2. 中国水产科学研究院长江中下游渔业生态环境评价与 资源养护重点实验室 中国水产科学研究院淡水渔业研究中心 江苏 无锡 214081
2. Key Laboratory of Fishery Ecological Environment Assessment and Research Conservation in Middle and Lower Reaches of the Yangtze River, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi, Jiangsu 214081, China
水产品是海洋和淡水渔业生产的动植物及其加工产品的统称,常见的水产品主要包括鱼、虾、蟹和贝。水产品富含蛋白质、维生素和矿质元素(姜晓东等, 2015; 赵亭亭等, 2018),具有极高的营养价值,其需求量逐年提高,传统捕捞的数量已完全无法满足消费者的需求,相关名特优新品种的养殖业也得到迅猛发展和高度关注。需要注意的是,随着消费者对水产品品质的追求及对知名产地或著名品牌水产品更加青睐,优质水产品,如阳澄湖中华绒螯蟹(Eriocheir sinensis)、波士顿龙虾(Homarus americanus)等可以售出更高的价格。鉴于此,不法商人常以假充真、以次充好来赚取非法利益。目前,除掺假行为外,原产地标识不清(Miller et al, 2010; Lamendin et al, 2015)及错贴标签(Wallstrom et al, 2020)的现象也大量存在。这种恶意行为必会损害消费者、生产者及行业的利益。
为使消费者合法权益得到保障,欧盟2065/2001号规定要求水产品标签必须注明水产品的获取方法(养殖或野生)、养殖国家与产地的地理位置等信息,但我国尚无如此严格的要求。养殖水产品冒充野生水产品以及普通产地水产品假冒知名地理标志保护产地水产品的现象频发,严重危害着原产地水产品的信誉和消费者的正当权益。在这种市场背景下,亟需进一步加快和强化对水产品的原产地溯源研究,以便建立更多、更有效且更为科学、客观的方法体系对水产品所标识的产地进行有效监督、鉴定,从而确保其产地或品牌来源信息真实。
矿质元素和稳定同位素分析是目前两类水产品产地溯源最受关注的方法(陈胜军等, 2019; 张政权等, 2020)。本文对矿质元素、稳定同位素指纹及其相关的多元统计分析技术在水产品溯源中的原理与应用进行综述与展望,以期为名特优新水产品产地溯源技术的发展提供思路。
1 元素微化学分析与水产品产地溯源 1.1 元素微化学溯源的技术原理矿质元素在生态环境中的分布很不均匀。不同的水、土壤、饲料和空气环境中的矿质元素组成和含量都有其各自的特征(Franke et al, 2005),且不同生境条件中的常量、微量元素会因为多种生命活动而进入动植物体内持续累积,导致来源于不同地区的生物体内元素含量存在较大差异(Anderson et al, 2005),故这种微化学“指纹”的差异可以成为很好的产地溯源指标(图 1)。基于该原理,如果对经济水产品中的元素含量和组成“指纹”进行精准定量检测,可以达到相关水产品产地溯源的目的。虽然由于饲料中常添加一些必需元素,水产品的某些矿质元素含量也有可能受到饲料的影响,但如果挑选饲料影响小的稳定元素,且对尽量多种的元素进行综合分析,则在进行产地判别时,可以有效避免受到人为因素(如污染、饵料等特殊情况)的干扰。
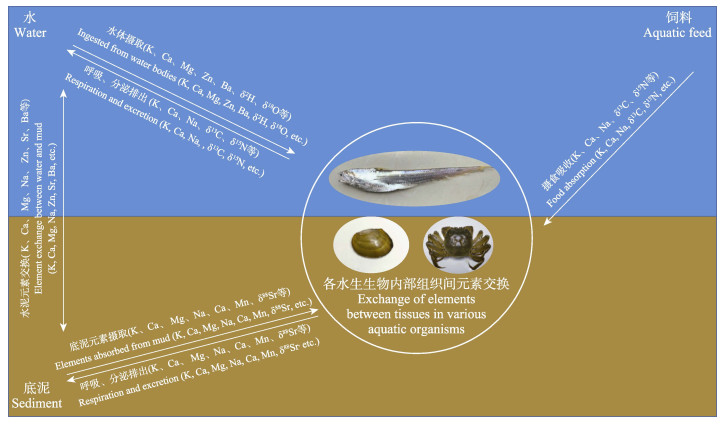
|
图 1 微化学“指纹”溯源原理模式 Fig.1 Schematic diagram of the traceability by microchemical "fingerprint" profiles |
在水产品的产地溯源应用中,鱼类应用最为广泛,因为鱼类在水体中生活的过程中,其耳石结构会持续地吸收水环境中的矿质元素并且不断富集(Campana et al, 2007)。耳石中的Sr与Ba元素的主要来源就是生活的水环境,因此,通过测定耳石的元素进行产地溯源是一种重要方式(Kerr et al, 2014)。Avigliano等(2017)运用电感耦合等离子体发射光谱仪(ICP-OES)对拉普拉塔河3个不同地区的条纹鲮脂鲤(Prochilodus lineatus)耳石的Sr、Ba、Zn与Ca元素的比值进行了测定,在后续的判别分析中,3个产地的判别准确率分别为82.1%、84.2%和77.8%,虽然将元素与几何形态学方法结合的判别更为准确,但单独使用元素指纹判别的准确率要明显优于只使用几何形态学方法。Miyan等(2016)通过电感耦合等离子体发射光谱仪(ICP-AES)测定了来自恒河及其支流的4个不同产地的胡子鲶(Clarias batrachus)矢耳石的Na、Mg、Ba和Cu等12种矿质元素的含量,结果显示,在4个不同产地中,除Na、Ca和Sr元素外,其余9种元素均存在显著性差异,通过12种元素对4个产地的判别分析准确率分别为88%、92%、96%和100%,总体的准确率达94%,产地判别效果非常好。该研究还对同样4个产地的样本进行几何形态学方法的产地判别,产地判别准确率仅为68.39%,与Avigliano等(2017)的研究结果相同,均表明矿质元素的判别效果显著优于几何形态学方法。耳石矿质元素含量同样被Maciel等(2020)应用于大西洋西南部4个河口的叉尾髯海鲶(Genidens genidens)的群体判别,电感耦合等离子体质谱仪(ICP-MS)和激光剥蚀电感耦合等离子体质谱仪(LA-ICP-MS)被用于测定耳石边缘与核心部分Ba、Cu和Mg等7种矿质元素与Ca的比值;边缘部分的综合判别率为75.9%,核心部分的综合判别率为86.2%,对群体的判别效果较好;通过耳石的几何形态学方法对4个群体判别的准确率为75.1%,低于矿质元素的判别效果。通过肌肉对不同地区鱼类的判别效果同样优异,Han等(2019)采集了中国2个不同地区同一年4个季节的鲑鱼(Oncorhynchus keta)样本,通过18种矿质元素的测定不仅可以将2个不同产地的鲑鱼进行产地鉴别,也进一步验证了多元素的产地溯源技术在对鲑鱼产地的鉴别中不会受到季节影响,证实了该技术的应用价值。
近年来,矿质元素分析也越来越多地应用在虾、蟹和贝类的研究中。杨文斌等(2012)通过ICP-MS测定了中华绒螯蟹第3步足的Na、Mg、Al等11种矿质元素的含量,对长江水系3个不同湖泊产的中华绒螯蟹进行产地判别的准确率达到93.3%。杨健等(2013)同样基于第3步足整体组织进行测定,选取Na、Mg等12种测得的矿质元素对相距较近的3个湖泊及距离较远的军山湖中华绒螯蟹进行产地判别,对4个产地的判别准确率达100%。赵鉴等(2014)分别选取辽河水系以及长江水系的4个不同产地,基于第3步足内Na、Mg等12种矿质元素进行产地判别,对4个产地的判别准确率同样可达100%。Varrà等(2021)使用四极感应耦合等离子体质谱仪(Q-ICP-MS)测定了地中海和大西洋3个不同地区的68尾普通乌贼(Sepia officinalis)样本的52种元素含量,主成分分析后选用了3种不同的判别分析方法对这3个产地的普通乌贼进行产地判别,3种方法的交叉验证判别准确率分别为100%、99%和100%,可以准确地对不同来源乌贼进行区分。
在贝类研究中,Zhao等(2016)对采集于中国3个省份海岸的菲律宾蛤仔(Ruditapes philippinarum)进行产地判别,采用ICP-MS测定蛤仔软组织及同步采集的生活水样与泥样的25种矿质元素的含量,3个产地的总体判别准确率为94.4%,具有较好的产地判别效果;相关性分析的结果显示,Na、Mg、Co、Cu等12种元素在蛤仔与水泥间存在显著相关性,有效证实水生生物体内的矿质元素会因为生活水体环境的变化而变化。Bennion等(2019)使用ICP-MS测定采集于爱尔兰西海岸3个海湾的4个不同产地的贻贝(Mytilus edulis)的As、Cd等9种元素,并进行产地判别,发现不同组织有不同的判别效果;但将清洁的贝壳、足部和角质层相结合之后,对4个不同地区(其中有2处采样地点的间距仅为6 km)的贻贝判别准确率可达到100%。Morrison等(2019)通过ICP-MS对采集于爱尔兰西海岸3个海湾共4个不同地点的扇贝(Pecten maximus)进行了产地的判别。其基于10种矿质元素、选取贝壳及软组织分别进行了矿质元素的产地判别研究。结果显示,贝壳的判别达到97.5%的判别准确率,具有满意的产地溯源效果。将软组织与贝壳数据相结合之后可进一步提高判别率至100%。Forleo等(2021)共采集了7个地区25个采样点的紫贻贝(Mytilus galloprovincialis)进行产地溯源研究,通过14种矿质元素的测定及分析,总体的产地溯源准确率达90%以上,可以有效应对市场上的假冒行为。Ricardo等(2020)利用矿质元素快速、准确、有效地进行了花蛤(Ruditapes philippinarum)的产地溯源。
矿质元素不仅被应用于不同产地的水产品的产地鉴别之中,也可以用于对不同养殖方式水产品的鉴别,可以极好地保护野生名贵水产品的信誉。Anderson等(2010)利用19种元素建立了5种不同的模型,尝试对2种生长方式(养殖与野生)的鲑[大西洋鲑(Salmo salar), 大鳞大马哈鱼(Oncorhynchus tshawytscha)和银大麻哈鱼(Oncorhynchus kisutch)]进行区分,虽然根据不同的模型,准确度略有不同,但能够成功地把不同产地与生产方法的鲑鱼区分开来。Zitek等(2010)使用激光剥蚀电感耦合等离子体质谱仪(LA-ICP-MS)对野生与人工养殖的鳟(Oncorhynchus mykiss和Salmo
trutta)的耳石进行元素测定,基于88Sr/43Ca、和23Na/43Ca等分析,可以100%区分野生与养殖的鳟。Li等(2015)通过ICP-AES对不同养殖模式的斑点叉尾

随着矿质元素“指纹”的研究种类与范围的不断扩大,矿质元素“指纹”的应用前景必会更加广阔,但我国目前特色水产品的产地溯源研究仍处于初始阶段,还有很多的特色水产品缺乏相关特征指标的研究,特色水产品的元素“指纹”图谱的建立仍然需要更多的样本量以及更多产地的数据支持。
2 稳定同位素微化学分析与水产品产地溯源 2.1 稳定同位素微化学“指纹”溯源的技术原理质子数相同、中子数不同的同一元素的不同核素被称为同位素,水产品体内某元素的比值受同位素分馏效应的影响。同位素分馏效应是指由于同位素质量不同,在物理、化学及生物化学作用过程中,同位素比值不同的两种物质或同种物质的两种相态间发生的同位素的自然分配效应(Nier, 1950)。同位素分馏效应受气候、环境、生物代谢等因素影响而存在差异(Branch et al, 2003; Liu et al, 2017)。同位素的波动范围相对较小,相对稳定,如同人类的指纹具有各自的特征信息,被称为“同位素指纹”。稳定同位素比质谱仪(IRMS)技术具有精确度高、运用少量样品即可进行同位素检测和区分,且没有放射性,不会造成二次污染的优点。该技术在草莓(Perini et al, 2017)等植物源性水果和牛羊肉(Zhao et al, 2015)等动物源性肉品中均得到了运用。近年来,在水产品溯源中也有了类似的应用(Pereira et al, 2019),特别是δ13C、δ15N、δ2H和δ18O等。
水产品体内的C稳定同位素组成与饲料的种类密切相关。δ13C会因为动物饲料中C3、C4植物比例的不同而发生变化。δ15N除受饲料内动植物占比的影响外,还受到土壤状况等多种因素的影响(Amundson et al, 2003)。水体中的δ2H、δ18O值会受到不同季节、海拔与生存纬度的影响而产生变化,因此,生活在不同水环境中的水产品的δ2H、δ18O值会有较大的差异(周毅等, 2017; Ramesh et al, 1992),也极具产地溯源的潜力。另外,Sr稳定同位素受到地质条件的影响较大,比H、O稳定同位素比值更稳定,可在H、O稳定同位素无法进行有效区分的水产品中进行溯源研究(Kelly et al, 2005)。
2.2 水产品产地溯源中的稳定同位素微化学分析进展稳定同位素技术已经越来越多地应用到鱼类的产地溯源研究。Kim等(2015)选取了11个不同产地的鲭(Scomber japonicas)、6个产地的小黄鱼(Larimichthys polyactis)及7个产地的鳕(Theragra chalcogramma)的肌肉组织,进行C、N稳定同位素的测定,结果显示,鲭与鳕的产地区分度很高,但小黄鱼的区分度极不理想。吴浩等(2021)对来自于6个不同国家的大西洋鲑进行产地溯源研究,选取三文鱼的肌肉、表皮、鳞片和骨骼测定了C、N、H、O和S稳定同位素比值,结果发现,表皮、鳞片和骨骼的C、N与S同位素产地判别准确率均达100%。在Molkentin等(2015)的研究中,通过C、N同位素还可以将野生鲑与养殖鲑进行准确区分,即使鲑鱼肉在烟熏后仍然可以准确的产地溯源。
除鱼类外,同位素溯源技术在贝、海参和虾蟹类的产地溯源中同样广受关注。Zhang等(2019)分别在同一年春季和秋季采集了中国黄海与渤海海域7个地点的虾夷扇贝等3种扇贝(Patinopecten yessoensis, Chlamys farreri和Argopecten irradians)进行了肌肉δ13C和δ15N测定,判别结果显示,对所采集的7个地点的扇贝的综合判别准确率达92%。Zhao等(2019)分别在不同季节采集了中国海域6个不同地点的虾夷扇贝,对所有样本的10种氨基酸进行了C、N同位素比值的测定,产地判别正确率亦可达100%。δ13C和δ15N还被应用在了茎柔鱼(Dosidicus gigas)的产地溯源研究中。Gong等(2018)与Liu等(2019)均采集了东太平洋不同地区的茎柔鱼进行产地溯源研究,通过δ13C和δ15N均达到了较好的产地溯源效果。后者的研究还证明了部分地区的茎柔鱼存在可能的种群关联性。Liu等(2017)对海参(Apostichopus japonicus)测定了特定脂肪酸内的δ13C和δ15N值,对采集于不同季节和不同地点的海参样本进行溯源。除长海岛与獐子岛的区分度可能因地理接近或食物相近而区分度不高外,其余产地的判别结果均可达79.0%。Li等(2018)采集了中国16个地区的凡纳滨对虾(Litopenaeus vannamei)测定δ13C和δ15N值,低盐度、高盐度与淡水产地虾的整体判别准确率为89.6%;研究还发现,δ13C值主要是因为咸水内的富集产生差异,而δ15N的差异主要是来源于饲料。Yin等(2020)则利用多接收器电感耦合等离子体质谱(MC-ICP-MS)测定了步足及背甲等组织中Sr稳定同位素比(87Sr/86Sr),尝试进行我国5个不同的产地中华绒螯蟹的溯源研究,结果显示,相同湖区的螃蟹与水样Sr稳定同位素比值相近,但不同湖区的值则存在显著差异,可进行产地溯源。结果也证实了Sr稳定同位素比值主要受水体环境而非饲料的影响。
稳定同位素溯源技术不仅可以对水产品进行产地鉴别,也可用来区分不同养殖方式的水产品。Camin等(2018)对20个意大利养殖场的130条意大利虹鳟进行鱼肉的H、C、O、N和S同位素分析,综合判别使用不同饲料养殖场虹鳟的准确率达91%;鱼体C、N和S同位素比值与饲料的比值呈正相关,而鱼体H和O同位素比值则与水体的比值呈正相关。Wang等(2018)对野生及不同养殖(传统养殖、有机养殖)条件的鲑进行溯源发现,C、N稳定同位素比值可精准确定上述3类养殖方式。Tulli等(2020)也通过C、N、H和O同位素区分了不同地区及不同养殖系统的欧洲鲈鱼(Dicentrarchus labrax)。
虽然稳定同位素技术在产地溯源中的应用已经取得了很多成功,但目前的研究深度仍然不足,许多研究仍局限于较少的产地范围及样本数量,在后续的相关研究中,对采样地区与样本数量需进一步扩大,以建立完整的同位素“指纹”数据库。
3 矿质元素和稳定同位素结合分析用于水产品产地的溯源 3.1 矿质元素和稳定同位素结合分析的技术原理不同环境条件中的常量、微量矿质元素会因为摄食、呼吸等生命活动在水生动物体内持续累积,导致来源于不同地区的水生动物体内元素含量会存在较大差异。对于同位素指标而言,水产品体内的C、N稳定同位素组成受到水生动物所食用饲料的密切影响;H、O同位素组成会因为水体环境的差异而形成产地上的较大的差异(Ramesh et al, 1992);Sr稳定同位素则受到地质条件的影响较大。不同的溯源指标因为影响因子的不同在进行产地溯源研究时会有极好的互相补充的效果,因此在进行产地溯源逇时,越来越多的研究将多种指标进行综合分析以取得比选取单一因素更好的溯源效果。
3.2 水产品产地溯源中的矿质元素和稳定同位素结合分析进展元素指纹和同位素指纹技术均在水产上具有广泛的应用潜力,而这2种技术的综合利用具有更好的产地溯源效果。2000年以来,将同位素比值和多元素分析相结合进行水产品产地溯源的研究不断增多。黄丽英等(2019)测定了δ13C和δ15N以及Na等10种矿质元素的含量以区别不同渔场产带鱼(Trichiurus haumela)。虽然4个渔场间的δ13C和δ15N值无显著差异,但将稳定同位素比值与矿质元素含量相结合后进行的主成分分析中,成功区分4个渔场的带鱼,且相互没有重叠。Luo等(2019)在区分长江水系8个不同产地中华绒螯蟹时,仅使用δ13C和δ15N值时判别准确率为82.3%,仅使用矿质元素的判别准确率为91.5%,将二者结合之后的判别准确率可达98.2%,判别效果有显著提升。骆仁军等(2020)在对东营、盘绵、营口3个地区的中华绒螯蟹的产地鉴别中发现,以δ13C和δ15N值为溯源指标进行产地鉴别,其原始正确率仅为66.7%,交叉判定正确率为65%,而利用多元分析方法结合稳定同位素和元素组成时,其产地溯源初始正确率高达96.7%,交叉判定正确率为91.7%。在对海参的产地溯源研究中,Kang等(2021)将C、N、O和H同位素比值与22种矿质元素含量相结合,对我国5个不同产地的海参进行溯源研究发现,判别分析的准确度可在96%以上。Liu等(2020)将鱼鳞的微量元素与C、N同位素比值结合,对野生、湖养和池养的鲤鱼(Cyprindae)进行产地区分,训练集的判别准确率高达100%。
在国外的相关研究中,Carter等(2015)对澳大利亚本地与亚洲邻国进口的斑节对虾(Penaeus monodon)等虾类的壳、几丁质及肌肉进行微量元素与同位素分析,结果显示,进口对虾与澳大利亚对虾等存在显著差异。同时,肌肉与几丁质间的同位素组成存在较强的相关性,表明可以从这2个成分中得到等价信息,只需要对其中1个进行分析即可得到产地结果。Ortea等(2015)将元素与同位素分析相结合,采集了3个属的7种对虾[斑节对虾、凡纳滨对虾、印度明对虾(Fenneropenaeus indicus)、墨吉明对虾(Fenneropenaeus merguiensis)、红对虾(Farfantepenaeus notialis)、阿根廷红虾(Pleoticus muelleri)和北极虾(Pandalus borealis)],将两种技术结合后成功地区分了不同种类、不同起源的对虾,同时还成功区分出野生与养殖虾。
稳定同位素技术与多矿质元素分析相联用,并与多种化学计量学分析方法相结合是产地溯源的研究热点。将矿质元素与同位素相结合进行产地溯源的研究,比使用单一方法具有更高的判别准确率。
4 结论近年来,矿质元素与稳定同位素技术在鱼类、甲壳类和贝类水产品的产地成功溯源的应用越来越多。随着人民对美好生活的需求日益增长、对水产品质量安全的问题更加关注,且随着现代渔业提质增效和绿色转型步伐的加速和乡村振兴国策的实施,水产品产地溯源和品牌保护机制将会得到进一步的健全。除矿质元素与稳定同位素技术外,形态学方法、分子生物学技术与仿生感官评价技术等在产地溯源中均有成功应用。综合利用更多元素和同位素微化学“指纹”以及在此基础上结合更多具有产地差异性的指标来开发更有效的技术应该是今后产地溯源研究的趋势,同时,建立从地区到国家层面的水产品产地溯源和原产地保护大数据库的工作同样需要得到高度重视。
AMUNDSON R, AUSTIN A T, SSHUUR E A G, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochemical Cycles, 2003, 17(1): 311-319 |
ANDERSON K A, SMITH B W. Use of chemical profiling to differentiate geographic growing origin of raw pistachios. Journal of Agricultural and Food Chemistry, 2005, 53: 410-418 DOI:10.1021/jf048907u |
ANDERSON K A, HOBBIE K A, SMITH B W. Chemical profiling with modeling differentiates wild and farm-raised salmon. Journal of Agricultural and Food Chemistry, 2010, 58(22): 11748-11774 |
AVIGLIANO E, DOMANICO A, SEBASTIÁN S, et al. Otolith elemental fingerprint and scale and otolith morphometry in Prochilodus lineatus provide identification of natal nurseries. Fisheries Research, 2017, 186: 1-10 DOI:10.1016/j.fishres.2016.07.026 |
BENNION M, MORRISON L, BROPHY D, et al. Trace element fingerprinting of blue mussel (Mytilus edulis) shells and soft tissues successfully reveals harvesting locations. Science of the Total Environment, 2019, 685: 50-58 DOI:10.1016/j.scitotenv.2019.05.233 |
BRANCH S, BURKE S, EVANS P, et al. A preliminary study in determining the geographical origin of wheat using isotope ratio inductively coupled plasma mass spectrometry with 13C, 15N mass spectrometry. Journal of Analytical Atomic Spectrometry, 2003, 18(1): 17-22 DOI:10.1039/B207055N |
CAMIN F, PERINI M, BONTEMPO L, et al. Stable isotope ratios of H, C, O, N and S for the geographical traceability of Italian rainbow trout (Oncorhynchus mykiss). Food Chemistry, 2018, 267: 288-295 DOI:10.1016/j.foodchem.2017.06.017 |
CAMPANA S E, VALENTIN A, SÉVIGNY J M, et al. Tracking seasonal migrations of redfish (Sebastes spp. ) in and around the Gulf of St. Lawrence using otolith elemental fingerprints. Canadian Journal of Fisheries and Aquatic Sciences, 2007, 64(1): 6-18 |
CARTER J F, TINGGI U, YANG X, et al. Stable isotope and trace metal compositions of Australian prawns as a guide to authenticity and wholesomeness. Food Chemistry, 2015, 170: 241-248 DOI:10.1016/j.foodchem.2014.08.037 |
CHEN S J, LIU X J, YANG X Q, et al. Analysis of characteristic elements and establishment of principal component evaluation model of abalone from different habitats. Progress in Fishery Sciences, 2019, 40(2): 83-90 [陈胜军, 刘先进, 杨贤庆, 等. 不同产地鲍鱼特征元素分析与主成分评价模型的建立. 渔业科学进展, 2019, 40(2): 83-90] |
FORLEO T, ZAPPI A, MELUCCI D, et al. Inorganic elements in Mytilus galloprovincialis shells: Geographic traceability by multivariate analysis of ICP-MS data. Molecules, 2021, 26(9): 2634 DOI:10.3390/molecules26092634 |
FRANKE B M, GREMAUD G, HADORN R, et al. Geographic origin of meat-element of an analytical approach to its authentication. European Journal of Food Technology, 2005, 221(3/4): 493-503 |
GONG Y, LI Y K, CHEN X J, et al. Potential use of stable isotope and fatty acid analyses for traceability of geographic origins of jumbo squid (Dosidicus gigas). Rapid Communications in Mass Spectrometry, 2018, 32(7): 583-589 DOI:10.1002/rcm.8071 |
HAN C, DONG S L, LI L, et al. The effect of the seasons on geographical traceability of salmonid based on multi- element analysis. Food Control, 2019, 109: 106893 |
HUANG L Y, FAN D J, ZHANG Y X, et al. Application of multi-element and stable isotope ratios in geographical traceability of Trichiurus haumela sold online. Chinese Journal of Analytical Chemistry, 2019, 47(3): 439-446 [黄丽英, 范栋杰, 张月星, 等. 元素含量及稳定同位素比值用于网销带鱼产地溯源. 分析化学, 2019, 47(3): 439-446] |
JIANG X D, LI H Y, WANG Y, et al. The analysis of nutrient components of Oncorhynchus keta skin. Progress in Fishery Sciences, 2015, 36(5): 145-150 [姜晓东, 李红艳, 王颖, 等. 大马哈鱼(Oncorhynchus keta)鱼皮的营养成分分析. 渔业科学进展, 2015, 36(5): 145-150] |
KANG X, ZHAO Y, LIU W, et al. Geographical traceability of sea cucumbers in China via chemometric analysis of stable isotopes and multi-elements. Journal of Food Composition and Analysis, 2021, 99(7): 103852 |
KELLY S, HEATON K, HOOGEWERFF J. Tracing the geographical origin of food: The application of multi- element and multi-isotope analysis. Trends in Food Science and Technology, 2005, 16(12): 555-567 DOI:10.1016/j.tifs.2005.08.008 |
KERR L A, CAMPANA S E. Chemical composition of fish hard parts as a natural marker of fish stocks. Stock Identification Methods (Second Edition), Elsevier Inc., 2014, 205-234 |
KIM H, SURESH K K, SHIN K H. Applicability of stable C and N isotope analysis in inferring the geographical origin and authentication of commercial fish (mackerel, yellow croaker and pollock). Food Chemistry, 2015, 172: 523-527 DOI:10.1016/j.foodchem.2014.09.058 |
LAMENDIN R, MILLER K, WARD R D. Labelling accuracy in Tasmanian seafood: An investigation using DNA barcoding. Food Control, 2015, 47: 436-443 DOI:10.1016/j.foodcont.2014.07.039 |
LI L, CLAUDE E B, DONG S L. Chemical profiling with modeling differentiates Ictalurid catfish produced in fertilized and feeding ponds. Food Control, 2015, 50: 18-22 DOI:10.1016/j.foodcont.2014.08.014 |
LI L, REN W J, DONG S L, et al. Investigation of geographic origin, salinity and feed on stable isotope profile of Pacific white shrimp (Litopenaeus vannamei). Aquaculture Research, 2018, 49(2): 1029-1036 DOI:10.1111/are.13551 |
LIU B L, CHEN X J, QIAN W J, et al. δ13C and δ15N in Humboldt squid beaks: Understanding potential geographic population connectivity and movement. Acta Oceanologica Sinica, 2019, 38(10): 53-59 DOI:10.1007/s13131-019-1487-2 |
LIU Y, ZHANG X, LI Y, et al. The application of compound- specific isotope analysis of fatty acids for traceability of sea cucumber (Apostichopus japonicus) in the coastal areas of China. Journal of the Science of Food and Agriculture, 2017, 97(14): 4912-4921 DOI:10.1002/jsfa.8367 |
LIU Z, YUAN Y W, ZHAO Y, et al. Differentiating wild, lake- farmed and pond-farmed carp using stable isotope and multi-element analysis of fish scales with chemometrics. Food Chemistry, 2020, 328: 127115 DOI:10.1016/j.foodchem.2020.127115 |
LUO R J, JIANG T, CHEN X B, et al. Determination of geographic origin of Chinese mitten crab (Eriocheir sinensis) using integrated stable isotope and multi-element analyses. Food Chemistry, 2019, 274: 1-7 DOI:10.1016/j.foodchem.2018.08.104 |
LUO R J, JIANG T, CHEN X B, et al. Potential assessment of stable isotopes and mineral elements for geographic origin identification of Eriocheir sinensis. Food Science, 2020, 41(2): 298-305 [骆仁军, 姜涛, 陈修报, 等. 基于稳定同位素和矿质元素的中华绒螯蟹产地鉴别潜力评价. 食品科学, 2020, 41(2): 298-305] |
MACIEL T R, AVIGLIANO E, CARVALHO B M D, et al. Population structure and habitat connectivity of Genidens genidens (Siluriformes) in tropical and subtropical coasts from Southwestern Atlantic. Estuarine Coastal and Shelf Science, 2020, 242: 106839 DOI:10.1016/j.ecss.2020.106839 |
MILLER D D, MARIANI S. Smoke, mirrors, and mislabeled cod: Poor transparency in the European seafood industry. Frontiers in Ecology and the Environment, 2010, 8(10): 517-521 DOI:10.1890/090212 |
MIYAN K, KHAN M A, PATEL D K, et al. Truss morphometry and otolith microchemistry reveal stock discrimination in Clarias batrachus (Linnaeus, 1758) inhabiting the Gangetic River system. Fisheries Research, 2016, 173: 294-302 DOI:10.1016/j.fishres.2015.10.024 |
MOLKENTIN J, LEHMANN I, OSTERMEYER U, et al. Traceability of organic fish-authenticating the production origin of salmonids by chemical and isotopic analyses. Food Control, 2015, 53: 55-66 DOI:10.1016/j.foodcont.2015.01.003 |
MORRISON L, BENNION M, GILL S, et al. Spatio-temporal trace element fingerprinting of king scallops (Pecten maximus) reveals harvesting period and location. Science of the Total Environment, 2019, 697: 134121 DOI:10.1016/j.scitotenv.2019.134121 |
NIER A O. A redeterrnination of the relative abundances of the isotopes of carbon, nitrogen, oxygen, argon, and potassium. Physical Review, 1950, 77(6): 789-793 DOI:10.1103/PhysRev.77.789 |
ORTEA I, GALLARDO J M. Investigation of production method, geographical origin and species authentication in commercially relevant shrimps using stable isotope ratio and/or multi-element analyses combined with chemometrics: An exploratory analysis. Food Chemistry, 2015, 170: 145-153 DOI:10.1016/j.foodchem.2014.08.049 |
PEREIRA L A, SANTOS R V, HAUSER M, et al. Commercial traceability of Arapaima spp. fisheries in the Amazon Basin: Can biogeochemical tags be useful? Biogeosciences, 2019, 16(8): 1781-1797 |
PERINI M, GIONGO L, GRISENTI M, et al. Stable isotope ratio analysis of different European raspberries, blackberries, blueberries, currants and strawberries. Food Chemistry, 2017, 239: 48-55 |
RAMESH R, SARIN M M. Stable isotope study of the Ganga (Ganges) River system. Journal of Hydrology, 1992, 139: 49-62 DOI:10.1016/0022-1694(92)90194-Z |
RICARDO F, MAMEDE R, BISPO R, et al. Cost-efficiency improvement of bivalve shells preparation when tracing their geographic origin through ICP-MS analysis of elemental fingerprints. Food Control, 2020, 118: 107383 DOI:10.1016/j.foodcont.2020.107383 |
TULLI F, MORENO-ROJAS J M, MESSINA C M, et al. The use of stable isotope ratio analysis to trace European sea bass (D. labrax) originating from different farming systems. Animals, 2020, 10: 2042 DOI:10.3390/ani10112042 |
VARRÀ O M, HUSAKOVÁ L, PATOČAK J, et al. Multi-element signature of cuttlefish and its potential for the discrimination of different geographical provenances and traceability. Food Chemistry, 2021, 356: 129687 DOI:10.1016/j.foodchem.2021.129687 |
WALLSTROM M A, MORRIS K A, CARLSON L V, et al. Seafood mislabeling in Honolulu, Hawaii. Forensic Science International: Reports, 2020, 2: 100154 DOI:10.1016/j.fsir.2020.100154 |
WANG Y V, WAN A, LOCK E J, et al. Know your fish: A novel compound-specific isotope approach for tracing wild and farmed salmon. Food Chemistry, 2018, 256: 380-389 DOI:10.1016/j.foodchem.2018.02.095 |
WU H, ZHOU X W, CHEN H Q, et al. Stable isotope fingerprints for tracing salmon of different origins. Food Science, 2021, 42(16): 304-311 [吴浩, 周秀雯, 陈海泉, 等. 不同产地三文鱼的稳定同位素指纹特征及原产地溯源. 食品科学, 2021, 42(16): 304-311 DOI:10.7506/spkx1002-6630-20200723-321] |
YANG W B, SU Y P, LIU H B, et al. A comparative study of morphological characteristics and elemental fingerprints of Chinese mitten crab Eriocheir sinensis from three lakes. Journal of Fishery Sciences of China, 2012, 19(1): 86-95 [杨文斌, 苏彦平, 刘洪波, 等. 长江水系3个湖泊中华绒蟹形态及元素"指纹"特征. 中国水产科学, 2012, 19(1): 86-95] |
YANG J, QIU C W, SU Y P, et al. Morphology and elemental distribution of Chinese mitten crab Eriocheir sinensis under different water conditions. Jiangsu Journal of Agricultural Sciences, 2013, 41(3): 187-191 [杨健, 邱楚文, 苏彦平, 等. 不同水域条件下中华绒螯蟹的形态和元素分布. 江苏农业科学, 2013, 41(3): 187-191] |
YIN H M, HUANG F, SHEN J, et al. Using Sr isotopes to trace the geographic origins of Chinese mitten crabs. Acta Geochimica, 2020, 39(3): 326-336 DOI:10.1007/s11631-020-00407-5 |
ZHANG X, CHENG J, HAN D, et al. Geographical origin traceability and species identification of three scallops (Patinopecten yessoensis, Chlamys farreri, and Argopecten irradians) using stable isotope analysis. Food Chemistry, 2019, 299: 125107 DOI:10.1016/j.foodchem.2019.125107 |
ZHANG Z Q, HUANG D M, CAI Y Q, et al. Geographical origin traceability of Chinese mitten crabs based on mineral elements and stable isotopes. Food Science, 2020, 41(24): 125-130 [张政权, 黄冬梅, 蔡友琼, 等. 矿物元素结合稳定同位素的中华绒螯蟹产地溯源. 食品科学, 2020, 41(24): 125-130 DOI:10.7506/spkx1002-6630-20191222-254] |
ZHAO H, ZHANG S. Effects of sediment, seawater, and season on multi-element fingerprints of Manila clam (Ruditapes philippinarum) for authenticity identification. Food Control, 2016, 66: 62-68 DOI:10.1016/j.foodcont.2016.01.045 |
ZHAO J, SU Y P, LIU H B, et al. Geographical origin difference of elemental "fingerprints" in Chinese mitten crab Eriocheir sinensis from the same/different water systems. Journal of Nuclear Agricultural Sciences, 2014, 28(7): 1253-1260 [赵鉴, 苏彦平, 刘洪波, 等. 不同/相同水系中华绒螯蟹元素"指纹"的产地差异性. 核农学报, 2014, 28(7): 1253-1260] |
ZHAO T T, ZHANG Y, CHEN C, et al. Analysis of nutrient components and evaluation of nutritive quality in flesh of three species of cultured groupers. Progress in Fishery Sciences, 2018, 39(6): 89-96 [赵亭亭, 张岩, 陈超, 等. 3种养殖石斑鱼的肌肉营养成分分析与品质评价. 渔业科学进展, 2018, 39(6): 89-96] |
ZHAO X, LIU Y, WANG G, et al. Tracing the geographical origins of Yesso scallop (Patinopecten yessoensis) by using compound-specific isotope analysis: An approach for overcoming the seasonal effect. Food Control, 2019, 102: 38-45 DOI:10.1016/j.foodcont.2019.03.016 |
ZHAO Y, ZHANG B, GUO B, et al. Combination of multi-element and stable isotope analysis improved the traceability of chicken from four provinces of China. CyTA-Journal of Food, 2015, 14(2): 1-6 |
ZHOU Y, WU H W, HE B, et al. Study on spatial and temporal variations of δ18O and δD in Yangtze River water and its factors. Resources and Environment in the Yangtze Basin, 2017, 26(5): 678-686 [周毅, 吴华武, 贺斌, 等. 长江水δ18O和δD时空变化特征及其影响因素分析. 长江流域资源与环境, 2017, 26(5): 678-686] |
ZITEK A, STURM M, WAIDBACHER H, et al. Discrimination of wild and hatchery trout by natural chronological patterns of elements and isotopes in otoliths using LA-ICP-MS. Fisheries Management and Ecology, 2010, 17(5): 435-445 DOI:10.1111/j.1365-2400.2010.00742.x |



