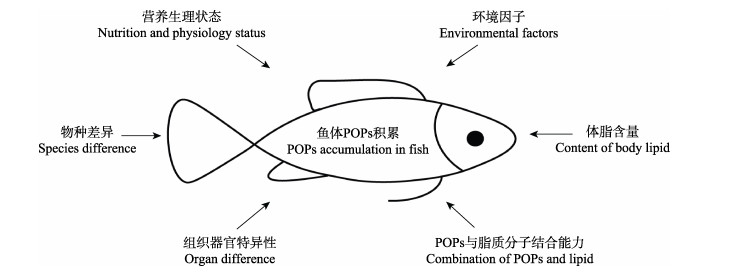
POPs具有亲脂疏水特性,主要累积在生物体的脂肪组织中,这也是POPs影响鱼类脂质代谢的物质前提。研究发现,在不同鱼种中,体脂含量较高的大西洋鲑鱼(Salmo salar)和鲱鱼(Clupea harengus)与体脂含量较少的鲈鱼(Perca fluviatilis)和鳕鱼(Gadus morhua)相比较,前二者体内的四氯联苯和OCPs污染物积累较多(Ewald et al, 1994)。在同一鱼种中,如大西洋鲑鱼,在其远距离迁徙时,体内脂肪含量会产生较大的波动,而PCBs在其体内的积蓄亦会随着体脂的变化,与体脂含量间呈现正相关性(Hamelink et al, 1971; Larsson et al, 1996; Sun et al, 2018)。因此,目前学界普遍认为鱼体POPs积累水平与其脂肪含量,尤其是与甘油三酯(TG)含量间密切相关(Jorgensen et al, 1997; Hardell et al, 2010; Li et al, 2018)。但也有少量研究表明,POPs在鱼体中的沉积量并非完全取决于体脂含量。比如,湖鳟(Salvelinus namaycush)不同亚种的肌肉脂肪含量存在显著差异,但是不同亚种体内的PCBs含量却无明显差异(Miller et al, 1992)。同样,处于非产卵期的鳟鱼体内脂肪含量与体内PCBs的沉积亦无相关性(Stow et al, 1997)。另有一些研究的结果表明,鱼体中POPs的沉积与鱼类的营养状态有关,并存在组织分布的特异性。比如,当鳎目鱼(Solea solea)饥饿后,虽然组织和器官中的脂肪含量降低,但PCBs含量却出现上升的趋势,且其肝脏中的PCBs含量比脑中的PCBs含量高3~4倍(Boon et al, 1985)。Bruner等(1994)认为,不同类型和分子大小的POPs与脂肪分子的结合能力存在差异,这种差异可造成不同POPs在脂肪组织间分配效率的不同。Bruner等(1994)研究表明,不同PAHs种类间,分子量较小的PAHs与脂肪分子的结合能力不够稳定,比较容易在机体中被清除,因此,其体内浓度与体脂含量的相关性较弱。在野外水环境的污染物研究中发现,水体中杀虫剂DDTs、林丹(γ-HCH)、七氯、Aldrin和Endrin的浓度与鱼体杀虫剂的累积量呈现正相关(Liu et al, 2012)。同样,来自于不同水域的鱼体POPs沉积量亦存在差异,如中国南海、渤海、东海和黄海中鱼体的PCBs平均含量分别为2、62.8、520和1008 ng/g ww (湿重)。其中,东海和黄海中鱼体PCBs沉积量显著高于南海和渤海(Liu et al, 2011)。在加拿大Detroit河中,蓝鳃鱼(Lepomis macrochirus)体内的PCBs含量随着季节更替而发生变化,在夏季,鱼体PCBs沉积量逐渐增加;在冬季,鱼体PCBs含量则维持稳定状态(Mcleod et al, 2014)。由此可见,水域和气候等环境因素同样影响鱼体POPs的沉积状态。综上,如图 1所示,尽管POPs在大部分鱼类中的积累与体脂含量存在正相关,但也和鱼的品种、发育阶段和营养状态等因素有关。
|
图 1 鱼体POPs沉积的影响因素 Fig.1 Influence factors of POPs accumulation in fish |
正因为POPs积蓄与鱼体脂肪含量间具有相关性,使得POPs对鱼体脂质代谢产生了较大的影响。一些研究发现,POPs可对鱼体的脂质代谢过程产生干扰作用,饲料中添加POPs可促进鱼体的脂肪积累。如用含有250 mg/L Aroclor 1248饲料分别投喂鲤鱼(Cyprinus carpio) 7、14和21 d,可显著增加鱼体TG、总胆固醇、游离胆固醇和游离脂肪酸含量(Ito et al, 1974)。相应地,在银鲑(Oncorhynchus kisutch)、三刺鱼(Gasterosteus aculeatus)和蓝鳍金枪鱼(Thunnus thynnus)中发现,以含500和50 mg/kg PCBs的饲料养殖银鲑,体内积累的PCBs可显著增加鱼体肝脏中的脂质含量,但躯壳中脂质含量与对照组相比显著降低(Leatherland et al, 1979; Maisano et al, 2016)。用含有多溴联苯醚(PBDEs)、多氯化萘(PCNs)、PCBs和壬基酚(NP)的饲料分别养殖雌性三刺鱼3.5月和海鲷(Sparus aurata) 21 d,所有POPs处理组鱼体肝脏中均可观察到显著的脂滴积累现象,并且上调NP处理组鱼体肝脏脂合成相关的脂肪酸合成酶(fas)和过氧化物酶增殖物激活受体γ(pparγ)的基因表达(Holm et al, 1993; Camevali et al, 2017)。除了饲料暴露之外,水体POPs暴露亦可对鱼体脂质代谢产生影响。如用0.1、0.2、0.5和1 mg/L杀虫剂Aldrin分别暴露胡子鲶(Clarias batrachus L.) 12、60和132 h后发现,随着暴露浓度的增加和暴露时间的延长,鱼血液中胆固醇的含量逐渐升高,但肝脏中的胆固醇含量则逐渐降低。在1 mg/L浓度132 h处理组中,胆固醇含量在血液中最高,在肝脏中最低。由此推测,在胡子鲶体内,Aldrin可显著提高胆固醇从肝脏向血液的转运效率(Bano, 1982)。在另一项类似的研究中,分别用2和8 mg/L的γ-BHC有机氯杀虫剂,以及1和4 mg/L的Malathion有机磷杀虫剂水体暴露胡子鲶4周后发现,Malathion可提高雄性胡子鲶肝脏脂质含量,γ-BHC则降低肝脏中磷脂合成。这2种杀虫剂均可抑制磷脂、脂肪酸和甘油酯从肝脏向性腺的转运功能,且在不改变胆固醇合成的前提下,均可降低酯化胆固醇向游离胆固醇的转化效率,以及降低游离脂肪酸向甘油酯的转变(Lal et al, 1987)。综合上述研究可知,不管是饲料暴露还是水体暴露,POPs均能对鱼体脂质代谢造成负面影响,往往导致体内甘油三酯的积累或磷脂含量的降低。由于脂质是鱼类性腺发育成熟过程中的重要营养素,因此,脂质代谢的紊乱和脂质组成的不合理,很可能导致鱼类生殖系统发育障碍,这或许是POPs造成水生动物生殖发育障碍的原因之一。
在哺乳动物的研究中,POPs中的三丁基锡(TBT)和三苯基锡(TPT)可特异地结合细胞核受体,如类视黄醇X受体(RXRs)和过氧化物酶体增殖物活化受体γ(PPARγ)。其中,RXRs和PPARs在细胞脂质代谢中具有重要作用,POPs与RXRs结合后,促进RXRs下游的脂合成基因固醇调节元件结合蛋白(srebp1)、脂肪酸转运蛋白(fatp)和fas的mRNA表达(Grün et al, 2006);而POPs与PPARγ结合后,通过调节RXR-PPARγ通路刺激脂肪细胞3T3分化,进而促进细胞内脂肪的积累和储存(Kanayama et al, 2004)。亦有研究表明,TBT通过激活腺苷酸活化蛋白激酶(AMPK)代谢通路进而调节脂质代谢(Nakatsu et al, 2008; Grün et al, 2009)。但在鱼类中,POPs影响脂质代谢的分子机制尚未得到完全阐明。
2 重金属对鱼类脂质代谢的影响重金属是被研究较多的环境污染物,然而,多年以来,研究者针对重金属对水生动物的毒理效应研究多集中于生殖、发育和器官畸形等方面,而重金属对鱼类内分泌与代谢,尤其是脂质代谢的影响研究则相对较少。近年来,一系列研究表明,重金属对水生动物的内分泌和代谢系统同样存在显著影响。因此,重金属也越来越多地被研究者认定为一类环境内分泌干扰物(Katti et al, 1984; Kopp et al, 1982; Richetti et al, 2011; Tulasi et al, 1992)。
2.1 Hg在鱼体中的沉积及对鱼类脂质代谢的影响Hg是环境中毒性最强的重金属之一,甲基汞(MeHg)是Hg在水环境中主要的存在形式,同时也是对动物和人体毒性最大的汞化物。目前,Hg已广泛存在于全球各大水体,中国东部沿海水体Hg含量为0.004~2.4 µg/L (李磊等, 2011; 张亚南等, 2013),中国内陆淡水水域的Hg含量则在0.19~36 ng/L不等(Guo et al, 2008; Wang et al, 2013)。水产养殖中,欧洲鲈鱼(Dicentrarchus labrax)肌肉中Hg含量最大值可达0.021 mg/kg ww (万慧珊等, 2017)。在关于MeHg暴露与2种印度鲶鱼(Wallagoo attu, Mystus aor)体脂质代谢关系的研究中发现,鱼体中富含脂肪的腹部肌肉可大量积累MeHg;随着鱼体脂肪含量的逐渐增加,体内腹部肌肉中MeHg的含量也随之提高,由此该研究提出MeHg在鱼体内的蓄积与鱼体脂肪含量具有显著的相关性(Pal et al, 2013)。但另一项对蓝鳍金枪鱼(Thunnus maccoyii)的研究结论则与之相反,此研究提出Hg在机体中的结合位点位于蛋白质片段上,当鱼体的蛋白含量下降时,与Hg结合的位点亦会随着蛋白质含量的下降而减少,因此,体内的Hg积累量会出现下降的趋势。而此时脂肪含量的增加会产生对Hg积蓄浓度的稀释效应,从而可能出现鱼体中脂肪含量较高,但Hg积蓄量却较低的现象(Balshaw et al, 2008)。综合上述研究,鱼体中的Hg积累是否与脂肪含量相关,至今在学界尚无明确定论。但已有研究表明,水环境中Hg对鱼体脂质代谢的影响较大。有研究表明,在水体中用0.2 mg/L氯化汞分别暴露印度囊鳃鲶(Heteropeneustes fossilis) 10、20和30 d,Hg处理组鱼脑中脂肪含量下降,但肝脏和肌肉中的脂肪含量增加,尤其在暴露20和30 d时,肌肉和肝脏中的脂肪含量分别达到最大值。此项研究中,Hg不仅改变了印度囊鳃鲶各组织中的脂肪含量,而且随着Hg暴露时间的延长,鱼体各组织中的磷脂和胆固醇含量与比例均发生明显变化,鱼脑、肝脏和肌肉出现显著的脂质过氧化现象(Bano et al, 1989)。由于磷脂在脑中具有十分重要的地位,又是细胞膜的主要成分之一,Hg对磷脂结构和成分的影响,以及所诱发的脂质过氧化,无疑是对鱼类脑部结构的破坏,这或许是Hg造成脑神经障碍的原因之一。
2.2 Pb对鱼类脂质代谢的影响Pb因其熔点较低、伸展性好及抗氧化性强等特性,已被大量使用在工业和制造业上,这也使得Pb成为在自然界分布最为广泛的重金属之一。中国东部沿海Pb含量为0.02~4.58 µg/L(孙维萍等, 2009),渤海和黄海的Pb含量平均值为2.62 µg/L (Kong et al, 2015)。Pb不属于动物的必需金属元素,长期高量暴露能够干扰动物的正常代谢和生殖发育(Rosen, 1989; Stowe et al, 1971)。在Pb影响胡子鲶脂质代谢的研究中发现,用5 mg/L的硝酸铅水体暴露胡子鲶150 d,Pb能够显著降低精巢、卵巢和脑中的脂肪和胆固醇的含量,但肝脏中的脂肪和胆固醇含量则显著增加(Katti et al, 1983)。相应地,在与之类似的研究中,用5 mg/L硝酸铅水体暴露攀鲈(Anabas testudineus) 30 d后发现,Pb暴露降低肝脏和卵巢中的脂肪、磷脂和胆固醇含量,但提高了肝脏和卵巢中游离脂肪酸含量和脂肪酶的活性,同时,显著提高血液中总脂、磷脂、胆固醇和游离脂肪酸含量(Tulasi et al, 1992)。与Hg的影响类似,高浓度的Pb暴露亦可造成脂质过氧化现象。研究表明,用50 µg/L的Pb水体暴露镜鲤(Cyprinus carpio) 30 d,可导致镜鲤脑中脂质过氧化水平升高至正常水平的225% (Shafiq Ur, 2003)。由此可见,Pb对鱼体各组织的脂肪含量和脂质代谢稳态造成干扰,并可能通过对性腺和脑中磷脂含量的影响损害鱼体的性腺发育和脑神经功能。此外,Pb暴露造成性腺中胆固醇含量的降低,也提示以胆固醇作为前体的鱼体激素合成与分泌会受到Pb暴露的影响,从而影响鱼类的生长、发育和性成熟(Singh et al, 1979)。
2.3 Cd对鱼类脂质代谢的影响环境中的Cd污染主要来源于有色金属开采和金属冶炼。中国东部沿海Cd含量为0.001~0.6 µg/L (方涛等, 2012; 李磊等, 2011),而在湘江流域中检测到的Cd含量可达5.21 mg/L(刘韵琴等, 2013)。黄渤海中的舌鳎(Cynoglossus robustus)和蓝点马鲛(Scomberomorus niphonius)体内的Cd与其他重金属相比,含量最高,可达0.2 mg/kg ww (韩丹丹等, 2018)。研究表明,Cd暴露可影响鱼类的生长发育,并造成组织器官损伤(Bais et al, 2012; Ismail et al, 2011)。在Cd对鱼类脂质代谢影响的研究中,用5 µg/L的Cd对欧洲鳗(Anguilla anguilla)进行水体暴露30 d后发现,Cd暴露组中,鱼体肌肉和肝脏中的脂肪含量虽然没有发生变化,但与脂肪合成与分解相关的酶类,包括葡萄糖六磷酸脱氢酶(G6PD)、六磷酸葡萄糖脱氢酶(6PGD)、苹果酸酶(ME)、异柠檬酸脱氢酶(ICDH)与脂肪组织脂肪酶(ATGL),其活性和相关基因的表达水平均显著上调。其中,与脂质降解相关的基因atgl表达水平比正常水平上调130倍(Pierron et al, 2007),表明较低浓度的Cd暴露对欧洲鳗的脂质代谢调控体系产生了显著的影响,尤其可能增加脂肪的分解机能。在较低浓度Cd暴露研究中发现,用0.68 mg/L的氯化镉水体暴露线鳢(Ophiocephalus striatus) 96 h,可导致线鳢肝脏中的脂质总含量显著降低(Bais et al, 2012)。而用较高浓度Cd对鱼类进行水体暴露时,则往往导致体脂增加。如用6.8 mg/L氯化镉暴露印度囊鳃鲶30 d后发现,Cd可导致鱼体肝脏中的脂肪显著积累(Sastry et al, 1979);用50 mg/L的氯化镉对胡子鲶进行水体暴露135 d后发现,Cd导致胡子鲶肝脏中的脂肪和胆固醇的含量显著提高,但在脑、卵巢和精巢中,脂肪和胆固醇含量均下降(Katti et al, 1984)。由此可见,不同浓度的Cd暴露对鱼体脂质代谢存在不同的影响。低浓度下,短期Cd暴露可上调鱼体脂肪降解能力,从而降低脂肪积累;而高浓度长期的Cd暴露则增加鱼体肝脏中的脂肪积累。多项Cd对水生动物的毒理研究表明,高浓度Cd暴露会损伤细胞线粒体的正常结构(张娜娜等, 2008)。由于线粒体是负责鱼体脂肪酸β-氧化分解的主要细胞器(Ning et al, 2016),高浓度Cd对鱼线粒体的破坏,就将显著降低鱼体的脂肪分解能力,从而导致脂肪严重积累。
2.4 Cu对鱼类脂质代谢的影响与前述Hg、Pb、Cd相比,Cu是鱼类必需微量元素之一,环境水体中也普遍含有Cu元素。中国东海海水中Cu含量平均值为4.6 µg/L (王长友等, 2009),长江口和杭州湾进海口海水中Cu浓度为0.13~2.72 µg/L(孙维萍等, 2009)。然而,近年来已有不少文献报道,水体中的Cu亦会对鱼类脂质代谢造成显著影响。如在矛尾复虾虎鱼(Synechogobius hasta)的研究上,用低于55 µg/L梯度浓度的Cu分别暴露30和60 d后发现,在30 d时,Cu可显著促进肝脏中脂肪积累,上调脂肪合成相关的脂肪酸合成酶(FAS)、苹果酸酶、异柠檬酸脱氢酶活性与过氧化物酶增殖物激活受体γ基因(pparγ)的表达,并降低脂肪分解相关的atgl基因表达;在60 d时,Cu则通过抑制鱼体脂肪合成,促进脂肪分解途径,最终降低肝脏中的脂肪含量(Huang et al, 2014)。基于不同浓度梯度的Cu暴露的研究表明,用2、24、71和198 µg/L Cu水体暴露黄颡鱼(Pelteobagrus fulvidraco) 42 d后,高浓度的Cu暴露可降低黄颡鱼肝脏脂肪合成酶活性和基因的表达,但显著提高脂蛋白水解酶(LPL)的活性及其mRNA水平;随着Cu暴露浓度的逐渐增加,肝脏和腹腔脂肪组织中的脂肪含量逐渐减少(Chen et al, 2013)。综合上述2项研究结果可知,鱼体肝脏中的脂肪含量受到水体中Cu浓度与暴露时间的影响,高浓度Cu的长期暴露,会严重干扰鱼体的脂肪代谢。研究也发现,不仅水体中的Cu暴露可对鱼体脂质代谢造成影响,饲料中过高的Cu含量亦对鱼体脂质代谢产生干扰。用含有92.45 mg/kg的过量Cu的配合饲料投喂黄颡鱼8周,肝脏中脂肪酸合成酶FAS酶活性下降,pparγ、atgl等与脂肪合成和分解相关基因的mRNA表达显著下调,同时肝脏脂肪显著降低;而在肌肉和腹腔脂肪组织中,高Cu饲料则不影响脂肪积累,且此两组织中的脂肪生成酶活性、脂肪合成与分解基因的表达亦无明显变化差异(Chen et al, 2015)。综合上述水体暴露和饲料暴露2种不同暴露方式的研究可见,不同Cu暴露方式对鱼体脂质代谢均会产生明显影响,但其作用效果有所不同,猜测与Cu经鳃、皮肤或消化道进入体内的效率差异有关,但其真正的机制尚待进一步探索。
2.5 重金属对鱼类脂质代谢的复合干扰效应当前,大部分研究重金属干扰鱼类脂质代谢的工作都聚焦于单一金属的代谢干扰效应,但是,在自然水体中,各类重金属是同时存在的。因此,在真实的水域环境中,重金属对水生动物的内分泌干扰效应必然是来自于多种物质的复合效应。在一项有关Cu和Cd对矛尾复虾虎鱼的复合内分泌干扰效应研究中,用Cu (77 µg/L)和Cd (79 µg/L)复合暴露矛尾复虾虎鱼30 d后,结果显示,Cu-Cd复合暴露与单独Cu或Cd暴露相比,前者可更大程度地提高肝脏中的脂肪积累,并更显著地上调葡萄糖六磷酸脱氢酶、六磷酸葡萄糖脱氢酶、苹果酸酶、异柠檬酸脱氢酶与脂肪酸合成酶活性(Song et al, 2014)。遗憾的是,多种重金属对鱼类代谢的复合干扰效应研究至今仍极其匮乏,当前的研究成果仍无法真实地评估实际水体中的重金属污染对水生动物的代谢干扰效应。
3 环境雌激素对鱼类脂质代谢的影响环境雌激素是一类具有雌激素活性的化学物质,主要包括天然雌激素和人工合成的雌激素物质。天然雌激素来源于人和家畜的排泄物,全世界70亿的人口可以排出大约30, 000 kg/年的雌激素,但家畜的雌激素排放量更为惊人,可达到83, 000 kg/年(Shrestha et al, 2012)。这些含有大量雌激素的排泄物或直接进入废水河流中,或作为天然有机肥料在农业上被使用。人工合成的雌激素包括炔雌醇(EE2)、双酚A (BPA)和壬基酚(NP)等,主要来源于杀虫剂和增塑剂等化学品的生产过程、人用和兽用激素类药物,以及人类的日常生活用品。这些天然或人工合成的雌激素通过各种途径最终汇集到水环境中,进而影响人类饮用水质量和水生动物的生长发育。在中国的31个主要城市采集到的62个饮用水样品中,检测到的17β雌二醇(17β-E2)的最高浓度可达1.7 ng/L (Fan et al, 2013)。BPA在国内外主要河流中的最高浓度为21 µg/L, 在垃圾处理渗液中的浓度可达到1.3~17200 µg/L (平均为269 µg/L) (Crain et al, 2007; Kang et al, 2007; Yamamoto et al, 2001)。目前已有多项研究表明,环境中的雌激素能够通过食物链的富集作用,最终对人体健康造成严重的危害,包括诱发心血管疾病、女性乳腺癌和男性前列腺癌症等(Linet, 2008; Nelles et al, 2011; Wocławekpotocka et al, 2013)。
在生态毒理领域,水体中的环境雌激素对水生动物生殖和发育的影响已有较多研究报道。当水中的雌激素含量在低浓度(0.3~10 ng/L)时,雄鱼精巢变小,精子数量下降(Rose et al, 2013),并同时提高卵黄蛋白原(vtg)的表达量,进而影响雄鱼的生殖能力,导致雄鱼发生雌性化现象(Larsen et al, 2008; Parrott et al, 2005; Xu et al, 2008)。水体雌激素含量过高时,甚至可能改变水生动物种群结构,威胁种群的生存(Blanchfield et al, 2015; Kidd et al, 2007)。然而,环境雌激素对水生动物脂质代谢的研究仍然较少。
在野外的生态调查中发现,加拿大圣克莱尔河中检测到的圆头红马吸口鱼(Moxostoma macrolepidotum)体内EE2含量与体脂含量之间存在明显的正相关(Al-Ansari et al, 2010)。研究发现,用100 µg/L的BPA暴露成年斑马鱼(Danio rerio) 60 d,可导致斑马鱼肝脏脂肪含量显著升高(Ngo et al, 2017)。用22 µg/L的17β-E2暴露虹鳟(Oncorhynchus mykiss) 21 d,可诱发鱼体的总脂肪及肝脏中的TG显著积累(Cakmak et al, 2006)。相应地,研究壬基酚(NP)对鱼类脂质代谢的影响时发现,用低于660 µg/L的不同梯度浓度的NP暴露虹鳟14 d,NP处理组的虹鳟肝脏中TG运输受阻,肝脏中积累大量的脂肪,导致严重的脂肪肝(Cakmak et al, 2003)。以上研究表明,环境雌激素对鱼类脂质代谢产生影响,主要表现为环境雌激素可诱发鱼体组织中的脂肪发生积累。但环境雌激素对鱼类脂质代谢的机制调控研究尚未阐明,在哺乳动物的相关研究中发现,雌激素可通过与雌激素受体α (ERα)结合激活信号传导与转录激活因子3 (STAT3)和腺苷酸活化蛋白激酶(AMPK)启动子,进而介导STAT3和AMPK下游脂肪酸代谢相关基因fas、硬脂酰CoA去饱和酶-1 (scd1)、乙酰辅酶A羧化酶(acc)和乙酰辅酶A酰基转移酶1 (acaa1)的mRNA表达变化(Gao et al, 2006; Palmisano et al, 2017; Pedram et al, 2013)。环境雌激素亦可与PPARγ相互作用,介导PPARγ下游的脂质代谢基因表达变化(Riu et al, 2011)。但在水生动物中,环境雌激素影响脂质代谢的分子机制尚未得到完全阐明。
4 药物对鱼类脂质代谢的影响越来越多的研究表明,多种药物都具有对环境生物的内分泌干扰效应。当前,随着全球药物消耗量的提高以及不恰当的处理,药物在海洋、表层水和地下水中的检出率随之增加,甚至在一些地方的饮用水中亦可检测出药物成分(Daughton et al, 1999; Heberer, 2002)。水体中的药物主要来自于制药厂、医院和家庭废水,并以复合药剂或机体排出的代谢产物2种形式存在于水环境中(Bound et al, 2005)。水中的主要药物包括β受体阻滞药、脂质调节类药、糖尿病治疗药、减轻心绞痛药以及止痛药和抗生素类药物等(Jones et al, 2002)。人、家畜和鱼类同属于脊椎动物,在基因同源性、生物体的生理结构和代谢模式上具有很高的相似性,因此,水中存在的多种药物成分在鱼体上同样具有作用靶点,从而给水生动物的生理和生殖发育带来不可忽视的潜在危害风险(Fent et al, 2006)。Owen等(2007)提出,鱼类对某些药物的敏感性高于哺乳动物,从而导致药物暴露可能对鱼类产生比哺乳动物更加强烈的机体反应。然而,药物对水生动物代谢的影响仍少有报道,相关数据极其匮乏。
目前,仅有的一些研究主要集中在脂质调节药物和抗生素对鱼类脂质代谢的影响上,其中,贝特类(Fibrates)药物的研究相对较多。贝特类药物属于人用降脂药物,是PPARα的激活剂,通过与PPARα结合介导下游的脂肪代谢通路,从而激活过氧化物酶体和线粒体中的脂肪酸β-氧化,促进脂肪分解(Araki et al, 2018; D'costa et al, 1975)。Corcoran等(2010)研究表明,贝特药物在水中的检出率为0.03~7 µg/L。在贝特类对鱼类脂质代谢的研究中发现,虹鳟分别腹腔注射环丙贝特(Ciprofibrate, 35 mg/kg鱼重)和吉非罗齐(Gemfibrozil, 152 mg/kg鱼重) 2~3周,肝脏中乙酰辅酶A氧化酶(ACO)活性显著提高,这表明环丙贝特和吉非罗齐可增加肝脏中过氧化物酶体的脂肪酸β氧化效率(Scarano et al, 1994; Yang et al, 1990)。Du等(2008)研究也发现,非诺贝特可降低草鱼(Ctenopharyngodon idella)的脂肪合成并提高脂肪酸氧化效率,从而降低血液中甘油三酯和胆固醇浓度,减少全鱼和肝脏中的脂肪和高度不饱和脂肪酸(EPA和DHA)的含量。然而,由于贝特类药物存在多种药型,它们在各地水体中的残留量差异较大,对不同鱼类的暴露效应也有所差异。考虑到目前临床上已有大量不同原理的血脂调节药物得到广泛使用,它们对水生动物的内分泌干扰效应仍有大量工作需要开展。
抗生素是人类在临床和动物养殖中使用最久、最广的一类药物。由于早年抗生素在临床和动物养殖中的滥用,使得抗生素在水体中的残留成为当前令人关注的全球性环境问题。然而,环境残留的抗生素对水生动物脂质代谢的影响至今尚未得到研究者的广泛关注。最近,本实验室研究表明,作为当前中国环境水体中残留的主要抗生素,氧四环素和磺胺甲恶唑在环境浓度(420、260 ng/L)以及合法水产治疗剂量(80、100 mg/kg/day)下对罗非鱼(Oreochromis niloticus)和斑马鱼的12周水体和饲料暴露,足以造成鱼体肠道健康、免疫机能和代谢系统的损伤。其中,2种抗生素均能下调罗非鱼体内的脂肪含量和脂肪酶活性,降低肠道和肝脏中的脂肪合成关键基因fas和脂肪酸分解关键基因cpt1的表达水平,显示这2种抗生素的低剂量长期暴露对鱼体的脂肪合成和降解均有抑制作用,并以此进一步影响鱼体的代谢与健康(Limbu et al, 2018; Zhou et al, 2018)。由此可以预料,水体中普遍存在的各类抗生素残留都可能对鱼类代谢存在影响。鉴于抗生素在当前水体环境中的广泛存在,这一领域的相关研究亟待得到重视,但相关研究仍极其缺乏。
综上可见,EDCs对鱼类脂质代谢具有显著而复杂的影响,为清晰地展示各种EDCs对鱼类脂质代谢表型的影响,本文将目前已知的相关影响总结于表 1中。然而,从表 1也可看出,大部分涉及环境污染物对脂代谢影响的研究主要集中于生理生化表型的描述,对其真正的分子调控机制的探索仍然较少;而就大量存在的水体环境污染物种类而言,也仍有大量污染物种类,尤其是新型污染物对鱼类代谢的影响,尚无相关研究。
|
|
表 1 EDCs对鱼类脂质代谢影响 Tab.1 The effects of EDCs on fish lipid metabolism |
本文综述了已有文献中关于EDCs对鱼类脂质代谢影响的研究,然而,这方面大部分研究仍主要集中在PCBs、OCPs、重金属等传统环境污染物上,而对于当前的新型环境污染物的研究较少。事实上,随着当前新型材料的研发与应用,水体中环境污染物的种类不断增加,比如自然界分布广泛的微塑料和纳米材料及一些新型代谢调节类药物和新型抗生素等,都开始进入毒理学家和生态学家的视野。然而,这些新型污染物对鱼类代谢的影响研究存在大量空白之处,因此,在进一步阐明传统EDCs对鱼类脂质代谢的作用机制的同时,后续研究也应着力关注当前新型环境污染物对鱼类脂质代谢的影响及其内分泌干扰机制,为更准确评价当前的环境健康与水生动物健康提供基础数据。
5.2 加强开展EDCs在低浓度下的慢性暴露和复合暴露研究客观地说,在当前大部分EDCs对水生动物的生态毒理研究中,所采用的实验浓度往往远高于实际水环境中的EDCs浓度,采用的暴露周期也相对较短,这并不能反映实际环境的状态。在实际水环境中的EDCs效应,往往更多来自于低剂量的慢性暴露,然而这方面的实验室模拟研究仍相对较少。因此,基于EDCs的实际环境浓度开展长期慢性暴露研究需要得到研究者的重视。同时,由于环境中EDCs对鱼体的危害不仅仅由单个污染物引发,而往往来自多种污染物的复合效应。因此,研究者在后续研究中,也需要加强研究多种DECs在低浓度的慢性复合效应,以使研究成果更接近真实的生态环境。
5.3 重视开展EDCs影响鱼类脂质代谢与关联生理过程的分子机制研究从本文综述可见,大部分的EDCs均会影响鱼体中的脂质代谢。目前的研究表明,EDCs往往通过干扰脂肪在鱼体器官中的合成、分解效率与转运效率(Katti et al, 1983、1984),最终导致肝脏、肌肉等组织器官中的脂肪异常积累,影响鱼体健康与食用安全(Cakmak et al, 2006; Ito et al, 1974; Pal et al, 2013)。但是,EDCs在鱼类脂质代谢和能量平衡调节中的具体作用机制、关键作用靶点等尚未得到阐明,这大大限制了人们对EDCs作用原理的理解。此外,EDCs对鱼类脂质代谢的影响并非仅仅影响脂质代谢本身,而更有可能介由脂质代谢异常进一步影响鱼体的生殖、发育以及抗病免疫过程等。然而,这些相关生理过程的关键连接过程、位点和作用机制均尚未阐明。因此,在今后的研究中,研究者应加强利用现代生物学技术,深入探讨EDCs影响脂质代谢及其关联生理过程的分子机制,这既有利于更深入地阐明鱼类代谢性疾病的发生原因,也为保障水产品的绿色安全提供重要的参考依据。
Al-Ansari AM, Saleem A, Kimpe LE, et al. Bioaccumulation of the pharmaceutical 17α-ethinylestradiol in shorthead redhorse suckers (Moxostoma macrolepidotum) from the St. Clair River, Canada. Environmental Pollution, 2010, 158(8): 2566-2571 DOI:10.1016/j.envpol.2010.05.020 |
Araki M, Nakagawa Y, Oishi A, et al. The peroxisome proliferator-activated receptor α (PPARα) agonist pemafibrate protects against diet-induced obesity in mice. International Journal of Molecular Sciences, 2018, 19(7): 2148 DOI:10.3390/ijms19072148 |
Bais UE, Lokhande MV. Effect of cadmium chloride on histopathological changes in the freshwater fish Ophiocephalus striatus (Channa). International Journal of Zoological Research, 2012, 8(1): 23-32 DOI:10.3923/ijzr.2012.23.32 |
Balshaw S, Edwards JW, Ross KE, et al. Mercury distribution in the muscular tissue of farmed southern bluefin tuna (Thunnus maccoyii) is inversely related to the lipid content of tissues. Food Chemistry, 2008, 111(3): 616-621 DOI:10.1016/j.foodchem.2008.04.041 |
Bano Y, Hasan M. Mercury induced time-dependent alterations in lipid profiles and lipid peroxidation in different body organs of cat-fish Heteropneustes fossilis. Journal of Environmental Science and Health Part B Pesticides, Food Contaminants, and Agricultural Wastes, 1989, 24(2): 145-166 DOI:10.1080/03601238909372641 |
Bano YA. Effects of aidrin on serum and liver constituents of freshwater caffish, Clarias batrachus L. Proceedings: Animal Sciences, 1982, 91(1): 27-32 DOI:10.1007/BF03186056 |
Baroiller JF, Guiguen Y, Fostier A. Endocrine and environmental aspects of sex differentiation in fish. Cellular and Molecular Life Sciences, 1999, 55(6-7): 910-931 |
Bernet D, Liedtke A, Bittner D, et al. Gonadal malformations in whitefish from lake thun: Defining the case and evaluating the role of EDCs. Chimia International Journal for Chemistry, 2008, 62(5): 383-388 DOI:10.2533/chimia.2008.383 |
Blanchfield P, Kidd K, Docker M, et al. Recovery of a wild fish population from whole-lake additions of a synthetic estrogen. Environmental Science and Technology, 2015, 49(5): 3136-3144 DOI:10.1021/es5060513 |
Boon JP, Duinker JC. Kinetics of polychlorinated biphenyl (PCB) components in juvenile sole (Solea solea) in relation to concentrations in water and to lipid metabolism under conditions of starvation. Aquatic Toxicology, 1985, 7(1-2): 119-134 DOI:10.1016/0166-445X(85)90040-2 |
Bound JP, Nikolaos V. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environmental Health Perspectives, 2005, 113(12): 1705-1711 DOI:10.1289/ehp.8315 |
Bruner KA, Fisher SW, Landrum PF. The role of the zebra mussel, Dreissena polymorpha, in contaminant cycling: I. The effect of body size and lipid content on the bioconcentration of PCBs and PAHs. Journal of Great Lakes Research, 1994, 20(4): 725-734 DOI:10.1016/S0380-1330(94)71190-4 |
Cabana H, Jones JP, Agathos SN. Elimination of endocrine disrupting chemicals using white rot fungi and their lignin modifying enzymes: A review. Engineering in Life Sciences, 2007, 7(5): 429-456 DOI:10.1002/(ISSN)1618-2863 |
Cakmak G, Togan I, Severcan F. 17Beta-estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquatic Toxicology, 2006, 77(1): 53-63 |
Cakmak G, Togan I, Uğuz C, et al. FT-IR spectroscopic analysis of rainbow trout liver exposed to nonylphenol. Applied Spectroscopy, 2003, 57(7): 835 DOI:10.1366/000370203322102933 |
Cao XQ, Lv XF, Cheng L. Analysis on removal efficiency of PCBs by typical wastewater treatment process. China Water and Wastewater, 2016, 32(21): 114-118 [ 曹秀芹, 吕小凡, 程琳. 典型污水处理工艺对多氯联苯去除效果的分析. 中国给水排水, 2016, 32(21): 114-118] |
Camevali O, Notarstefano V, Olivotto I, et al. Dietary administration of EDC mixtures: A focus on fish lipid metabolism. Aquatic Toxicology, 2017, 185: 95-104 DOI:10.1016/j.aquatox.2017.02.007 |
Chen QL, Luo Z, Pan YX, et al. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquatic Toxicology, 2013, 136(3): 72-78 |
Chen QL, Luo Z, Wu K, et al. Differential effects of dietary copper deficiency and excess on lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2015, 184: 19-28 DOI:10.1016/j.cbpb.2015.02.004 |
Corcoran J, Winter MJ, Tyler CR. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Critical Reviews in Toxicology, 2010, 40(4): 287-304 DOI:10.3109/10408440903373590 |
Crain DA, Eriksen M, Iguchi T, et al. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reproductive Toxicology, 2007, 24(2): 225-239 DOI:10.1016/j.reprotox.2007.05.008 |
Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: Agents of subtle change?. Environmental Health Perspectives, 1999, 107(Suppl 6): 907-938 DOI:10.1289/ehp.99107s6907 |
D'Costa MA, Angel A. Inhibition of hormone-stimulated lipolysis by clofibrate. A possible mechanism for its hypolipidemic action. Journal of Clinical Investigation, 1975, 55(1): 138-148 DOI:10.1172/JCI107904 |
Du ZY, Clouet P, Degrace P, et al. Hypolipidaemic effects of fenofibrate and fasting in the herbivorous grass carp (Ctenopharyngodon idella) fed a high-fat diet. British Journal of Nutrition, 2008, 100(6): 1200-1212 DOI:10.1017/S0007114508986840 |
Du ZY. Causes of fatty liver in farmed fish: A review and new perspectives. Journal of Fisheries of China, 2014, 38(9): 1628-1638 [ 杜震宇. 养殖鱼类脂肪肝成因及相关思考. 水产学报, 2014, 38(9): 1628-1638] |
Rose E, Paczolt KA, Jones AG. The effects of synthetic estrogen exposure on premating and postmating episodes of selection in sex-role-reversed Gulf pipefish. Evolutionary Applications, 2013, 6(8): 1160-1170 DOI:10.1111/eva.2013.6.issue-8 |
Ewald G, Larsson P. Partitioning of 14C-labelled 2, 2′, 4, 4′-tetrachlorobiphenyl between water and fish lipids. Environmental Toxicology and Chemistry, 1994, 13(10): 1577-1580 DOI:10.1002/etc.v13:10 |
Fan Z, Hu J, An W, et al. Detection and occurrence of chlorinated byproducts of bisphenol a, nonylphenol, and estrogens in drinking water of China: Comparison to the parent compounds. Environmental Science and Technology, 2013, 47(19): 10841-10850 DOI:10.1021/es401504a |
Fang T, Li DJ, Tang JL, et al. Evaluation on distribution of heavy metal elements in sediments and bed material environment in Yangtze River Estuary and its adjacent sea. Yangtze River, 2012, 43(10): 68-71 [ 方涛, 李道季, 唐静亮, 等. 长江口及近海区沉积物重金属与底质环境评价. 人民长江, 2012, 43(10): 68-71 DOI:10.3969/j.issn.1001-4179.2012.10.018] |
Fent K, Weston AA, Caminada D. Ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 2006, 76(2): 122-159 DOI:10.1016/j.aquatox.2005.09.009 |
Gao H, Bryzgalova G, Hedman E, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: A possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Molecular Endocrinology, 2006, 20(6): 1287-1299 DOI:10.1210/me.2006-0012 |
Grün F, Blumberg B. Endocrine disrupters as obesogens. Molecular and Cellular Endocrinology, 2009, 304(1): 19-29 |
Grün F, Watanabe H, Zamanian Z, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Molecular Endocrinology, 2006, 20(9): 2141-2155 DOI:10.1210/me.2005-0367 |
Guo Y, Feng X, Li Z, et al. Distribution and wet deposition fluxes of total and methyl mercury in Wujiang River Basin, Guizhou, China. Atmospheric Environment, 2008, 42(30): 7096-7103 DOI:10.1016/j.atmosenv.2008.06.006 |
Hamelink JL, Waybrant RC, Ball RC. A proposal: Exchange equilibria control the degree chlorinated hydrocarbons are biologically magnified in lentic environments. Transactions of the American Fisheries Society, 1971, 100(2): 207-214 DOI:10.1577/1548-8659(1971)100<207:AP>2.0.CO;2 |
Han DD, Zhao F, MU WL, et al. Hazard analysis and screening of the prior heavy metals of priority pollution in fish in the Yellow Sea and the Bohai Sea. Progress in Fishery Sciences, 2018, 39(1): 46-53 [ 韩丹丹, 赵峰, 牟伟丽, 等. 黄渤海鱼类优先监控重金属污染物的筛选与评价. 渔业科学进展, 2018, 39(1): 46-53] |
Hardell S, Tilander H, Welfinger-Smith G, et al. Levels of polychlorinated biphenyls (PCBs) and three organochlorine pesticides in fish from the Aleutian Islands of Alaska. PloS One, 2010, 5(8): e12396 DOI:10.1371/journal.pone.0012396 |
Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicology Letters, 2002, 131(1-2): 5-17 DOI:10.1016/S0378-4274(02)00041-3 |
Holm G, Norrgren L, Andersson T, et al. Effects of exposure to food contaminated with PBDE, PCN or PCB on reproduction, liver morphology and cytochrome P450 activity in the three-spined stickleback, Gasterosteus aculeatus. Aquatic Toxicology, 1993, 27(1-2): 33-50 DOI:10.1016/0166-445X(93)90045-3 |
Huang C, Chen QL, Luo Z, et al. Time-dependent effects of waterborne copper exposure influencing hepatic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquatic Toxicology, 2014, 155(4): 291-300 |
Huang CH, Xiao TY, Hu Y, et al. Analysis on research status of fatty liver disease in aquaculture fish. Chinese Journal of Animal Nutrition, 2014, 26(7): 1715-1722 [ 黄春红, 肖调义, 胡毅, 等. 养殖鱼类脂肪肝研究现状分析. 动物营养学报, 2014, 26(7): 1715-1722 DOI:10.3969/j.issn.1006-267x.2014.07.001] |
Huang GY, Ying GG, Liu S, et al. Regulation of reproduction- and biomarker-related gene expression by sex steroids in the livers and ovaries of adult female western mosquitofish (Gambusia affinis). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 2012, 162(1): 36-43 DOI:10.1016/j.cbpa.2012.02.006 |
Ismail A, Yusof S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Marine Pollution Bulletin, 2011, 63(5-12): 347-349 DOI:10.1016/j.marpolbul.2011.02.014 |
Ito Y, Murata T. Studies on the influence of PCB on aquatic organisms-Ⅳ: Changes in serum lipid contents and formation of lipid peroxide in the tissues of carp administered with PCB orally. Nippon Suisan Gakkaishi, 1974, 40(3): 261-265 DOI:10.2331/suisan.40.261 |
Jones OAH, Voulvoulis N, Lester JN. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Research, 2002, 36(20): 5013-5022 DOI:10.1016/S0043-1354(02)00227-0 |
Jørgensen EH, Burkow IC, Foshaug HKB, et al. Influence of lipid status on tissue distribution of the persistent organic pollutant octachlorostyrene in Arctic charr (Salvelinus alpinus). Comparative Biochemistry and Physiology, 1997, 118(3): 311-318 |
Kanayama T. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator- activated receptor/retinoid X receptor pathway. Molecular Pharmacology, 2004, 67(3): 766-774 DOI:10.1124/mol.104.008409 |
Kang JH, Aasi D, Katayama Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Critical Reviews in Toxicology, 2007, 37(7): 607-625 DOI:10.1080/10408440701493103 |
Katti SR, Sathyanesan AG. Changes in tissue lipid and cholesterol content in the catfish Clarias batrachus (L.) exposed to cadmium chloride. Bulletin of Environmental Contamination and Toxicology, 1984, 32(4): 486-490 |
Katti SR, Sathyanesan AC. Lead nitrate induced changes in lipid and cholesterol levels in the freshwater fish Clarias batrachus. Toxicology Letters, 1983, 19(1-2): 93-96 DOI:10.1016/0378-4274(83)90267-9 |
Kidd KA, Blanchfield PJ, Mills KH, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(21): 8897-8901 DOI:10.1073/pnas.0609568104 |
Kong P, Luo W, Lu Y, et al. Distribution and bioaccumulation of lead in the coastal watersheds of the Northern Bohai and Yellow Sea in China. Environmental Geochemistry and Health, 2015, 37(3): 491-506 DOI:10.1007/s10653-014-9664-x |
Koponen K, Myers MS, Ritola O, et al. Histopathology of feral fish from a PCB-contaminated freshwater lake. Ambio, 2001, 30(3): 122-126 DOI:10.1579/0044-7447-30.3.122 |
Kopp SJ, Glonek T, Perry HM, et al. Cardiovascular actions of cadmium at environmental exposure levels. Science, 1982, 217(4562): 837-839 DOI:10.1126/science.6213041 |
Lal B, Singh TP. Impact of pesticides on lipid metabolism in the freshwater catfish, Clarias batrachus, during the vitellogenic phase of its annual reproductive cycle. Ecotoxicology and Environmental Safety, 1987, 13(1): 13-23 DOI:10.1016/0147-6513(87)90038-8 |
Larsen MG, Hansen KB, Henriksen PG, et al. Male zebrafish (Danio rerio) courtship behaviour resists the feminising effects of 17α-ethinyloestradiol—morphological sexual characteristics do not. Aquatic Toxicology, 2008, 87(4): 234-244 DOI:10.1016/j.aquatox.2008.02.003 |
Larsson P, Backe C, Bremle G, et al. Persistent pollutants in a salmon population (Salmo salar) of the southern Baltic Sea. Canadian Journal of Fisheries and Aquatic Sciences, 1996, 53(1): 62-69 DOI:10.1139/f95-157 |
Le Bihanic F, Clérandeau C, Le Menach K, et al. Developmental toxicity of PAH mixtures in fish early life stages. Part Ⅱ: adverse effects in Japanese medaka. Environmental Science and Pollution Research, 2014, 21(24): 13732-13743 DOI:10.1007/s11356-014-2676-3 |
Leatherland JF, Sonstegard RA, Holdrinet MV. Effect of dietary Mirex and PCB's on hepatosomatic index, liver lipid, carcass lipid and PCB and Mirex bioaccumulation in yearling coho salmon, Oncorhynchus kisutch. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 1979, 63(2): 243-246 DOI:10.1016/0306-4492(79)90068-6 |
Li J, Haffner GD, Paterson G, et al. Importance of growth rate on mercury and polychlorinated biphenyl bioaccumulation in fish. Environmental Toxicology and Chemistry, 2018, 37(6): 1655-1667 DOI:10.1002/etc.4114 |
Li L, Ping XY, Shen XQ. Spatial and temporal distribution and pollution evaluation of the dissolved heavy metals in the Changjiang Estuary. Journal of Zhejiang University, 2011, 38(5): 541-549 [ 李磊, 平仙隐, 沈新强. 春、夏季长江口溶解态重金属的时空分布特征及其污染评价. 浙江大学学报(理学版), 2011, 38(5): 541-549 DOI:10.3785/j.issn.1008-9497.2011.05.013] |
Limbu SM, Zhou L, Sun SX, et al. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environment International, 2018, 115: 205-219 DOI:10.1016/j.envint.2018.03.034 |
Lin T, Li J, Xu Y, et al. Organochlorine pesticides in seawater and the surrounding atmosphere of the marginal seas of China: Spatial distribution, sources and air-water exchange. Science of the Total Environment, 2012, 435-436(7): 244-252 |
Linet MS. Invited commentary: Postmenopausal unopposed estrogen and breast cancer risk in the women's health initiative--before and beyond. American Journal of Epidemiology, 2008, 167(12): 1416-1420 DOI:10.1093/aje/kwn089 |
Liu L, Li H, Wang Z, et al. Insights into spatially and temporally co-occurring polybrominated diphenyl ethers in sediments of the East China Sea. Chemosphere, 2015, 123: 55-63 DOI:10.1016/j.chemosphere.2014.12.022 |
Liu WX, He W, Qin N, et al. Residues, distributions, sources, and ecological risks of OCPs in the water from Lake Chaohu, China. Scientific World Journal, 2012, 897697 http://www.ncbi.nlm.nih.gov/pubmed/23251107
|
Liu YL. Concentration characteristics and loading of organochlorine pesticides (OCPs) in the West River, south China. Journal of Shenzhen Institute of Information Technology, 2008, 6(4): 76-80 [ 刘艳霖. 西江流域有机氯农药含量组成特征及年通量. 深圳信息职业技术学院学报, 2008, 6(4): 76-80 DOI:10.3969/j.issn.1672-6332.2008.04.018] |
Liu YP, Li JG, Zhao YF, et al. Polybrominated diphenyl ethers (PBDEs) and indicator polychlorinated biphenyls (PCBs) in marine fish from four areas of China. Chemosphere, 2011, 83(2): 168-174 DOI:10.1016/j.chemosphere.2010.12.045 |
Liu YQ, Liu YG, HU XJ, et al. Adsorption of Cr(Ⅵ) by modified chitosan from heavy-metal polluted water of Xiangjiang River, China. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3095-3103 [ 刘韵琴, 刘云国, 胡新将, 等. 改性壳聚糖对湘江河水中重金属污染物Cr(Ⅵ)的吸附. 中国有色金属学报(英文版), 2013, 23(10): 3095-3103] |
Maisano M, Cappello T, Oliva S, et al. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the Mediterranean Sea. Marine Environmental Research, 2016, 121: 40-48 DOI:10.1016/j.marenvres.2016.03.003 |
McLeod A, Leadley TA, Drouillard KG, et al. Effect of season and habitat on PCB bioaccumulation by caged bluegill sunfish deployed in a Great Lakes area of concern. Bulletin of Environmental Contamination and Toxicology, 2014, 93(1): 1-6 |
Milestone CB, Orrego R, Scott PD, et al. Evaluating the potential of effluents and wood feedstocks from pulp and paper mills in Brazil, Canada, and New Zealand to affect fish reproduction: Chemical profiling and in vitro assessments. Environmental Science and Technology, 2012, 46(3): 1849-1858 DOI:10.1021/es203382c |
Miller MA, Madenjian CP, Masnado RG. Patterns of organochlorine contamination in lake trout from Wisconsin waters of the Great Lakes. Journal of Great Lakes Research, 1992, 18(4): 742-754 DOI:10.1016/S0380-1330(92)71333-1 |
Nakatsu Y, Kotake Y, Hino A, et al. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicology and Applied Pharmacology, 2008, 230(3): 358-363 DOI:10.1016/j.taap.2008.03.021 |
Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Review of Endocrinology and Metabolism, 2011, 6(3): 437-451 DOI:10.1586/eem.11.20 |
Ngo MT, Doan TT-P, Nguyen CT, et al. Chronic exposure of μg/L range Bisphenol A to adult zebrafish (Danio rerio) leading to adipogenesis. AIP Conference Proceedings, 2017, 1878(1): 020028 |
Ning LJ, He AY, Li JM, et al. Mechanisms and metabolic regulation of PPARα activation in Nile tilapia (Oreochromis niloticus). Biochimica et Biophysica Acta (BBA)- Molecular and Cell Biology of Lipids, 2016, 1861(9): 1036-1048 |
Owen SF, Giltrow E, Huggett DB, et al. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquatic Toxicology, 2007, 82(3): 145-162 DOI:10.1016/j.aquatox.2007.02.007 |
Pal M, Ghosh M. Relationship of methyl mercury accumulation with lipid and weight in two river cat fish species, Wallagoo attu and Mystus aor, from West Bengal, India. Environmental Monitoring and Assessment, 2013, 185(1): 31-37 DOI:10.1007/s10661-012-2530-3 |
Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Advances in Experimental Medicine and Biology,, 2017, 1043: 227-256 DOI:10.1007/978-3-319-70178-3 |
Parrott JL, Blunt BR. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environmental Toxicology, 2005, 20(2): 131-141 DOI:10.1002/(ISSN)1522-7278 |
Pedram A, Razandi M, O'Mahony F, et al. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Science Signaling, 2013, 6(276): 36 |
Pierron F, Baudrimont M, Bossy A, et al. Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquatic Toxicology, 2007, 81(3): 304-311 |
Richetti SK, Rosemberg DB, Ventura-Lima J, et al. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. NeuroToxicology, 2011, 32(1): 116-122 DOI:10.1016/j.neuro.2010.11.001 |
Riu A, Grimaldi M, le Marine A, et al. Peroxisome proliferator- activated receptor γ is a target for halogenated analogs of bisphenol A. Environmental Health Perspectives, 2011, 119(9): 1227-1232 DOI:10.1289/ehp.1003328 |
Rosen JF. Metabolic abnormalities in lead toxic children: Public health implications. Bulletin of the New York Academy of Medicine, 1989, 65(10): 1067-1084 |
Sastry KV, Gupta PK. The effect of cadmium on the digestive system of the teleost fish, Heteropneustes fossilis. Environmental Research, 1979, 19(2): 221-230 DOI:10.1016/0013-9351(79)90050-1 |
Scarano LJ, Calabrese EJ, Kostecki PT, et al. Evaluation of a rodent peroxisome proliferator in two species of freshwater fish: Rainbow trout (Onchorynchus mykiss) and Japanese medaka (Oryzias latipes). Ecotoxicology and Environmental Safety, 1994, 29(1): 13-19 DOI:10.1016/0147-6513(94)90026-4 |
Shafiq ur R. Lead-exposed increase in movement behavior and brain lipid peroxidation in fish. Journal of Environmental Science and Health. Part A, Toxic/hazardous substances & environmental engineering, 2003, 38(4): 631-643 |
Shrestha SL, Casey FX, Hakk H, et al. Fate and transformation of an estrogen conjugate and its metabolites in agricultural soils. Environmental Science and Technology, 2012, 46(20): 11047-11053 DOI:10.1021/es3021765 |
Singh AK, Singh TP. Seasonal fluctuation in lipid and cholesterol content of ovary, liver and blood serum in relation to annual sexual cycle in Heteropneustes fossilis (Bloch). Endokrinologie, 1979, 73(1): 47-54 |
Song YF, Luo Z, Pan YX, et al. Effects of copper and cadmium on lipogenic metabolism and metal element composition in the javelin goby (Synechogobius hasta) after single and combined exposure. Archives of Environmental Contamination and Toxicology, 2014, 67(2): 167-180 DOI:10.1007/s00244-014-0011-0 |
Stow CA, Jackson LJ, Amrhein JF. An examination of the PCB: Lipid relationship among individual fish. Canadian Journal of Fisheries and Aquatic Sciences, 1997, 54(5): 1031-1038 |
Stowe HD, Goyer RA. Reproductive ability and progeny of F1 lead-toxic rats. Fertility and Sterility, 1971, 22(11): 755-760 DOI:10.1016/S0015-0282(16)38586-7 |
Sun SX, Hua XM, Deng YY, et al. Tracking pollutants in dietary fish oil: From ocean to table. Environmental Pollution, 2018, 240: 733-744 DOI:10.1016/j.envpol.2018.05.027 |
Sun WP, Pan JM, Lv HY, et al. Distribution of dissolved trace metals in summer and winter of 2006 in Changjiang River Estuary and Hangzhouwan Bay. Journal of Marine Sciences, 2009, 27(1): 37-43 [ 孙维萍, 潘建明, 吕海燕, 等. 2006年夏冬季长江口、杭州湾及邻近海域表层海水溶解态重金属的平面分布特征. 海洋学研究, 2009, 27(1): 37-43 DOI:10.3969/j.issn.1001-909X.2009.01.006] |
Tang Z, Huang Q, Yang Y, et al. Organochlorine pesticides in the lower reaches of Yangtze River: Occurrence, ecological risk and temporal trends. Ecotoxicology and Environmental Safety, 2013, 87: 89-97 DOI:10.1016/j.ecoenv.2012.10.001 |
Tri TM, Anh DH, Hoai PM, et al. Emerging endocrine disrupting chemicals and pharmaceuticals in Vietnam: A review of environmental occurrence and fate in aquatic and indoor environments. 2016, 10.1021/bk-2016-1244.ch010
|
Trudeau V, Tyler C. Endocrine disruption. General and Comparative Endocrinology, 2007, 153(1-3): 13-14 DOI:10.1016/j.ygcen.2007.06.003 |
Tulasi SJ, Reddy PUM, Rao JVR. Accumulation of lead and effects on total lipids and lipid derivatives in the freshwater fish Anabas testudineus (Bloch). Ecotoxicology and Environmental Safety, 1992, 23(1): 33-38 DOI:10.1016/0147-6513(92)90019-Y |
Wan HS, Cheng B, Song XH, et al. Contamination and accumulation of heavy metals in Dicentrarchus labrax cultured in recirculating aquatic systems. Progress in Fishery Sciences, 2017, 38(5): 83-91 [ 万慧珊, 程波, 宋晓红, 等. 循环水养殖欧洲鲈鱼(Dicentrarchus labrax)重金属污染状况与富集分布特征. 渔业科学进展, 2017, 38(5): 83-91] |
Wang B, Yu G, Huang J, et al. Probabilistic ecological risk assessment of OCPs, PCBs, and DLCs in the Haihe River, China. Scientific World Journal, 2010, 10(1): 1307-1317 |
Wang CY, Wang XL, Sun BY, et al. Primary study on ecological criterion of heavy metals in East China Sea. Marine Environmental Science, 2009, 28(5): 544-548 [ 王长友, 王修林, 孙百晔, 等. 东海主要重金属生态基准浓度初步研究. 海洋环境科学, 2009, 28(5): 544-548 DOI:10.3969/j.issn.1007-6336.2009.05.016] |
Wang S, Xing D, Wei Z, et al. Spatial and seasonal variations in soil and river water mercury in a boreal forest, Changbai Mountain, Northeastern China. Geoderma, 2013, 206: 123-132 DOI:10.1016/j.geoderma.2013.04.026 |
Wania F. Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environmental Science and Technology, 2003, 37(7): 1344-1351 DOI:10.1021/es026019e |
Wocławek-Potocka I, Mannelli C, Boruszewska D, et al. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. International Journal of Endocrinology, 2013, 650984 https://www.ncbi.nlm.nih.gov/pubmed/23710176/
|
Xu H, Yang J, Wang Y, et al. Exposure to 17α- ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquatic Toxicology, 2008, 88(1): 1-8 |
Yamamoto T, Yasuhara A, Shiraishi H, et al. Bisphenol A in hazardous waste landfill leachates. Chemosphere, 2001, 42(4): 415-418 DOI:10.1016/S0045-6535(00)00079-5 |
Yang JH, Kostecki PT, Calabrese EJ, et al. Induction of peroxisome proliferation in rainbow trout exposed to ciprofibrate. Toxicology and Applied Pharmacology, 1990, 104(3): 476-482 DOI:10.1016/0041-008X(90)90169-U |
Zhang L, Shi S, Dong L, et al. Concentrations and possible sources of polychlorinated biphenyls in the surface water of the Yangtze River Delta, China. Chemosphere, 2011, 85(3): 399-405 DOI:10.1016/j.chemosphere.2011.07.064 |
Zhang NN, Mao WP, Wei CJ, et al. Influence of cadmium on structure and functions of mitochondria in hepatic cells. National Medical Journal of China, 2008, 88(19): 1350-1353 [ 张娜娜, 毛伟平, 魏传静, 等. 镉对人肝细胞WRL-68离体线粒体结构及功能的影响. 中华医学杂志, 2008, 88(19): 1350-1353 DOI:10.3321/j.issn:0376-2491.2008.19.015] |
Zhang YN, He Q, Chen JM, et al. Heavy metals' process in water and pollution risk assessment in surface sediments of the Zhujiang River Estuary. Acta Oceanologica Sinica, 2013, 35(2): 178-186 [ 张亚南, 贺青, 陈金民, 等. 珠江口及其邻近海域重金属的河口过程和沉积物污染风险评价. 海洋学报, 2013, 35(2): 178-186 DOI:10.3969/j.issn.0253-4193.2013.02.019] |
Zhou L, Limbu SM, Shen M, et al. Environmental concentrations of antibiotics impair zebrafish gut health. Environmental Pollution, 2018, 235(1): 245-254 |



