2. 青岛海洋科学与技术国家试点实验室海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
3. 上海海洋大学水产与生命学院 上海 201306;
4. 浙江海洋大学水产学院 浙江 舟山 316022;
5. 荣成楮岛水产有限公司 山东 荣成 264312
2. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China;
3. College of Fisheries and Life Science, Shanghai Ocean University/National Demonstration Center for Experimental Fisheries Science Education, Shanghai 201306, China;
4. College of Fisheries, Zhejiang Ocean University, Zhoushan, Zhejiang 316022, China;
5. Rongcheng Chudao Aquaculture Corporation, Rongcheng, Shandong 264312, China
与红树林和珊瑚礁同为三大典型海洋生态系的海草系统是浅海生态系中最丰富最重要的系统之一,它们极具初级生产力,构成多种海洋食物链的基础部分,大大增加了周围环境的生物多样性,维持着浅海生态环境的健康稳定(Nordlund et al, 2016; Unsworth et al, 2019; Syukur et al, 2021)。同时,由于海草系统吸收隔离了大量的CO2,并通过沉积碳累积成为重要的蓝碳系统。虽然,海草床面积不足海洋总面积的0.1% (Duarte et al, 2013),但它们碳埋藏速率极高,达48~112 Tg C/yr,埋藏量占海洋总碳埋藏的10%~18% (McLeod et al, 2011)。海草床强大的吸收CO2和储存有机碳(Corg)的能力,一方面源于它们所具有的将CO2转化为植物生物量的能力;另一方面,源于海草床可以通过减少水流和沉积物再悬浮来捕获来自生态系统内外的有机颗粒(Oreska et al, 2018; Barcelona et al, 2021)。
沉积碳汇是海草床生态系统碳汇最为主要的组成部分,远高于生物量碳(Fourqurean et al, 2012)。全球海草床的同位素综合分析发现,海草床表层沉积碳中仅有约50%的贡献来自海草,其余则来自草床捕获的其他颗粒碳,如浮游植物碳等(Kennedy et al, 2010)。近年来的研究显示,该值仍然对海草自身碳贡献甚至存在一定程度的高估(Oreska et al, 2018),即多数草床中,外源有机质(如浮游植物、悬浮颗粒物及毗邻系统中的红树等)占主导地位(Chen et al, 2017; Reef et al, 2017; Oreska et al, 2018)。主要是因为海草与周围系统因水体流动而时刻进行着物质和能量的交换,有机碳是主要载体。印尼的北苏拉威西的海草沉积碳中,来自邻近红树林系统的碳是表层沉积碳的主要来源(Chen et al, 2017)。尽管海草碳在一些草床内直接贡献较小,但由于海草本身极高的初级生产力,海草凋落物和释放的光合溶解有机碳在水动力作用下被不断的带出草床,最终被埋藏在系统外或输送至深海。这部分来自海草的初级生产力碳,是海草碳封存贡献中被低估的部分,这导致草床碳汇量约被低估30% (Duarte et al, 2017)。
潮间带和浅潮下带是海草生长分布的环境,其周边也是发展贝类养殖的区域。毗邻的海草系统和养殖系统间不可避免的存在以碳为载体的关键生源要素交换(史洁等, 2010; Li et al, 2016)。桑沟湾是我国北方海草分布区之一,总面积约为472 hm2,其中,鳗草为主要种类,鳗草床面积约为395 hm2,主要分布于楮岛海区和八河港海区(李政等, 2020)。同时,该湾也是我国典型的养殖海湾,长期的规模化贝类养殖活动赋予了该区域养殖生态系统和海草床生态系统毗邻的生态场景,在该场景下,海草床有机碳来源的分析是解析草床碳汇特征的重要方面。本研究基于稳定同位素技术分析了桑沟湾主要海草区沉积物有机碳的来源,评估了其有机碳储量,拟为深入探究我国海草碳汇特征提供科学依据。
1 材料与方法 1.1 取样地点与站位布设2021年8―9月,对桑沟湾楮岛鳗草床区和八河港鳗草床区及楮岛草床周边区域开展取样调查。楮岛草床位于桑沟湾的南岸湾口,八河港草床位于西南部湾底,根据李政等(2020)的调查,2个区域鳗草床面积分别为154.12和229.96 hm2。由于桑沟湾内部主要进行贝类养殖,楮岛草床和八河港草床距贝类筏式养殖区距离约0.3~2.0 km (图1)。
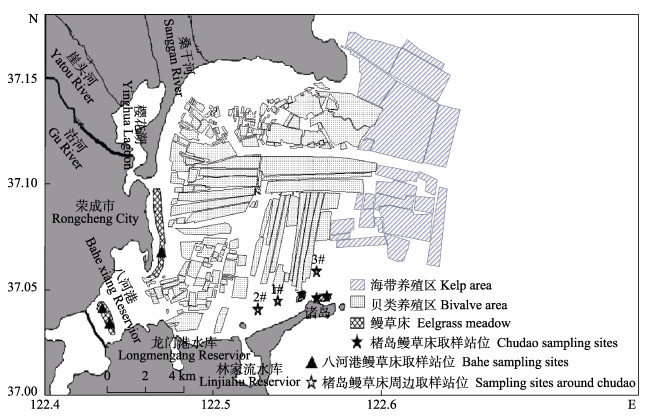
|
图 1 研究区域及采样站位 Fig.1 Location of the study area Sanggou Bay and sampling sites |
草床碳储量调查的站位布设按照Howard等(2014)的方法开展,使用随机取样站位法,根据2个地点的草床特征,各分为3个小区,在小区中随机选择取样站位,用以分析底质特征、有机碳来源和含量特征;海草对周围区域有机碳贡献的研究以楮岛鳗草床为例进行开展,即在其周边水平方向1.0和2.0 km处各设1个取样站位(图1,1#和2#),垂直方向1.5 km处设1个取样站位(图1,3#),用以评估海草对周围区域有机碳的贡献。
1.2 样品采集2处草床样品的采集在大潮退潮期间进行,各站位利用柱状采样器(φ=5 cm)手动取样。取样深度为40~50 cm,其中,0~30 cm的部分中,每3 cm分段用于样品分析(n=3),并在各站位按照《海洋调查规范》的方法进行底质取样(每站位样品数量n=3)和鳗草生物量取样(25 cm×25 cm,n=6)。
楮岛鳗草床周围布设的取样站位,利用柱状采样器(ϕ=4 cm)取表层0~3 cm的底质样品。将草床内发现的海草、浮游植物和大型藻3种主要自养生物作为有机碳来源的主要端元。此外,考虑到周围为典型的贝类养殖区,在水动力的作用下,贝类生物沉积水动力也是草床潜在的贡献源。同时,鉴于八河港和楮岛区域较小的季节性河流输入和占比较小的底栖微藻,因此,未将陆源有机碳和底栖微藻有机碳纳入计算。取样方式为在各站位随机收集草床为25 cm×25 cm内的所有大型藻[漂流大型藻:孔石莼(Ulva pertusa)及附着杂藻,n=3]作为混合样品;贝类生物沉积取样方式为取草床附近养殖的贝类[养殖种类为牡蛎(Ostrea gigas)],洗刷干净后至岸上的流水系统,流水4 h,收集足量沉积物。浮游植物样由64 μm的浮游生物网取得(虽然含少量浮游动物,但与其摄食的浮游植物同位素相似)(Yoshii et al, 1999; Post, 2002),以上所有样品在0℃低温下带回实验室。
1.3 样品分析本研究取柱状样中表层0~3 cm层分样,烘干(60℃下烘干72 h)并称重,于0.1 mol/L的HCl中酸化,去除无机碳,多次洗净后进入连接元素分析仪(Elementar, 美国)的同位素质谱仪(ISOprime 100)进行δ13C和δ15N和有机碳含量的测定。草床碳储量的分析按照Howard等(2014)的方法,即0~30 cm的分层柱状样根据取样器内径计算体积,在60℃下烘干72 h至恒重,并称重量,用以计算底质容重与碳密度,按照上述同样的方式称重、酸化,进入元素分析仪进行有机碳的测定。楮岛草床周边站位的表层底质样及鳗草、浮游植物和大型藻等同位素δ13C和δ15N分析如上。海草样品在去除附着生物后烘干,定量生物量。底质分析的样品按照《海洋操作规范》进行湿筛分与激光粒度仪(Multisizer 3)分析。
1.4 数据分析计算 1.4.1 表层沉积物有机碳来源分析利用混合模型(Parnell et al, 2010)对来自主要初级生产者与贝类生物沉积物的贡献进行分析,分析过程在R程序包SIAR (Stable Isotope Analysis in R)下进行,计算各成分的贡献率,其中,0.95置信区间下的平均贡献率在文中详述。楮岛草床周围取样点的有机碳来源按照同样的方式进行分析。2处鳗草床沉积物特征以中值粒径(ϕ)、分选系数和粉砂含量(%)为主要参数。
1.4.2 草床有机碳储量估算碳储量以两个方面的数据进行表征。首先是沉积物碳储量,为单位面积的0~30 cm的各层沉积物中有机碳的累加(Lavery et al, 2013),单位为Mg C/hm2 (1 Mg=106 g);总碳储量为单位面积碳储量与草床面积的乘积。其次是年平均碳埋藏量,参考刘赛等(2014)的桑沟湾楮岛和八河港海区的沉积速率为0.59 cm/yr和2.08 cm/yr,以0~30 cm碳储量除以底质沉积30 cm所用的时间,得到年均碳埋藏量,单位为Mg。
2 结果 2.1 2处鳗草床沉积物特征及鳗草密度与生物量楮岛鳗草床分布于砂质和粉砂质砂区,表层(0~ 3 cm)有机碳含量为0.15% (表1),有机质含量为1.63%,容重为1.07,平均植株密度约578株/m2,平均总生物量为446.2 g DW/m2;八河港鳗草床底质类型为砂,有机碳含量较低,为0.11%,有机质含量为0.98%,平均植株密度为467株/m2,生物量为410.2 g DW/m2。
|
|
表 1 桑沟湾鳗草床底质与鳗草特征 Tab.1 Sediment and eelgrass meadows characteristics in Sanggou Bay |
楮岛草床和八河港草床内鳗草碳氮同位素值相近(图2),分别为(–12.42±0.24)‰与(6.45±0.73)‰和(–12.14±0.13)‰与(6.56±0.54)‰,但表层沉积物的δ13C与δ15N有较为明显的不同,其平均值分别为(–20.67±0.37)‰和(4.54±0.96)‰与(–21.64±0.32‰)和(5.09±0.80)‰。混合模型的结果显示,2处草床内来自鳗草的贡献均较低(图3),分别为8.3%和17.1%;浮游植物的贡献在楮岛草床内为41.4%,在八河港为34.0%;贝类生物沉积的贡献率分别为25.3%和23.9%;2处草床内大型藻对表层有机碳的贡献度相似,为25.0%和24.9%。
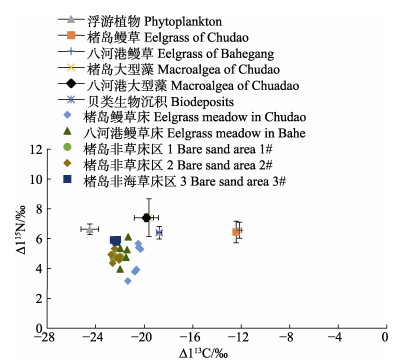
|
图 2 调查点表层沉积物与各有机碳源的碳氮稳定同位素(δ13C和δ15N)特征 Fig.2 Characteristics of carbon and nitrogen stable isotopes (δ13C and δ15N) in surface sediments and organic carbon sources at the survey sites |
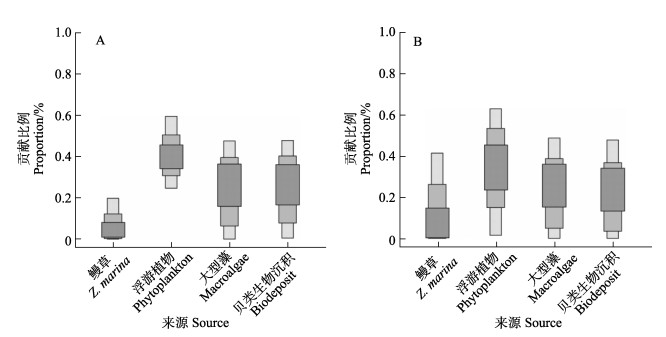
|
图 3 4种主要有机碳来源(鳗草、浮游植物、大型藻和贝类生物沉积)对桑沟湾楮岛鳗草床(A)和八河港(B)表层沉积物(0~3 cm)的相对贡献 Fig.3 Relative contribution of four main organic matter sources (eelgrass, phytoplankton, seaweed and biodeposit) to the surface sediment (0~3 cm) in Chudao (A) and Bahegang (B) eelgrass meadows |
楮岛鳗草床周围3个站位表层沉积物的δ13C相近,δ15N的差异较为明显(图2),浮游植物是主要的贡献者,平均贡献率为47.0%~63.8% (图4),以距离海草床最近的1#站位(距离草床1.0 km),浮游植物的贡献相对最小,为47.0%,鳗草的贡献相对最大,为10.7%,大型藻类的贡献率为21.9%,贝类沉积的贡献率为20.4%;距离草床的2#、3#站位(距离分别为2.0 km与1.5 km),鳗草的贡献率减小至5.2%和6.7%,来自贝类生物沉积的贡献率约14.6%~16.5%,浮游植物的贡献率升高至57.6%~63.8%,大型藻类与贝类沉积贡献率相近,为14.7%~16.3%。
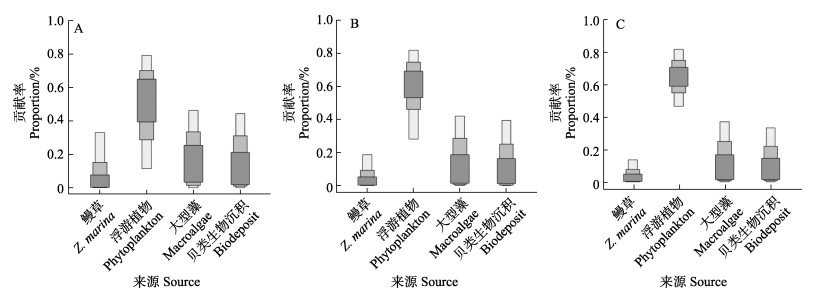
|
图 4 4种主要有机碳来源对楮岛鳗草床周围3个站位(A:1#;B:2#;C:3# )表层沉积物(0~3 cm)的相对贡献 Fig.4 Relative contribution of four main organic carbon sources to the surface sediment (0~3 cm) in eelgrass meadows (A: 1#; B: 2#; C: 3#) |
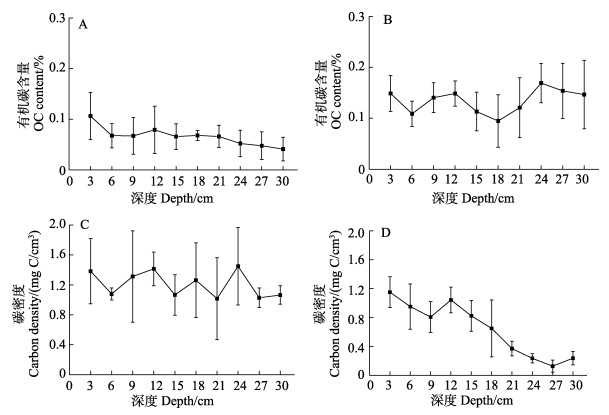
楮岛鳗草床底质中,0~30 cm有机碳含量波动较大(图5A),变化范围为0.09%~0.15%,平均为(0.13± 0.02)%;八河港底质则呈较为明显的从表层至底层含量逐渐降低的趋势(图5B),其含量为(0.04~ 0.11)%,平均为(0.066±0.018)%。楮岛草床底质碳密度的含量变化为1.03~1.45 mg C/cm3,平均为(1.20±0.17) mg C/cm3 (图5C);八河港草床碳密度从表层至底层急剧降低(图5D),平均含量为(0.64± 0.37) mg C/cm3。
|
图 5 桑沟湾鳗草床0~30 cm沉积物中有机碳含量剖面 Fig.5 Sediment profiles of organic carbon content A:楮岛;B:八河港;C:楮岛;D:八河港 A: Chudao; B: Bahegang; C: Chudao; D: Bahegang |
由楮岛草床和八河港草床沉积物为0~30 cm的平均碳密度计算得到碳储量分别为3.75 Mg C/m2和2.02 Mg C/m2(1 Mg=106 g,即1 t)。由2处的鳗草床的面积计算得到桑沟湾总的碳储量为1 041.5 Mg。根据楮岛和八河港海域平均沉积速率分别为0.59 cm/yr和2.08 cm/yr (刘赛等, 2014),得到每年的碳埋藏量的平均值分别为11.36 Mg/yr和32.15 Mg/yr (表2)。
|
|
表 2 桑沟湾鳗草床有机碳储量与年均碳埋藏速率 Tab.2 Carbon stocks and annual areal carbon accumulation in eelgrass (Z. marina) meadows of Sanggou Bay |
海草碳是蓝碳的直接贡献者,但在不同草床及不同区域,其贡献比例的估算值具有较大的差异。Kennedy等(2010)研究表明,计算得到的全球草床50%的有机碳来自海草,这一数值因区域而异,既与草床内潜在贡献源的多样性和复杂性有关,也与特定草床的结构和组成有关(Oreska et al, 2018)。桑沟湾鳗草床δ13C的变化为(–20.31~21.99)‰,与鳗草自身的–12.30‰相比,差值为–8.2‰,呈现较为典型的外源性有机碳的特征(Kennedy et al, 2010);Röhr等(2018)和Stankovic等(2021)分析表明,2处草床表层有机碳中来自鳗草自身的贡献较小,仅为8.3%~17.1%,而北温带鳗草床中,鳗草的贡献率为20%~40%。一方面,鳗草自身较低的碳贡献可能与2处草床均处于近岸水动力较强的环境有关(毛兴华等, 1988),在较强的水动力作用下,叶片脱落物因较强浮力更多的被携带出草床,因此,海草自身对草床表层的碳贡献相对较小(高亚平等, 2016; Rahayu et al, 2019)。另一方面,较强的动力使周围养殖贝类的生物沉积被再悬浮作用携带至草床,大型藻类也因阻力被滞留在草床底部(Dahl et al, 2018),这从楮岛鳗草床比周围站位具有更高的来自大型藻类和贝类生物沉积的比例可以体现(图4)。同时,从距离草床1.5~2.0 km的2#、3#站位浮游植物碳占比达57.6%~63.8%,远高于草床内34.0%~41.4%的比例,也反映出海草床较强的滞留大型藻和生物沉积等大颗粒物高于以2~20 μm为主的浮游植物细小颗粒(李凤雪等, 2020)的能力,尤其在桑沟湾草床斑块区较多,大型藻类等更易进入斑块区(Ricart et al, 2017)并在此积聚。
滤食性养殖贝类耦合水体与沉积环境。浮游植物等颗粒有机碳被消化吸收后,以生物沉积的形式在表层底质沉降(Kautsky et al, 1987),并随再悬浮作用扩散至其他系统,因此,在桑沟湾规模化养殖使得贝类生物沉积对表层沉积碳的贡献远高于其他海区,并成为了养殖碳汇的重要组成部分(聂梦晨等, 2022)。而海草由于其冠层结构带来的机械阻碍降低了水流速度,更多的悬浮颗粒尤其是大颗粒在草床内沉降并累积(Oreska et al, 2018; Barcelona et al 2021),但在桑沟湾,贝类沉积同时也成为了海草碳汇的重要组成部分。同时,除了碳的输入,贝类沉积对海草的生长亦有潜在的促进作用(吴亚林等, 2018)。近年来,随着研究的深入,海草与草床内开展的贝类养殖间的关系被逐渐揭示,贝类对海草的正面作用或负面作用取决于养殖方式。通常草床内进行筏式养殖会因遮光等作用降低海草的密度、覆盖度和繁殖力,而底播养殖虽存在与海草的空间竞争,使得海草密度降低,但由于营养的输入,促进了海草的生长和繁殖(Ferriss et al, 2019)。Reusch等(1994)和Vinthe等(2008)的研究也表明,贝类沉积带来的营养输入,促进了海草的生长。桑沟湾内的贝类养殖在海草系统外部,不仅不存在遮光等负面作用,来自贝类沉积的输送,还可能在一定程度上对海草生长具有有潜在的促进作用。
3.2 草床有机碳储量及影响因素沉积有机碳是海草最为重要的组成部分,草床有机碳储量备受关注。桑沟湾2处主要海草床表层有机碳密度平均为0.92 mg C/cm3,仅为世界鳗草床平均值2721 mg C/cm2的1/10。Röhr等(2016)研究表明,桑沟湾鳗草床碳密度与芬兰Fårö和Kolaviken海区相近,但远低于温带鳗草床的平均值(11.4±4.3) mg C/cm3 (Röhr et al, 2018),与我国北方沿海海草系统相比,也处于低值,远低于东营潮间带的矮鳗草床的碳密度(12.31 mg C/cm3)(Yue et al, 2021),此外,与我国广西海草床的碳密度相比也处于较低的水平(李梦, 2018)。
近年的研究报道中,无论从世界范围内大的区域尺度,还是从局部地区的中小尺度,有机碳储量都存在巨大的差异(Lavery et al, 2013; Röhr et al, 2018)。以波罗的海和地中海鳗草床为例,二者碳储量相差15倍。Röhr等(2018)对温带鳗草床碳储量的分析发现,引起碳储量差异的主要原因是草床的底质特性(如泥含量、容重和分选系数)和环境特性(水深与盐度),二者对差异的解释占62%。其他的研究则表明,底质特性尤其是粒度分布特征是草床碳储量最为重要的预测因子(Miyajima et al, 2015; Dahl et al, 2016; Mazarrasa et al, 2021)。与碳储量较高的区域相比,桑沟湾草床的底质粒度相对较大,而泥含量相对较低(表1);与朝鲜半岛南部Dongdae Bay的鳗草床相比,桑沟湾草床泥含量甚至不足其1/30 (Kim et al, 2022)。通常泥含量小于10%的草床,其碳储量均<1 kg C/m2,而2~10 kg C/m2的区域其泥含量在30%以上,甚至高达80%。这是由于在细颗粒的泥或粉砂中,更容易因氧的扩散受阻而处于厌氧环境,微生物的分解作用减弱,使得各个来源的有机碳更易积累。因此,桑沟湾较低的碳储量也与其底质特性相关。目前,桑沟湾的海草分布仅余400 hm2左右,且多处斑块状,草床面积大小是影响碳积聚速率的重要因素(Ricart et al, 2017),因此,较低的碳储量也可能与目前的草床规模有关。尽管如此,桑沟湾鳗草床的初级生产力水平处于高值,年均值达543.5 g C/(m2·yr),这部分以植物体尤其是叶片组织固定下来的有机碳,虽未能在草床内积聚,多被输送至草床外,但这部分仍然是蓝碳的重要组成部分,至少在楮岛草床周围2.0 km内,海草的有机碳贡献率仍在5.2%~10.7% (图4),成为向周边区域输送碳的。
3.3 草床修复对蓝碳扩增的重要意义海草的衰退会引起沉积有机碳的大量损失(Marbà et al, 2015; Thorhaug et al, 2017; Stankovic et al, 2021)。研究发现,即便在实验尺度上,去除海草后的18个月内,表层有机碳含量显著降低,0~5 cm的碳损失量约2.21 Mg/C hm2 (Githaiga et al, 2019)。值得注意的是,草床修复是抑制该趋势的有效手段。在佛吉尼亚、牡蛎港和墨西哥湾,海草修复均显著增加了碳储量,其碳储量与自然草床相近,甚至更高(Greiner et al, 2013; Marbà et al, 2015; Thorhaug et al, 2017)。尤其是墨西哥湾其表层为20 cm的有机碳扩增量达(20.96± 8.59) Mg/hm2 (Thorhaug et al, 2017),其修复1~2年的草床也可实现蓝碳的有效扩增。因此,加大对桑沟湾海草的保护,并进行有效的移植修复是提高桑沟湾海草碳储量,同时,发挥诸如增加生物多样性与环境健康的维持(Heiss et al, 2000; Heck et al, 2003; Lamb et al, 2017; Hossain et al, 2019)等更多生态服务功能的重要手段。
BARCELONA A, OLDHAM C, COLOMER J, et al. Particle capture by seagrass canopies under an oscillatory flow. Coastal Engineering, 2021, 169: 103972 DOI:10.1016/j.coastaleng.2021.103972 |
CHEN G, AZKAB M H, CHMURA G L, et al. Mangroves as a major source of soil carbon storage in adjacent seagrass meadows. Scientific Reports, 2017, 7(1): 1-10 DOI:10.1038/s41598-016-0028-x |
DAHL M, DEYANOVA D, GÜTSCHOW S, et al. Sediment properties as important predictors of carbon storage in Zostera marina meadows: A comparison of four European areas. PLoS One, 2016, 11(12): e0167493 DOI:10.1371/journal.pone.0167493 |
DAHL M, INFANTES E, CLEVESJö R, et al. Increased current flow enhances the risk of organic carbon loss from Zostera marina sediments: Insights from a flume experiment. Limnology and Oceanography, 2018, 63(6): 2793-2805 DOI:10.1002/lno.11009 |
DUARTE C M, KENNEDY H, MARBÀ N, et al. Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies. Ocean and Coastal Management, 2013, 83: 32-38 DOI:10.1016/j.ocecoaman.2011.09.001 |
DUARTE C M, KRAUSE-JENSEN D. Export from seagrass meadows contributes to marine carbon sequestration. Frontiers in Marine Science, 2017, 4: 13 |
DUARTE C M. Allometric scaling of seagrass form and productivity. Marine Ecology Progress Series. Oldendorf, 1991, 77(2): 289-300 |
GAO Y P, JIANG Z J, FANG J H, et al. Ecological function of seagrass Zostera marina L. bed in sea cucumber pond. Journal of Fisheries of China, 2016, 40(6): 925-932 [高亚平, 蒋增杰, 房景辉, 等. 刺参围堰养殖中大叶藻的生态功能. 水产学报, 2016, 40(6): 925-932] |
GITHAIGA M N, FROUWS A M, KAIRO J G, et al. Seagrass removal leads to rapid changes in fauna and loss of carbon. Frontiers in Ecology and Evolution, 2019, 7: 62 DOI:10.3389/fevo.2019.00062 |
GREINER J T, MCGLATHERY K J, GUNNELL J, et al. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS One, 2013, 8(8): e72469 DOI:10.1371/journal.pone.0072469 |
HECK Jr K L, HAYS G, ORTH R J. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series, 2003, 253: 123-136 DOI:10.3354/meps253123 |
HEISS W M, SMITH A M, PROBERT P K. Influence of the small intertidal seagrass Zostera novazelandica on linear water flow and sediment texture. New Zealand Journal of Marine and Freshwater Research, 2000, 34(4): 689-694 DOI:10.1080/00288330.2000.9516970 |
HOSSAIN M S, HASHIM M. Potential of earth observation (EO) technologies for seagrass ecosystem service assessments. International Journal of Applied Earth Observation and Geoinformation, 2019, 77: 15-29 DOI:10.1016/j.jag.2018.12.009 |
HOWARD, J, S. HOYT, K. ISENSEE E, et al. Coastal blue carbon: Methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrass meadows. Arlington: Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature, 2014
|
KAUTSKY N, EVANS S. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in a Baltic coastal ecosystem. Marine Ecology Progress Series, 1987, 201-212 |
KENNEDY H, BEGGINS J, DUARTE C M, et al. Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochemical Cycles, 2010, 24: GB4026 |
KIM S H, SUONAN Z, QIN L Z, et al. Variability in blue carbon storage related to biogeochemical factors in seagrass meadows off the coast of the Korean peninsula. Science of the Total Environment, 2022, 813: 152680 DOI:10.1016/j.scitotenv.2021.152680 |
LAMB J B, VAN DE WATER, JEROEN A J M, et al. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science, 2017, 355: 731-733 DOI:10.1126/science.aal1956 |
LAVERY P S, MATEO M á, SERRANO O, et al. Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service. PLoS One, 2013, 8(9): e73748 DOI:10.1371/journal.pone.0073748 |
LI F X, JIANG Z J, GAO Y P, et al. Distribution of size-fractionated phytoplankton and its relationship with environmental variables in Sanggou Bay. Progress in Fishery Sciences, 2020, 41(1): 31-40 [李凤雪, 蒋增杰, 高亚平, 等. 桑沟湾浮游植物粒径结构及其与环境因子的关系. 渔业科学进展, 2020, 41(1): 31-40] |
LI M. Carbon storage in the seagrass sediments of Guangxi, China. Master´s Thesis of Guangxi Normal University, 2018 [李梦. 广西海草床沉积物碳储量研究. 广西师范学院硕士研究生学位论文, 2018]
|
LI R, LIU S, ZHANG J, et al. Sources and export of nutrients associated with integrated multi-trophic aquaculture in Sanggou Bay, China. Aquaculture Environment Interactions, 2016, 8: 285-309 DOI:10.3354/aei00177 |
LI Z, LI W T, YANG X L, et al. Distribution and ecological characteristics of seagrass beds in Rongcheng Sanggou Bay, Weihai. Marine Sciences, 2020, 44(10): 52 [李政, 李文涛, 杨晓龙, 等. 威海荣成桑沟湾海域海草床分布现状及其生态特征. 海洋科学, 2020, 44(10): 52] |
LIU S, YANG Q, YANG S, et al. The long-term records of carbon burial fluxes in sediment cores of culture zones from Sanggou Bay. Acta Oceanologica Sinica, 2014, 36(8): 30-38 [刘赛, 杨茜, 杨庶, 等. 桑沟湾养殖海域沉积物中碳埋藏通量的长期记录. 海洋学报, 2014, 36(8): 30-38 DOI:10.3969/j.issn.0253-4193.2014.08.004] |
MAO X H, ZHANG W X, ZHANG J Z. Comprehensive investigation and research on the aquaculture environment of Sanggou Bay. Qingdao: Qingdao Press, 1988 [毛兴华, 张为先, 张建中. 桑沟湾增养殖环境综合调查研究. 青岛: 青岛出版社, 1988]
|
MAZARRASA I, LAVERY P, DUARTE C M, et al. Factors determining seagrass blue carbon across bioregions and geomorphologies. Global Biogeochemical Cycles, 2021, 35(6): e2021GB006935 |
MATEO M, CEBRIÁN J, DUNTON K, et al. Carbon flux in seagrass ecosystems. Seagrasses:Biology, ecology and conservation, 2006, 159-192 |
MCLEOD E, CHMURA G L, BOUILLON S, et al. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment, 2011, 9(10): 552-560 DOI:10.1890/110004 |
MIYAJIMA T, HORI M, HAMAGUCHI M, et al. Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Global Biogeochemical Cycles, 2015, 29(4): 397-415 DOI:10.1002/2014GB004979 |
NIE M C, HUANG C L, SUI Q, et al. Carbon and nitrogen stable isotope analysis and source analysis of organic matter in sediments of Sanggou Bay. Progress in Fishery Sciences, 2022, 43(5): 84-97 [聂梦晨, 黄翠玲, 隋琪, 等. 桑沟湾沉积物有机质的碳氮稳定同位素分析及其来源解析. 渔业科学进展, 2022, 43(5): 84-97] |
NORDLUND M L, KOCH E W, BARBIER E B, et al. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS One, 2016, 11(10): e0163091 DOI:10.1371/journal.pone.0163091 |
ORESKA M P J, WILKINSON G M, MCGLATHERY K J, et al. Non-seagrass carbon contributions to seagrass sediment blue carbon. Limnology and Oceanography, 2018, 63(S1): 3-18 |
PARNELL A C, INGER R, BEARHOP S, et al. Source partitioning using stable isotopes: Coping with too much variation. PLoS One, 2010, 5(3): e9672 DOI:10.1371/journal.pone.0009672 |
POST D M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology, 2002, 83(3): 703-718 DOI:10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2 |
POTOUROGLOU M, WHITLOCK D, MILATOVIC L, et al. The sediment carbon stocks of intertidal seagrass meadows in Scotland. Estuarine, Coastal and Shelf Science, 2021, 258: 107442 DOI:10.1016/j.ecss.2021.107442 |
RAHAYU Y P, SOLIHUDDIN T, KUSUMANINGTYAS M A, et al. The sources of organic matter in seagrass sediments and their contribution to carbon stocks in the Spermonde Islands, Indonesia. Aquatic Geochemistry, 2019, 25(3): 161-178 |
REEF R, ATWOOD T B, SAMPER‐VILLARREAL J, et al. Using eDNA to determine the source of organic carbon in seagrass meadows. Limnology and Oceanography, 2017, 62(3): 1254-1265 DOI:10.1002/lno.10499 |
RICART A M, PÉREZ M, ROMERO J. Landscape configuration modulates carbon storage in seagrass sediments. Estuarine, Coastal and Shelf Science, 2017, 185: 69-76 DOI:10.1016/j.ecss.2016.12.011 |
RÖHR M E, BOSTRÖM C, CANAL-VERGÉS P, et al. Blue carbon stocks in Baltic Sea eelgrass (Zostera marina) meadows. Biogeosciences, 2016, 13(22): 6139-6153 DOI:10.5194/bg-13-6139-2016 |
RöHR M E, HOLMER M, BAUM J K, et al. Blue carbon storage capacity of temperate eelgrass (Zostera marina) meadows. Global Biogeochemical Cycles, 2018, 32(10): 1457-1475 DOI:10.1029/2018GB005941 |
SHI J, WEI H, ZHAO L, et al. Study on ecosystem model of multi-species culture in Sanggou Bay: Ⅱ Simulation of ecosystem and the circulation of nutrients. Progress in Fishery Sciences, 2010, 31(4): 36-42 [史洁, 魏皓, 赵亮, 等. 桑沟湾多元养殖生态模型研究: Ⅱ生态环境模拟与生源要素循环. 渔业科学进展, 2010, 31(4): 36-42 DOI:10.3969/j.issn.1000-7075.2010.04.005] |
STANKOVIC M, AMBO-RAPPE R, CARLY F, et al. Quantification of blue carbon in seagrass ecosystems of Southeast Asia and their potential for climate change mitigation. Science of the Total Environment, 2021, 783: 146858 DOI:10.1016/j.scitotenv.2021.146858 |
SYUKUR A, HIDAYATI B N, IDRUS A, et al. The suitability of seagrass ecological function for the survival of the bivalvia on the East Coast of Lombok, Indonesia. IOP Conference Series:Earth and Environmental Science. IOP Publishing, 2021, 712(1): 012033 DOI:10.1088/1755-1315/712/1/012033 |
THORHAUG A, POULOS H M, LÓPEZ–PORTILLO J, et al. Seagrass blue carbon dynamics in the Gulf of Mexico: Stocks, losses from anthropogenic disturbance, and gains through seagrass restoration. Science of the Total Environment, 2017, 605: 626-636 |
UNSWORTH R K F, NORDLUND L M, CULLEN-UNSWORTH L C. Seagrass meadows support global fisheries production. Conservation Letters, 2019, 12(1): e12566 DOI:10.1111/conl.12566 |
VINTHER H F, HOLMER M. Experimental test of biodeposition and ammonium excretion from blue mussels (Mytilus edulis) on eelgrass (Zostera marina) performance. Journal of Experimental Marine Biology and Ecology, 2008, 364(2): 72-79 DOI:10.1016/j.jembe.2008.07.003 |
WOOLLER M, SMALLWOOD B, JACOBSON M, et al. Carbon and nitrogen stable isotopic variation in Laguncularia racemosa (L. ) . (white mangrove) from Florida and Belize:Implications for trophic level studies. Hydrobiologia, 2003, 499(1): 13-23 |
WU Y L, GAO Y P, LÜ X N, et al. Ecological contribution of Ruditapes philippinarum in the seagrass meadow of Sanggou Bay. Progrees in Fishery Sciences, 2018, 39(6): 126-133 [吴亚林, 高亚平, 吕旭宁, 等. 桑沟湾楮岛大叶藻床区域菲律宾蛤仔的生态贡献. 渔业科学进展, 2018, 39(6): 126-133] |
YOSHII K, MELNIK N G, TIMOSHKIN O A, et al. Stable isotope analyses of the pelagic food web in Lake Baikal. Limnology and Oceanography, 1999, 44(3): 502-511 DOI:10.4319/lo.1999.44.3.0502 |
YUE S, ZHANG X, XU S, et al. The super typhoon Lekima (2019) resulted in massive losses in large seagrass (Zostera japonica) meadows, soil organic carbon and nitrogen pools in the intertidal Yellow River Delta, China. Science of the Total Environment, 2021, 793: 148398 DOI:10.1016/j.scitotenv.2021.148398 |



