2. 西南大学水产学院 重庆 400715;
3. 南京农业大学无锡渔业学院 江苏 无锡 214081
2. College of Fisheries, Southwest University, Chongqing 400715, China;
3. Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081, China
在自然界中,鱼类常改变其生理和行为特征以应对不断变化的环境条件,捕食是影响个体生存的主要环境因素之一(Killen et al, 2016; Lima, 1998a; Spiegel et al, 2013)。猎物应对捕食者的存在会使其产生生理应激和能量代谢,已有研究表明,猎物与捕食者相遇会减少猎物的进食和其他与健康相关的活动(Barcellos et al, 2007; Breves et al, 2005; Woodley et al, 2003)。在捕食胁迫环境下,鱼在感知到压力后会启动应激反应,使其能克服压力恢复体内平衡(Schreck et al, 2016)。应激的程度主要取决于压力的强度和持续时间(Lima et al, 1999; 张宇婷等, 2021)。若捕食者出现间歇性,并且猎物鱼在遭遇捕食后生理状态在短时间内恢复正常,此时的应激反应可以促进猎物鱼的生理改变以更好地适应环境(Cooke et al, 2003)。然而,在压力重复或持续且无法避免的情况下,猎物鱼的正常生理反应机制可能会受到损害,生理应激可能对免疫系统、生长或繁殖产生长期的负面影响,并降低猎物鱼的适应性和生存能力(Barton, 2002; Colson et al, 2015; Redfern et al, 2017)。已有研究表明,捕食胁迫会对鱼类造成生理应激,但不同鱼种甚至相同鱼种的不同群体对胁迫的应激程度和应激方式都有较大的差异,仍需要更多的物种特异性研究来确定捕食胁迫的长度和强度对鱼类生理应激的影响。
捕食胁迫导致鱼体产生应激反应的一个重要表现为血液中应激激素含量的升高(Clinchy et al, 2004; Frid et al, 2002; Lima, 1998b)。皮质醇是鱼体在受到外界刺激后分泌的一种重要应激激素,能灵敏反映鱼体的胁迫状况(Clinchy et al, 2011; Wendelaar et al, 1997)。鱼类在面临被捕食的风险产生应激并做出行为和生理改变时,鱼体的营养状况和代谢机能也会发生改变(Benson et al, 2019)。因此,捕食胁迫下鱼体应激的另一个重要表现为鱼体内血液生化指标的变化。血液的生化组成包括血糖(glucose,GLU)、血脂、血清蛋白以及酶等成分,是评价环境应激时鱼类的健康状况、营养状况及对环境的适应状况的重要指标(洪磊等, 2004)。GLU作为鱼体内主要供能物质,其含量与鱼类代谢水平和营养状况直接相关,当其含量不足时,血脂和血清蛋白也会被鱼体利用,三者之间存在着相互转换关系(陈剑杰等, 2009)。除此之外,血清中的许多酶成分也是反映鱼体胁迫状况的重要指标(于淼等, 2008)。
青鱼(Mylopharyngodon piceus)、草鱼(Ctenoph-aryngodon idellus)、鲢(Hypophthalmichthys molitrix)、鳙(Aristichthys nobilis)合称为“四大家鱼”,是中国长江流域常见的鲤科(Cyprinidae)鱼类。多年来,由于水力建设、环境污染、过度捕捞等诸多原因导致其野外种群数量急剧下降(Duan et al, 2009; 刘飞等, 2019),此外,在自然水域中,捕食者的普遍存在也对其数量的增长造成威胁。目前,尚不清楚当捕食压力持续存在时,“四大家鱼”幼鱼如何调整其生理过程以应对捕食压力?多数与捕食胁迫相关的研究通常仅选择血液激素中皮质醇(cortisol,COR)水平来反映鱼类生理反应的变化,而没有对捕食胁迫下鱼类的能量代谢进行多方面评估。因此,为了系统探讨鱼类的生理反应和捕食胁迫的内在联系,本研究选取了“四大家鱼”幼鱼为研究对象,分析了不同捕食胁迫水平对血清COR和血液生化指标的影响。研究捕食胁迫条件下,“四大家鱼”幼鱼的生理应激和能量代谢对捕食压力的适应性,可为深入研究环境变化引起的机体应激反应提供理论依据,也可为增殖放流前的捕食驯化提供数据参考。
1 材料与方法 1.1 实验鱼来源与驯养本实验于2021年5—7月在长江“四大家鱼”监利老江河原种场开展,猎物鱼幼鱼(青鱼、草鱼、鲢和鳙)及捕食者[乌鳢(Channa argus)和南方大口鲶(Silurus soldatovi meridionalis)]均取自老江河原种场,其中,“四大家鱼”幼鱼为原种场人工繁殖鱼苗。实验鱼分别放入规格为4 m×2 m×1.2 m的长方形水泥养殖池中驯养14 d。驯养期间,“四大家鱼”幼鱼每天09:00和17:00投喂2次商业饲料,投喂量为池内实验鱼体重总和的3%;乌鳢、南方大口鲶与不用于实验的“四大家鱼”幼鱼混养,不额外投喂饲料,用于混养投喂捕食者的“四大家鱼”幼鱼与用于测定的实验鱼取自同一批次幼鱼,比例及规格均相近。养殖用水取自老江河,驯养温度为25~29 ℃,溶解氧(DO)为7~8 mg/mL。
1.2 实验设计14 d驯养结束后,每种实验鱼各随机选择体长和体重相近的样本[青鱼:(8.81±0.22) cm,(11.73±0.62) g,n=400;草鱼:(8.33±0.19) cm,(10.23±0.72) g,n=400;鲢:(8.43±0.15) cm,(9.10±0.36) g,n=400;鳙:(8.50±0.11) cm,(11.10±0.36) g,n=400]分类别按捕食胁迫程度将每种鱼随机分为3组,无捕食组(对照组)、低捕食组(隔网胁迫)和高捕食组(直接捕食)共12组。为尽量保证除捕食胁迫外的其余因素一致,将低胁迫组与高胁迫组放置在同一水体中。从图 1可以看出,D、E(D、E为对照组,D、E组各放入100尾实验鱼)、H(低捕食组)、I(高捕食组)各放入100尾同种实验鱼。对照组中,拦网两侧不放入捕食者,捕食组中将乌鳢[(33.64±0.86) cm,(456.82±10.21) g]和南方大口鲶[(35.13±0.64) cm,(421.43±6.12) g]各1尾作为共同捕食者一起放入。低捕食组实验鱼与捕食者被拦网隔开,无直接接触;高捕食组实验鱼与捕食者处于拦网同侧,捕食者可直接捕食高捕食组实验鱼(图 1)。捕食胁迫期间,“四大家鱼”幼鱼的投喂同驯养期间一致,捕食者不额外投喂,其食物来源为混养的用于测定的实验鱼。捕食胁迫处理0、7、14 d后,随机选取身体健康、大小接近的实验鱼,分别测定其体长、体重、COR和血液生化指标(n=9)。捕食胁迫期间为微流水养殖,水深保持在0.6 m左右,水温为(27.75±1.05) ℃,DO为(7.82±0.84) mg/L,pH为8.16±0.05,氨氮(NH4+-N)含量 < 0.10 mg/L。

|
图 1 实验装置示意 Fig.1 Schematic diagram of the test device A:进水口;B:出水口;C:尼龙拦网(孔径为0.5 cm× 0.5 cm);D、E:无捕食组(对照组),各放100尾实验鱼,不放入捕食者;F:捕食者(乌鳢和南方大口鲶各1尾);H:低捕食组,实验鱼(n=100)与捕食者分开隔网可见;I:高捕食组,实验鱼(n=100)中放入双捕食者:乌鳢和南方大口鲶各1尾。 A: Water inlet; B: Water outlet; C: Nylon block (0.5 cm× 0.5 cm); D and E: No predation group (control group), each with 100 experimental fish, but no predators; F: Predators (one snakehead and one southern catfish); H: Low predation group, experimental fish (n=100) and predators were separated by net but can see each other; I: High predation group, two predators, one snakehead and one southern catfish, were added to experimental fish (n=100). |
采样前,将鱼停食24 h;取样时,先采用0.1 g/mL的MS-222分别将“四大家鱼”幼鱼麻醉,使用1 mL的一次性无菌注射器从实验鱼尾静脉采血,采集的血液于离心管中4 ℃下静置4 h后,4 000 r/min低温离心10 min至完全分层,收集上层澄清透明的血清。血清生化指标采用英诺华DS-261全自动分析仪进行测定。检测指标包括总蛋白(total protein, TP)、白蛋白(albumin, ALB)、球蛋白(globulin, GLB)、GLU、总胆固醇(tholesterol, CHO)、甘油三酯(triglyceride, TG)、碱性磷酸酶(alkaline phosphatase, ALP)。COR采用酶联免疫法ELISA进行测定,试剂盒购自南京建成科技有限公司。
1.4 数据分析实验数据采用Excel 2019软件进行整理和统计,使用Origin 2021软件和SPSS 26.0软件进行统计分析及绘图。捕食胁迫程度和捕食胁迫时长对COR和血液生化指标的影响采用双因素协方差分析(two-way ANOVA),以体重作为协方差。若差异显著,利用Duncan法进行多重比较。所有数据结果均以平均值±标准误(Mean±SE)表示,P < 0.05为差异显著。
2 结果与分析因考虑到高捕食组存在猎物鱼被捕食情况,捕食胁迫前,各实验组猎物鱼数量在取样所需基础上已增加放养密度,捕食胁迫期间不补充,仅记录各实验组死亡数目。14 d胁迫实验结束后,统计高捕食组猎物鱼被摄食量并计算被摄食率(表 1)。从表 1可以看出,各实验组均存在实验鱼死亡现象,主要为较小规格的实验鱼卡在拦网上无法挣脱导致其死亡,除此之外,鱼类无异常行为。
|
|
表 1 捕食胁迫期间“四大家鱼”幼鱼的存活情况 Tab.1 Survival of juvenile "four major Chinese carps" during predation stress |
7 d胁迫处理后,青鱼、草鱼、鲢和鳙低捕食组COR水平升高幅度分别为9.65%、7.33%、18.71%和17.13%。高捕食胁迫组升高幅度分别为18.57%、26.28%、48.76%和46.79%;14 d胁迫处理后,低捕食胁迫组升高幅度分别为36.28%、31.95%、28.29%和32.36%,高捕食胁迫组升高幅度分别为47.39%、54.44%、57.98%和49.89%。
在捕食胁迫7 d后,低捕食组的COR水平升高,但与无捕食组相比差异不显著;高捕食组青鱼、鲢和鳙的COR水平显著高于对照组,且高捕食组鲢和鳙的COR水平显著高于低捕食组;捕食胁迫14 d后,低捕食组和高捕食组的COR水平均显著高于无捕食组,其中,高捕食组鲢的COR水平显著高于低捕食组。
在不同捕食胁迫时长中,低捕食组“四大家鱼”幼鱼的COR水平在胁迫14 d后显著高于低捕食0 d,其中,青鱼和草鱼的COR水平在低捕食胁迫14 d显著高于低捕食7 d;高捕食组的COR水平在捕食胁迫7和14 d后均显著高于0 d,其中,青鱼和草鱼14 d捕食胁迫后,COR水平显著高于7 d (P < 0.05) (表 2和图 2)。
|
|
表 2 捕食胁迫程度(无捕食、低捕食和高捕食)和捕食胁迫时长(0、7、14 d)对“四大家鱼”幼鱼COR和血液生化指标的双因素方差统计分析 Tab.2 Summary of the two-way ANOVA model on serum cortisol and biochemical parameters of juvenile "four major Chinese carps" exposed to different degree (no predation, low predation, high predation) and duration(0 d, 7 d and 14 d) of predation stress |

|
图 2 捕食胁迫程度(无捕食、低捕食和高捕食)和捕食胁迫时长(0、7和14 d)对“四大家鱼”幼鱼COR水平的影响 Fig.2 The levels of serum cortisol in no predation, low predation, and high predation groups of juvenile "four major Chinese carps" in 0, 7 and 14 d after predation stress 不同大写字母表示在特定的捕食胁迫程度内(无捕食、低捕食和高捕食)不同时长(0、7和14 d)之间显著差异(P < 0.05);不同的小写字母表示在特定的捕食胁迫时长(0、7和14 d)内不同的捕食胁迫程度(无捕食、低捕食和高捕食)之间显著差异(P < 0.05)。图中数据为平均值±标准误(Mean±SE),下同。 Different uppercase letters indicated that there were significant differences among different exposure duration (0 d, 7 d and 14 d) within specific predation stress (no predation, low predation, high predation) (P < 0.05). Different lowercase letters indicated that there were significant differences amongdifferent levels of predation stress (no predation, low predation, and high predation) within the specific duration of predation stress (0, 7and14 d) (P < 0.05). Data in the figure are mean±standard error (Mean±SE), the same as below. |
不同的捕食胁迫程度和胁迫时长对“四大家鱼”幼鱼的TP、ALB和GLB浓度影响均不显著(表 2和图 3)。

|
图 3 捕食胁迫程度(无捕食、低捕食、高捕食)和捕食胁迫时长(0、7和14 d)对“四大家鱼”幼鱼TP (A)、ALB (B)和GLB (C)浓度的影响 Fig.3 The levels of total protein, albumin and globulin in no predation, low predation, high predation groups of juvenile "four major Chinese carps" in 0 d, 7 d and 14 d after predation stress |
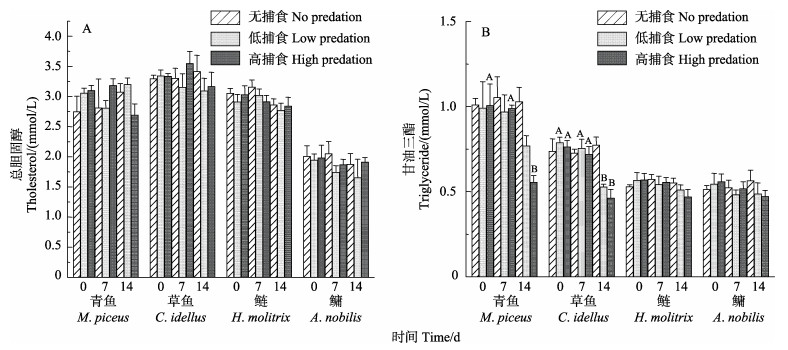
不同的捕食胁迫程度和胁迫时长对“四大家鱼”幼鱼的血清胆固醇浓度影响不显著,但TG浓度降低。7 d胁迫处理后,青鱼、草鱼、鲢和鳙低捕食组TG浓度的下降幅度分别为2.25%、4.21%、4.32%和11.48%;高捕食胁迫组下降幅度分别为1.82%、5.73%、2.51%和7.40%;14 d胁迫处理后,低捕食胁迫组下降幅度分别为为22.41%、33.00%、10.00%和10.59%,高捕食胁迫组下降幅度分别为44.93%、39.58%、17.56%和15.64%。
在不同捕食胁迫中,青鱼、草鱼低捕食组和高捕食组的TG浓度与无捕食组相比无显著差异。但在不同的捕食胁迫时长中,低捕食组草鱼和高捕食组的青鱼、草鱼在捕食胁迫14 d后的TG浓度显著低于0和7 d,其中,0和7 d的TG浓度相比差异不显著(表 2和图 4)。
|
图 4 捕食胁迫程度(无捕食、低捕食、高捕食)和捕食胁迫时长(0、7和14 d)对“四大家鱼”幼鱼CHO (A)和TG (B)浓度的影响 Fig.4 The levels of cholesterol and triglycerides in no predation, low predation, high predation groups of juvenile "four major Chinese carps" in 0, 7 and 14 d after predation stress. |
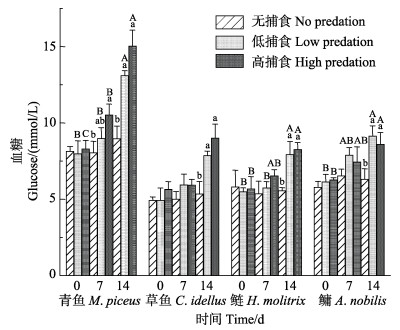
7 d胁迫后,青鱼、草鱼、鲢和鳙低捕食组GLU浓度升高幅度分别为12.43%、20.52%、4.66%和28.60%,高捕食胁迫组GLU浓度升高幅度分别为26.78%、5.03%、14.96%和18.68%;14 d胁迫处理后,低捕食胁迫组GLU浓度升高幅度分别为64.08%、59.45%、44.36%和49.05%,高捕食胁迫组血糖浓度升高幅度分别为81.00%、59.68%、45.02%和37.06%(表 2)。
在不同捕食胁迫中,捕食胁迫7 d后,低捕食组“四大家鱼”幼鱼的GLU浓度上升,但与无捕食组相比差异不显著(P>0.05),高捕食组仅青鱼的GLU浓度显著高于无捕食组;捕食胁迫14 d后,低捕食组和高捕食组的GLU浓度均显著高于无捕食组,其中,高捕食组和低捕食组相比无显著差异(P>0.05)(图 5)。
|
图 5 捕食胁迫程度(无捕食、低捕食、高捕食)和捕食胁迫时长(0、7和14 d)对“四大家鱼”幼鱼GLU浓度的影响 Fig.5 The levels of glucose in no predation, low predation, high predation groups of juvenile "four major Chinese carps" in 0 d, 7 d and 14 d after predation stress |
在不同捕食胁迫时长中,草鱼的GLU浓度升高但差异不显著。低捕食组青鱼和鲢捕食胁迫14 d的GLU浓度显著高于0和7 d;低捕食组鳙胁迫14 d的GLU浓度显著高于0 d。青鱼、鲢和鳙低捕食胁迫0和7 d的GLU浓度相比无显著差异(P>0.05);高捕食组青鱼捕食胁迫0、7和14 d的GLU浓度随着捕食时长的增加显著升高,高捕食组鲢和鳙捕食胁迫14 d的GLU浓度显著高于0 d (表 2和图 5)。
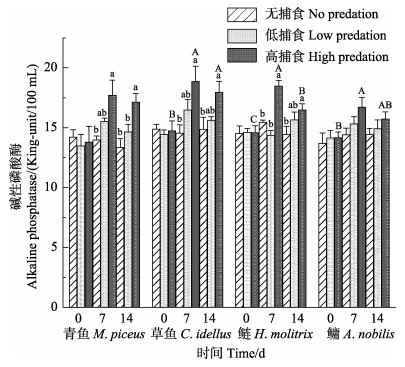
2.2.4 ALP与无捕食组相比,ALP在捕食组中浓度升高。在不同的捕食胁迫程度中,“四大家鱼”幼鱼低捕食组的ALP浓度与无捕食组相比无显著差异,鳙的ALP浓度在不同捕食胁迫程度下有升高趋势,但差异不显著(P>0.05)。捕食胁迫7 d后,高捕食组青鱼、草鱼和鲢的ALP浓度显著高于无捕食组,且高捕食组鲢的ALP浓度显著高于低捕食组;捕食胁迫14 d后,高捕食组青鱼、草鱼和鲢的ALP浓度显著高于无捕食组,且高捕食组青鱼的ALP浓度显著高于低捕食组(P < 0.05)。
在不同的捕食胁迫时长中,低捕食组“四大家鱼”幼鱼捕食胁迫0、7和14 d的ALP浓度相比无显著差异。青鱼的ALP浓度在不同捕食胁迫时长中有升高趋势,但差异不显著(P>0.05)。高捕食组草鱼、鲢和鳙的ALP浓度在捕食胁迫7 d后显著高于0 d,鳙在14 d捕食胁迫后,ALP浓度降低至无捕食组水平,草鱼和鲢在14 d捕食胁迫后,ALP浓度呈下降趋势但仍显著高于0 d (P < 0.05) (表 2和图 6)。
|
图 6 捕食胁迫程度(无捕食、低捕食和高捕食)和捕食胁迫时长(0、7和14 d)对“四大家鱼”ALP幼鱼浓度的影响 Fig.6 The levels of alkaline phosphatase in no predation, low predation, and high predation groups of juvenile "four major Chinese carps" in 0 d, 7 d and 14 d after predation stress |
应激反应的核心是下丘脑–垂体–肾上腺(HPA)轴的激活以及随后的糖皮质激素(GC)的分泌(Busch et al, 2009; Hansen et al, 2016; Tarlow et al, 2007)。COR是关键的应激糖皮质激素,其水平的升高被视为鱼类应激的信号(Kortan et al, 2011; Oliveira et al, 2017; Sapolsky et al, 2000)。本研究中,COR水平随着捕食胁迫程度和胁迫时长的增加显著升高,表明“四大家鱼”幼鱼暴露于捕食者或捕食线索时均产生应激反应,和周龙艳等(2021)对胭脂鱼(Myxocyprinus asiaticus)和中华倒刺鲃(Spinibarbus sinensis)进行捕食胁迫的研究结果一致。不同捕食压力水平下,COR水平表现为无捕食组 < 低捕食组 < 高捕食组,0 d < 7 d < 14 d,表明“四大家鱼”幼鱼会根据捕食风险调整其生理反应,且与捕食者直接接触的应激程度大于嗅觉和视觉等感官接触。COR水平的增加有利于鱼体抵抗外界不良因子胁迫(Brown et al, 2005)。不同捕食胁迫时长处理后,COR水平表现为0 d < 7 d < 14 d,这可能是长期压力导致COR代谢增加的转变,进而导致体内基底COR水平升高(Dallman et al, 1992; Pottinger et al, 2000),表明在应激情况下,猎物鱼倾向于以增强自身生存能力的方式改变生理反应。捕食压力下,COR水平升高也反映了与应激有关的能量需求的增加(Schreck et al, 1985),COR升高能刺激糖原(Janssens et al, 1988)、蛋白质(Vijayan et al, 1989)和脂质(Davis et al, 1985)的代谢,为机体应对捕食胁迫提供能量,有利于鱼类提高游泳能力和在捕食者攻击下更高的生存率(Fu et al, 2017; Van et al, 1998)
3.2 捕食胁迫下“四大家鱼”幼鱼的能量代谢鱼类的血液生化指标始终处于动态平衡中,直接反映了鱼类的内分泌水平和代谢情况(Congleton et al, 2006; Dawood et al, 2017; Wagner et al, 2004)。蛋白质是血清中的重要组成部分,在鱼类的生理学和免疫系统中发挥重要作用(Kumar et al, 2005)。TP含量常被用作鱼体对环境应激反应的指示物(Khan et al, 2016; Vaziriyan et al, 2018)。ALB是重要的特异性蛋白,被认为是鱼类健康状况良好的指标(Ergonul et al, 2012; Rehulka, 1993)。GLB是由生物体免疫器官产生的,其浓度变化反映机体的抵抗力(Xia et al, 2018; Yousefi et al, 2019)。许氏平鲉(Sebastes schlegelii)和半滑舌鳎(Cynoglossus semilaevis)受到急性高温胁迫后,血清TP和ALB水平显著降低,GLB分泌增多(张亚晨等, 2015; 孙学亮等, 2010)。本研究中,“四大家鱼”幼鱼的血清蛋白浓度(TP、ALB和GLB)相比均无显著差异(表 2和图 3),表明“四大家鱼”幼鱼的健康状况良好,免疫功能也并未受到影响。也可能是相比于急性胁迫,血清蛋白对有较大时间跨度的捕食胁迫敏感程度低,或者是本研究中捕食胁迫的强度不够,不足以引起血清蛋白发生显著变化。
血清胆固醇和TG是机体重要的能量来源。TG是脂类代谢的主要物质,其含量的变化也反映了机体能量的波动(何志刚等, 2016; Peres et al, 2014)。有研究发现,环境胁迫会促进脂类分解,导致血清胆固醇和TG浓度降低(Vijayan et al, 1990; 张亚晨等, 2015)。本研究中,“四大家鱼”幼鱼的胆固醇浓度与无捕食组相比无显著差异,表明胆固醇可能不是捕食胁迫下鱼类应激的敏感指标。TG浓度随着胁迫时长增加而下降,其中,青鱼和草鱼在捕食组胁迫处理后,TG浓度显著降低(表 2和图 4B)。一方面可能是捕食压力使鱼类代谢率增加,导致体内TG消耗加快(Lulijwa et al, 2021; Rossi et al, 2017)。青鱼和草鱼相比鲢和鳙TG浓度下降显著,可能是因为青鱼和草鱼的游泳能力更强,能量消耗需求高(王晓等, 2022);另一方面可能是由于捕食胁迫下,鱼类需要花费更多的时间保持警惕,更少的时间觅食,进而导致能量摄入减少(Belgrad et al, 2016; Elvidge et al, 2020; 钱云霞等, 2002)。
GLU是鱼类各种生命活动所需能量的直接来源,作为鱼体内主要供能物质,其含量与鱼类代谢水平和营养状况直接相关(Barton et al, 1988)。GLU在常态下含量比较恒定,而随着机体的活动和环境的变化,其含量也会发生变化。本研究中,“四大家鱼”幼鱼的GLU浓度显著上升(表 2和图 5)。在捕食压力下,COR水平升高促使肝糖原发生糖酵解,导致GLU浓度的上升(Vijayan et al, 2003),在满足捕食胁迫下,由于呼吸速率、代谢水平的上升而产生的对能量更高需求(Hawkins et al, 2004; Xu et al, 2019)。此外,GLU的升高也是“四大家鱼”幼鱼对捕食胁迫作出应激反应的表现(Lawrence et al, 2018),且随着捕食胁迫程度和胁迫时长的增加,应激程度也相应增加。
ALP是一类膜结合糖蛋白,直接参与磷酸基团的转移和代谢过程,通常用于评估质膜的完整性(Akanji et al, 1993)。本研究中,“四大家鱼”幼鱼在捕食胁迫后,ALP浓度升高,其中,高捕食组的ALP浓度显著升高(表 2和图 6)。这可能是因为捕食胁迫对“四大家鱼”幼鱼肝脏细胞的ALP有激活作用,ALP浓度升高有助于鱼类在捕食压力下保持细胞膜的完整性,ALP浓度升高也表明在捕食胁迫下鱼体代谢增强(Kong et al, 2012; Guo et al, 2021)。随着捕食时长的增加,ALP浓度呈先升高后下降的趋势,可能是因为鱼体对捕食环境的适应性结果。
AKANJI M A, OLAGOKE O A, OLOYEDE O B. Effect of chronic consumption of metabisulphite on the integrity of rat kidney cellular system. Toxicology, 1993, 81(3): 173-179 DOI:10.1016/0300-483X(93)90010-P |
BARCELLOS L J G, RITTER F, KREUTZ L C, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture, 2007, 272(1/2/3/4): 774-778 |
BARTON B A, SCHRECK C B, FOWLER L G. Fasting and diet content affect stress-induced changes in plasma glucose and cortisol in juvenile chinook salmon (Oncorhynchus tshawytscha). Progressive Fish-Culturist, 1988, 50(1): 16-22 DOI:10.1577/1548-8640(1988)050<0016:FADCAS>2.3.CO;2 |
BARTON B A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integrative and Comparative Biology, 2002, 42: 517-525 DOI:10.1093/icb/42.3.517 |
BELGRAD B A, GRIFFEN B D. Predator-prey interactions mediated by prey personality and predator hunting mode. Proceedings of the Royal Society Biological Sciences, 2016, 283(1828): 20160408 DOI:10.1098/rspb.2016.0408 |
BENSON C W, SHEA B D, DONOVAN D, et al. Physiological consequences of varying large shark exposure on striped bass (Morone saxatilis). Canadian Journal of Zoology, 2019, 97(12): 1195-1202 DOI:10.1139/cjz-2019-0173 |
BREVES J P, SPECKER J L. Cortisol stress response of juvenile winter flounder (Pseudopleuronectes americanus, Walbaum) to predators. Journal of Experimental Marine Biology and Ecology, 2005, 325(1): 1-7 DOI:10.1016/j.jembe.2005.04.019 |
BROWN C, GARDNER C, BRAITHWAITE V A. Differential stress responses in fish from areas of high-and low-predation pressure. Journal of Comparative Physiology. B: Biochemical, Systemic, and Environmental Physiology, 2005, 175(5): 305-312 DOI:10.1007/s00360-005-0486-0 |
BUSCH D S, HAYWARD L S. Stress in a conservation context: A discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biological Conservation, 2009, 142(12): 2844-2853 DOI:10.1016/j.biocon.2009.08.013 |
CHEN J J, CAO J L, LUO Y J. Effects of starvation on blood physiological and biochemical indices in Clarias fuscus. Journal of Anhui Agricultural Sciences, 2009, 37(5): 2014-2015 [陈剑杰, 曹谨玲, 罗永巨. 饥饿对胡子鲇血液生理生化指标的影响. 安徽农业科学, 2009, 37(5): 2014-2015] |
CLINCHY M, ZANETTE L, BOONSTRA R, et al. Balancing food and predator pressure induces chronic stress in songbirds. Proceedings of the Royal Society. B: Biological Sciences, 2004, 271(1556): 2473-2479 DOI:10.1098/rspb.2004.2913 |
CLINCHY M, ZANETTE L, CHARLIER T D, et al. Multiple measures elucidate glucocorticoid responses to environmental variation in predation threat. Oecologia, 2011, 166(3): 607-614 DOI:10.1007/s00442-011-1915-2 |
COLSON V, VALOTAIRE C, GEFFROY B, et al. Egg cortisol exposure enhances fearfulness in larvae and juvenile rainbow trout. Ethology, 2015, 121(12): 1191-1201 DOI:10.1111/eth.12437 |
CONGLETON J L, WAGNER T. Blood-chemistry indicators of nutritional status in juvenile salmonids. Journal of Fish Biology, 2006, 69(2): 473-490 DOI:10.1111/j.1095-8649.2006.01114.x |
COOKE S J, STEINMETZ J, DEGNER J F, et al. Metabolic fright responses of different-sized largemouth bass (Micropterus salmoides) to two avian predators show variations in nonlethal energetic costs. Canadian Journal of Zoology, 2003, 81(4): 699-709 DOI:10.1139/z03-044 |
DALLMAN M F, AKANA S F, SCRIBNER K A, et al. Stress, feed-back and facilitation in the hypothalamo-pituitary- adrenal axis. Journal of Neuroendocrinology, 1992, 4(5): 517-526 DOI:10.1111/j.1365-2826.1992.tb00200.x |
DAVIS K B, TORRANCE P M, Parker N C, et al. Growth, body composition and hepatic tyrosine aminotransferase activity in cortisol-fed channel catfish, Ictalurus punctatus Rafinesque. Journal of Fish Biology, 1985, 27(2): 177-184 DOI:10.1111/j.1095-8649.1985.tb04019.x |
DAWOOD M, KOSHIO S, ISHIKAWA M, et al. Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiology and Biochemistry, 2017, 43(1): 179-192 DOI:10.1007/s10695-016-0277-4 |
DUAN X B, LIU S P, HUANG M G, et al. Changes in abundance of larvae of the four domestic Chinese carps in the middle reach of the Yangtze River, China, before and after closing of the Three Gorges Dam. Environmental Biology of Fishes, 2009, 86(1): 13-22 DOI:10.1007/s10641-009-9498-z |
ELVIDGE C K, COOKE S J. Predation risk mediates cognitive constraints following physical exertion in schoolmaster snapper. Physiology and Behavior, 2002, 214: 112767 |
ERGONUL M B, ATASAGUN S, KOCATURK K. Alterations in the hematological and biochemical parameters and plasma ion concentrations of common carp (Cyprinus carpio L.) after short term exposure to sub-lethal concentrations of lead. Veteriner Fakultesi Dergisi, 2012, 18(2): 297-302 |
FRID A, DILL L. Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology, 2002, 6(1): 94-109 |
FU C, FU S, WU Q, et al. Predation threat modifies relationships between metabolism and behavioural traits but not their ecological relevance in Chinese bream. Marine and Freshwater Behaviour and Physiology, 2017, 50(5/6): 329-344 |
GUO J, PU Y, ZHONG L, et al. Lead impaired immune function and tissue integrity in yellow catfish (Peltobargus fulvidraco) by mediating oxidative stress, inflammatory response and apoptosis. Ecotoxicology and Environmental Safety, 2021, 226: 112857 DOI:10.1016/j.ecoenv.2021.112857 |
HANSEN W K, BATE L J, LANDRY D W, et al. Feather and faecal corticosterone concentrations predict future reproductive decisions in harlequin ducks (Histrionicus histrionicus). Conservation Physiology, 2016, 4(1): cow015 DOI:10.1093/conphys/cow015 |
HAWKINS L A, ARMSTRONG J D, MAGURRAN A E. Predator-induced hyperventilation in wild and hatchery Atlantic salmon fry. Journal of Fish Biology, 2004, 65(s1): 88-100 DOI:10.1111/j.0022-1112.2004.00543.x |
HE Z G, WANG J L, WU Y A, et al. Effect of dietary lipid levels on serum biochemical indices, immune responses and antioxidant capability of juvenile Furong crucian carp (Furong carp♀× red crucian carp♂). Acta Hydrobiologica Sinica, 2016, 40(4): 655-662 [何志刚, 王金龙, 伍远安, 等. 饲料脂肪水平对芙蓉鲤鲫幼鱼血清生化指标、免疫反应及抗氧化能力的影响. 水生生物学报, 2016, 40(4): 655-662] |
HONG L, ZHANG X M. Effects of environmental stress on physiological function of fish. Advances in Marine Science, 2004, 22(1): 114-121 [洪磊, 张秀梅. 环境胁迫对鱼类生理机能的影响. 海洋科学进展, 2004, 22(1): 114-121] |
JANSSENS P A, WATERMAN J. Hormonal regulation of gluconeogenesis and glycogenolysis in carp (Cyprinus carpio) liver pieces cultured in vitro. Comparative Biochemistry and Physiology Part A: Physiology, 1988, 91(3): 451-455 DOI:10.1016/0300-9629(88)90617-2 |
KHAN A, SHAH N, GUL A, et al. Comparative study of toxicological impinge of glyphosate and atrazine (Herbicide) on stress biomarkers; blood biochemical and haematological parameters of the freshwater common carp (Cyprinus carpio). Polish Journal of Environmental Studies, 2016, 25(5): 1995-2001 DOI:10.15244/pjoes/62698 |
KILLEN S S, FU C, WU Q, et al. The relationship between metabolic rate and sociability is altered by food deprivation. Functional Ecology, 2016, 30(8): 1358-1365 DOI:10.1111/1365-2435.12634 |
KONG X G, WANG S P, JIANG H X, et al. Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquatic Toxicology, 2012, 120/121: 119-125 DOI:10.1016/j.aquatox.2012.05.005 |
KORTAN J, BLAHOVA J, KRUZIKOVA K, et al. Stress responses of carp pond fish stock upon hunting activities of the great cormorant (Phalacrocorax carbo sinensis L). Aquaculture Research, 2011, 42(3): 322-330 DOI:10.1111/j.1365-2109.2010.02624.x |
KUMAR S, SAHU N P, PAL A K, et al. Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish and Shellfish Immunology, 2005, 19(4): 331-344 DOI:10.1016/j.fsi.2005.03.001 |
LAWRENCE M J, ELIASON E J, BROWNSCOMBE J W, et al. Influence of supraphysiological cortisol manipulation on predator avoidance behaviors and physiological responses to a predation threat in a wild marine teleost fish. Integrative Zoology, 2018, 13(2): 206-218 DOI:10.1111/1749-4877.12282 |
LIMA S L, BEDNEKOFF P A. Temporal variation in danger drives anti-predator behavior: The predation risk allocation hypothesis. American Naturalist Devoted to the Conceptual Unification of the Biological Sciences, 1999, 153(6): 649-659 |
LIMA S L. Stress and decision-making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Advances in the Study of Behavior, 1998, b, 27(8): 215-290 |
LIMA, S L. Nonlethal effects in the ecology of predator-prey interactions-what are the ecological effects of anti-predator decision-making?. Bioscience, 1998a, 48(1): 25-34 DOI:10.2307/1313225 |
LIU F, LIN P C, LI M Z, et al. Situations and conservation strategies of fish resources in the Yangtze River basin. Acta Hydrobiologica Sinica, 2019, 43(S1): 144-156 [刘飞, 林鹏程, 黎明政, 等. 长江流域鱼类资源现状与保护对策. 水生生物学报, 2019, 43(S1): 144-156] |
LULIJWA R, YOUNG T, SYMONDS J E, et al. Uncoupling thermotolerance and growth performance in chinook salmon: Blood biochemistry and immune capacity. Metabolites, 2021, 11(8): 547 DOI:10.3390/metabo11080547 |
OLIVEIRA T A, IDALENCIO R, KALICHAK F, et al. Stress responses to conspecific visual cues of predation risk in zebrafish. PeerJ, 2017, 5(9): e3739 |
PERES H, SANTOS S, OLIVATELES A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiology and Biochemistry, 2014, 40(5): 1339-1347 DOI:10.1007/s10695-014-9928-5 |
POTTINGER T, CARRICK T, APPLEBY A, et al. High blood cortisol levels and low cortisol receptor affinity: Is the chub, Leuciscus cephalus, a cortisol-resistant teleost?. General and Comparative Endocrinology, 2000, 120(1): 108-117 DOI:10.1006/gcen.2000.7544 |
QIAN Y X, CHEN H Q, SUN J F, et al. Effects of starvation on hematological and blood biochemical indices in cultured Lateolabrax japonicus. Journal of Fishery Sciences of China, 2002, 9(2): 133-137 [钱云霞, 陈惠群, 孙江飞, 等. 饥饿对养殖鲈鱼血液生理生化指标的影响. 中国水产科学, 2002, 9(2): 133-137 DOI:10.3321/j.issn:1005-8737.2002.02.010] |
REDFERN J C, COOKE S J, LENNOX R J, et al. Effects of maternal cortisol treatment on offspring size, responses to stress, and anxiety-related behavior in wild largemouth bass (Micropterus salmoides). Physiology and Behavior, 2017, 180: 15-24 DOI:10.1016/j.physbeh.2017.08.001 |
REHULKA J. Erythrodermatitis of carp (Cyprinus carpio L): An electrophoretic study of blood serum protein fraction levels. Acta Veterinaria Brno, 1993, 62(3/4): 187-197 |
ROSSI A, BACCHETTA C, CAZENAVE J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecological Indicators, 2017, 79: 361-370 DOI:10.1016/j.ecolind.2017.04.042 |
SAPOLSKY R, ROMERO L, MUNCK A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 2000, 21(1): 55-89 |
SCHREC L, TORT A P, FARRELL C J. Biology of stress in fish-fish physiology. San Diego: Academic Press, 2016, 1-34 |
SCHRECK C B, PATINO R, PRING C K, et al. Effects of rearing density on indices of smoltification and performance of coho salmon, Oncorhynchus kisutch. Aquaculture, 1985, 45(1/2/3/4): 345-358 |
SPIEGEL O, HAREL R, GETZ W M, et al. Mixed strategies of griffon vultures' (Gyps fulvus) response to food deprivation lead to a hump-shaped movement pattern. Movement Ecology, 2013, 1(1): 5 DOI:10.1186/2051-3933-1-5 |
SUN X L, XING K Z, CHENG C X, et al. The effects of acute temperature stress on blood parameters in half-smooth tongue-sole (Cynoglossus semilaevis). Fisheries Science, 2010, 29((7): 387-393 [孙学亮, 邢克智, 陈成勋, 等. 急性温度胁迫对半滑舌鳎血液指标的影响. 水产科学, 2010, 29((7): 387-393] |
TARLOW E M, BLUMSTEIN D T. Evaluating methods to quantify anthropogenic stressors on wild animals. Applied Animal Behaviour Science, 2007, 102(3): 429-451 |
VAN W J, KOMEN J. The effects of chronic stress on growth in fish: A critical appraisal. Comparative Biochemistry and Physiology. Part A: Molecular and Integrative Physiology, 1998, 120(1): 107-112 DOI:10.1016/S1095-6433(98)10017-X |
VAZIRIYAN M, BANAEE M, HAGHI B N, et al. Effects of dietary exposure to aflatoxins on some plasma biochemical indices of common carp (Cyprinus carpio). Iranian Journal of Fisheries Sciences, 2018, 17(3): 487-502 |
VIJAYAN M M, LEATHERLAND J F. High stocking density affects cortisol secretion and tissue distribution in brook charr, Salvelinus fontinalis. Journal of Endocrinology, 1990, 124(2): 311-318 DOI:10.1677/joe.0.1240311 |
VIJAYAN M M, LEATHERLAND J. Cortisol-induced changes in plasma-glucose, protein, and thyroid-hormone levels, and liver-glycogen content of coho salmon (Oncorhynchus- kisutch walbaum). Canadian Journal of Zoology, 1989, 67(11): 2746-2750 DOI:10.1139/z89-389 |
VIJAYAN M M, RAPTIS S, SATHIYAA R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. General and Comparative Endocrinology, 2003, 132(2): 256-263 DOI:10.1016/S0016-6480(03)00092-3 |
WAGNER T, CONGLETON J L. Blood chemistry correlates of nutritional condition, tissue damage, and stress in migrating juvenile Chinook salmon (Oncorhynchus tshawytscha). Canadian Journal of Fisheries and Aquatic Sciences, 2004, 61(7): 1066-1074 DOI:10.1139/f04-050 |
WANG X, LIAO D Y, YU L X, et al. Effect of temperature gradient on the critical swimming speed of four major Chinese carps. Progress in Fishery Sciences, 2022, 43(3): 33-44 [王晓, 廖冬芽, 俞立雄, 等. 温度梯度对四大家鱼临界游泳速度的影响. 渔业科学进展, 2022, 43(3): 33-44] |
WENDELAAR BONGA S E. The stress response in fish. Physiological Reviews, 1997, 77(3): 591-625 DOI:10.1152/physrev.1997.77.3.591 |
WOODLEY C M, PETERSON M S. Measuring responses to simulated predation threat using behavioral and physiological metrics: The role of aquatic vegetation. Oecologia, 2003, 136(1): 155-160 DOI:10.1007/s00442-003-1236-1 |
XIA S L, LI X F, PRUDENCE A K, et al. Effects of dietary glucose and starch levels on the growth, apparent digestibility, and skin-associated mucosal non-specific immune parameters in juvenile blunt snout bream (Megalobrama amblycephala). Fish and Shellfish Immunology, 2018, 79: 193-201 DOI:10.1016/j.fsi.2018.05.001 |
XU J J, FU S J, FU C. Physiological and behavioral stress responses to predators are altered by prior predator experience in juvenile qingbo (Spinibarbus sinensis). Biology Open, 2019, 8(5): bio041012 |
YOUSEFI M, HOSEINI S M, VATNIKOV Y A, et al. Rosemary leaf powder improved growth performance, immune and antioxidant parameters, and crowding stress responses in common carp (Cyprinus carpio) fingerlings. Aquaculture, 2019, 505: 473-480 DOI:10.1016/j.aquaculture.2019.02.070 |
YU M, FAN Q X, CHENG P, et al. Effects of acute crowding stress on cortisol and several biochemical indexes in Cyprinus carpio serum. Freshwater Fisheries, 2008, 38(4): 20-24 [于淼, 樊启学, 程鹏, 等. 急性拥挤胁迫对鲤血液中皮质醇及几项生化指标的影响. 淡水渔业, 2008, 38(4): 20-24] |
ZHANG Y C, WEN H S, LI L M, et al. Effect of acute temperature stress on serum cortisol and hematological physiology of gestated Sebastes schlegelii. Journal of Fisheries of China, 2015, 39(12): 1872-1882 [张亚晨, 温海深, 李兰敏, 等. 急性温度胁迫对妊娠期许氏平鲉血清皮质醇和血液生理指标的影响. 水产学报, 2015, 39(12): 1872-1882] |
ZHANG Y T, YANG J, GENG L W, et al. Effects of salinity stress on antioxidant enzymes and serum cortisol in Luciobarbus capito. Progress in Fishery Sciences, 2021, 42(1): 56-63 [张宇婷, 杨建, 耿龙武, 等. 盐度胁迫对大鳞鲃抗氧化酶和血清皮质醇的影响. 渔业科学进展, 2021, 42(1): 56-63] |
ZHOU L Y, LI X M, FU S J. The effect of predation acclimation on swimming behavior, stress and immune responses of juvenile Myxocyprinus asiaticus and Spinibarbus sinensis. Acta Hydrobiologica Sinica, 2021, 45(5): 1112-1119 [周龙艳, 李秀明, 付世建. 捕食驯化对胭脂鱼和中华倒刺鲃游泳行为、应激和免疫功能的影响. 水生生物学报, 2021, 45(5): 1112-1119] |



