2. 中国水产科学研究院黄海水产研究所农业农村部海洋渔业可持续发展重点实验室 山东 青岛 266071;
3. 崂山实验室海洋渔业科学与食物产出过程功能实验室 山东 青岛 266071;
4. 上海海洋大学水产与生命学院 上海 201306
2. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture and Rural Affairs, Qingdao 266071, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao 266071, China;
4. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China
游泳对鱼类的生存具有重要意义,直接影响其躲避捕食者和敌害、寻找和捕获猎物、求偶繁殖以及洄游的能力(Martinezl et al, 2003; Brian Langerhans et al, 2010; Handelsman et al, 2010)。鱼类的游泳方式根据保持特定速度运动的时间,一般分为持续式游泳(sustained swimming)、延长式游泳(prolonged swimming)和爆发式游泳(burst swimming)三类(Brett, 1964)。鱼类低速进行的游泳运动被称为持续式游泳,其持续的时间一般为数小时或者数天甚至是数月,被形象地称为鱼类的“马拉松运动”(Beamish, 1978);爆发式游泳只能维持很短的时间(<20 s),短期高性能的能力有助于鱼类捕获猎物、躲避捕食者的猎杀(Brian Langerhans et al, 2010);延长式游泳持续时间通常介于持续式游泳与爆发式游泳之间,约为20 s~ 200 min (Brett, 1964)。硬骨鱼类游泳运动依靠骨骼肌来支持,其骨骼肌约占鱼体质量的40%~60% (Bone, 1978),可为不同速度及不同持续时间的运动提供动力。骨骼肌的基本构成单位是肌纤维(李本相等, 2019)。根据颜色的不同,可分为红肌纤维和白肌纤维(梁婷玉等, 2018)。红肌纤维收缩慢、耐力强,线粒体、糖原和肌红蛋白含量高,以有氧代谢为主,可以有效地利用氧气产生ATP,也被称为慢肌纤维,其主要功能是为鱼类游泳过程提供稳定和持续的动力;白肌纤维收缩快,但很快会疲劳,以酵解代谢为主,又称为快肌纤维,主要为鱼类的快速游泳行为(如捕食和逃跑)提供动力(Moss et al, 1971; Akolkar et al, 2010; Jackson et al, 2013)。
目前,很多研究指出,鱼类的游泳习性与快、慢肌的构成存在着较强的关联性,例如,有学者对不同游泳习性的淡水硬骨鱼红肌和白肌的比例进行研究,发现持续式游泳的鱼类红肌比例更高(Kiessling et al, 2006; Cediel et al, 2008)。有研究也指出,红肌适合于持续而缓慢的收缩,白肌适合于快速而短暂的收缩(Zhang et al, 2011; Jiao et al, 2019)。此外,有研究证明,鱼类缓慢游泳时,只有红肌纤维活跃;而在快速或剧烈运动时,白肌纤维活跃(Drazen et al, 2013; Wu et al, 2018)。这些研究多集中在对鱼类游泳运动与肌纤维类型相关的研究,对不同游泳习性鱼类的快、慢肌纤维组织学特征差异却鲜见报道。肌纤维的组织学特性主要包括肌纤维的形态、直径和密度等(尹丽卿等, 2015; 关文静等, 2008),是描述鱼体骨骼肌组织学结构特征的重要指标。
本研究选取以鲐(Scomber japonicus)为代表种的持续式游泳习性鱼类、以大黄鱼(Larimichthys crocea)为代表种的延长式游泳习性鱼类和以褐牙鲆(Paralichthys olivaceus)为代表种的爆发式游泳习性鱼类,利用石蜡切片苏木精–伊红染色法和形态计量法对3种游泳类型鱼类的快、慢肌纤维组织学特性进行定性描述和定量分析,旨在比较3种游泳习性鱼类快、慢肌纤维组织学结构特征,阐释游泳习性与骨骼肌肌纤维组织学特征的相关性,以期为系统研究硬骨鱼类骨骼肌运动生理学提供基础资料。
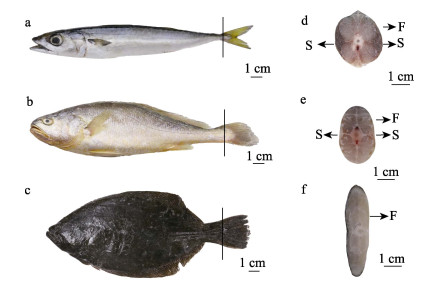
1 材料与方法 1.1 实验材料鲐为采自北黄海的野生样品;大黄鱼为浙江舟山近海网箱养殖;褐牙鲆采自山东威海养殖场。所有实验用鱼均为健康成鱼,每种鱼类各取样3尾,基本生物学信息见表 1,取样部位如图 1a、b、c所示。因尾鳍是鱼类游泳的主要驱动器官,它的运动特征直接影响鱼类的游泳习性(Histand, 1977; Bone, 1966),研究尾部肌肉组织学特征可以更直接地反映不同鱼类游泳习性的差异。因此,本研究选取尾部(尾鳍前端)快、慢肌组织(图 1d、e、f),将快、慢肌组织横切成约0.5 cm ×1.0 cm×1.0 cm的组织块,浸入环保型GD固定液(主要成分为福尔马林、冰醋酸和无水乙醇)中,固定48 h后保存于70%酒精中备用。
|
|
表 1 实验用鱼的基本生物学信息 Tab.1 Basic biological information of experimental fish |
固定的快、慢肌肉组织经75%、85%、90%、95%、100%和100%酒精梯度脱水,二甲苯透明,透蜡及包埋后用LEICA RM 2016轮转式切片机切片,切片厚度为5 μm,苏木精–伊红(Hematoxylin- Eosin, HE)染色,中性树胶封片之后在生物显微镜(OLYMPUS BX3, 日本)下观察拍照,观察其形态特征。
|
图 1 3种鱼取样部位示意图 Fig.1 Schematic diagram of sampling sites of the three fish species a:鲐;b:大黄鱼;c:褐牙鲆;d:鲐尾鳍横截面;e:大黄鱼尾鳍横截面;f:褐牙鲆尾鳍横截面。S:慢肌;F:快肌。 a: S. japonicus; b: L. crocea; c: P. olivaceus; d: Tail fin transverse sections of S. japonicus; e: Tail fin transverse sections of L. crocea; f: Tail fin transverse sections of P. olivaceus. S: Slow-twitch muscle; F: Fast-twitch muscle. |
肌纤维直径计算参考崔长艳(2017)的指标测定方法,在10×40倍光学显微镜下拍照,随机选择10个视野,用ImageJ直线工具分别测量长轴和短轴,求其平均值为肌纤维直径。肌纤维密度的测量参考崔长艳(2017)的指标测定的方法,在10×20倍光学显微镜下拍照,以“记上不记下,记左不记右”为原则,随机选取无重叠、无断裂清晰的10个视野,使用ImageJ软件统计10个视野内的肌纤维面积,统计每个视野内肌纤维的根数,换算成每平方毫米的肌纤维根数,作为被测样本的肌纤维密度。
1.2.3 数据处理使用Excel 2019软件对数据进行前期处理,再用SPSS 26.0软件对实验数据进行方差分析及多重比较,实验数据以平均值±标准差(Mean±SD)表示。同一物种内快、慢肌纤维间组织学可量化指标进行配对样本t检验,3种鱼类之间肌纤维可量化指标进行独立样本t检验(unpaired student´s t-test),以P < 0.05为差异显著、P < 0.01为差异极显著,采用GraphPad Prism 9软件作图。
2 结果 2.1 快、慢肌纤维形态特征3种鱼的慢肌均分布在侧线位置附近的皮下浅层,快肌分布于躯干深层。但不同的是,鲐的慢肌的横截面呈三角形;大黄鱼的慢肌呈波浪状;而褐牙鲆的慢肌紧靠皮下浅层且颜色浅,所以在横截面上不能明显观察到(图 1d、e、f)。
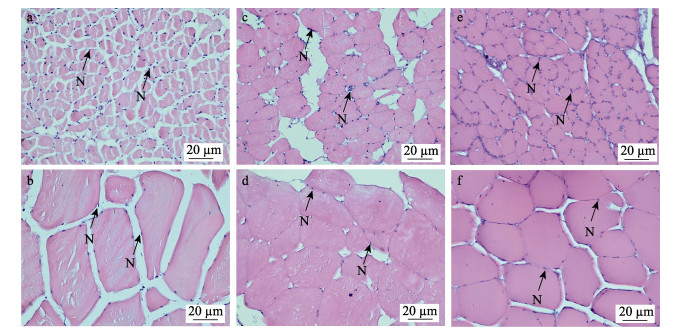
图 2为实验所得3种鱼类骨骼肌纤维的显微观测结果。肌质内嗜酸性的肌纤维被伊红染成淡红色;细胞核分布在肌质的周边,被碱性染料苏木精染成蓝紫色,每个肌细胞含多个细胞核。3种鱼类的骨骼肌细胞形态在组织学水平上可以见到明显差异:3种鱼的快、慢肌纤维细胞均呈不规则形状,其中,鲐的慢肌纤维细胞呈多角状,快肌纤维细胞呈多边形(图 2a、b);大黄鱼的慢肌纤维细胞和快肌纤维细胞均呈长椭圆形,且细胞边缘都较圆润(图 2c、d);褐牙鲆的快、慢肌纤维细胞均呈扁椭圆形(图 2e、f)。此外,与另外2种鱼类相比,鲐快、慢肌纤维细胞间隙大,肌细胞排列松散;而褐牙鲆快、慢肌纤维细胞间隙小,肌细胞排列更紧密。
|
图 2 3种鱼类骨骼肌纤维光学显微结构 Fig.2 Optical microstructure in muscle of skeletal muscle fibers of three fish species a:鲐慢肌纤维横切面;b:鲐快肌纤维横切面;c:大黄鱼慢肌纤维横切面;d:大黄鱼快肌纤维横切面;e:褐牙鲆慢肌纤维横切面;f:褐牙鲆快肌纤维横切面;N:细胞核。 a: The slow-twitch muscle fibers transverse section of S. japonicus; b: The fast-twitch muscle fibers transverse section of S. japonicus; c: The slow-twitch muscle fibers transverse section of L. crocea; d: The fast-twitch muscle fibers transverse section of L. crocea; e: The slow-twitch muscle fibers transverse section of P. olivaceus; f: The fast-twitch muscle fibers transverse section of P. olivaceus; N: Nucleus. |
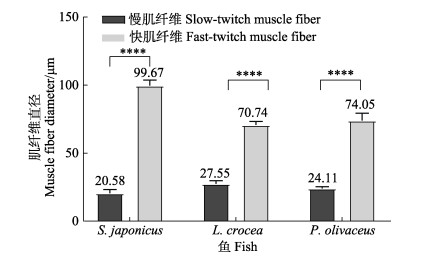
如图 3可知,鲐、大黄鱼和褐牙鲆的快肌纤维直径均极显著大于慢肌纤维(P < 0.01)。鲐、大黄鱼和褐牙鲆的快肌纤维直径分别约为其慢肌纤维的4.84、2.57和3.07倍。此外,大黄鱼的慢肌纤维直径约为鲐的1.34倍、褐牙鲆的1.14倍;鲐快肌纤维直径约为大黄鱼的1.41倍、褐牙鲆的1.35倍。
|
图 3 3种鱼类快、慢肌纤维直径 Fig.3 Diameter of fast-twitch and slow-twitch muscle fiber of three fish species ****表示差异极显著(P < 0.01)。下同。 **** indicates highly significant difference. The same as below. |
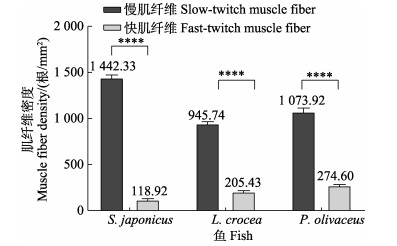
由图 4可知,本研究中的3种鱼类的慢肌纤维密度均极显著大于快肌纤维(P < 0.01)。鲐、大黄鱼和褐牙鲆的慢肌纤维密度分别约为其快肌纤维的12.13、4.60和3.91倍。此外,鲐慢肌纤维密度约为大黄鱼的1.53倍、褐牙鲆的1.34倍;褐牙鲆快肌纤维密度约为鲐的2.31倍、大黄鱼的1.34倍。
3 讨论以往骨骼肌纤维多被描述为圆柱形(沈元新等, 1984; 王力, 2020),但本研究发现,3种游泳习性鱼类的快、慢肌纤维形态有别:鲐的慢肌纤维细胞呈多角形,快肌纤维细胞呈多边柱形;而大黄鱼快、慢肌纤维细胞均呈长椭圆形;褐牙鲆的快、慢肌纤维细胞均呈扁椭圆形。这种不同鱼类物种间骨骼肌细胞形态的差异在其他鱼类中也有报道,例如,刘希良(2013)利用琥珀酸脱氢酶(SDH)染色法对翘嘴鳜(Siniperca chuatsi)的研究结果显示,翘嘴鳜背肌浅层边缘圆润的肌纤维细胞为慢肌纤维,而呈棱角多边形的深层肌纤维细胞为快肌纤维。梁向阳(2011)利用石蜡切片和电镜切片对3种不同倍性鲫的快肌纤维显微结构进行观察时也发现,肌纤维横截面均呈多角形。可见硬骨鱼类骨骼肌的快、慢肌纤维细胞存在多种形态。
|
图 4 3种鱼类快、慢肌纤维密度 Fig.4 Fiber density of fast-twitch muscle and slow-twitch muscle of three fish species |
本研究中,3种不同游泳习性鱼类的快肌纤维直径均极显著大于慢肌纤维(P < 0.01),这种快肌纤维直径与慢肌纤维直径的差异在其他鱼类中也有报道。Rowlerson等(2001)提出,大多数鱼类的快肌纤维的最大直径可达100~300 μm,而慢肌纤维的直径则相对较小。朱琼等(2011)通过制作石蜡切片对鳜的快、慢肌纤维比较发现,其快肌纤维直径为50.0~91.5 μm,而慢肌纤维直径为12.2~44.2 μm。另外,对其他脊椎动物的快、慢肌纤维直径进行研究也发现了差异,例如,白伟良等(2002)研究显示,从犬(Canis lupus familiaris)肌纤维横断面观测,快肌纤维直径较大,为(32.7±5.8) μm,慢肌纤维直径较小,为(28.3±7.9) μm。张霖等(1992)利用SDH与AchE结合染色法对不同负荷训练小鼠(Mus musculus)肌纤维组织学的研究结果显示,不同负荷训练模式下其快肌纤维直径均大于慢肌纤维。解祥学等(2011)对不同品种肉牛(Bos taurus)肌纤维组织学的研究结果显示,6个品种牛不同部位均是快肌纤维直径大于慢肌纤维直径。陈宽维等(2002)对7个品种肉鸡(Gallus gallus)肌纤维的研究结果显示,快肌纤维直径较粗,且比慢肌纤维粗20%左右。可见不论是高等脊椎动物还是较低等脊椎动物,肌纤维直径均表现为快肌纤维大于慢肌纤维。
从本研究结果可以看出,3种游泳习性鱼类的快肌纤维和慢肌纤维在形态、直径和密度上存在差异。3种鱼的快、慢肌纤维的形状各不相同,3种鱼的快肌纤维直径均显著大于慢肌纤维。此外,本研究将不同游泳习性鱼类的快、慢肌纤维密度进行对比发现,鲐的慢肌纤维密度最高,而褐牙鲆的快肌纤维密度最高,这种差异可能与快、慢肌纤维的功能有关系。Martinez等(2003)研究证明,硬骨鱼骨骼肌可为不同游泳速度和持续时间提供动力。慢肌收缩慢,依靠有氧代谢来支持活动,它含有高水平的线粒体以提供稳定和持续的动力;相比之下,快肌收缩速度快、线粒体含量低、爆发力强,更依靠无氧代谢来支持快速游动(Pinte et al, 2021)。Jobling等(1993)研究发现,鱼类在有氧运动训练中主要运用慢肌有氧代谢供能,进行无氧运动训练后主要动用快肌供能。本研究以鲐为代表的持续式游泳习性鱼类所进行的长距离洄游需要有氧运动,因此,鲐的慢肌纤维密度高可能是为了适应这一游泳习性。而以褐牙鲆为代表的爆发式游泳习性鱼类在捕食和避敌中进行的爆发高速的游泳活动则需要无氧运动(李秀明, 2013),因此,褐牙鲆快肌纤维密度高。
总之,不同种类的鱼类在不同环境和生态条件下适应不同的游泳习性,因此,在肌肉结构和功能方面也会有所差异。这些差异可能是为了更好地适应其生存环境和生活习性而发生的适应性进化。同时,不同种类的鱼类肌肉对不同种类的运动负荷也有不同的适应反应,这也可能会导致肌纤维形态和密度的差异。因此,鱼类肌纤维形态、直径和密度的差异与其游泳习性密切相关。此外,不同物种甚至不同营养水平和环境因素等也可能影响肌纤维特征。比如,野生花鲈(Lateolabrax maculatus)的肌纤维密度高于养殖花鲈(Periago et al, 2005);饥饿导致团头鲂(Megalobrama amblycephala)的红、白肌纤维细胞小型化,饥饿后再投喂可以使肌纤维细胞加快肥大(陈丽萍, 2013);缺氧环境也会使鱼类的肌纤维直径增长缓慢(Kiessling et al, 1991)。另外,Johnston等(2000)对大西洋鲑(Salmo salar)研究发现,快肌纤维的直径与运动存在相关性,即运动会增加肌纤维的直径。孙文波等(2023)也指出,禾花鲤(Cyprinuscarpio var. Quanzhounensis)的运动强度大,可引起肌纤维密度变大。因此,本研究结果可为进一步开展硬骨鱼的骨骼肌适应性进化和运动生理学等研究提供重要参考。
AKOLKAR D B, KINOSHITA S, YASMIN L, et al. Fibre type-specific expression patterns of myosin heavy chain genes in adult torafugu Takifugu rubripes muscles. Journal of Experimental Biology, 2010, 213(1): 137-145 DOI:10.1242/jeb.030759 |
BAI W L, WANG W, HE P, et al. Type and motor end plate distribution of canine thyroarytenoid muscle. Journal of China Medical University, 2002, 31(6): 426-427 [白伟良, 王玮, 何平, 等. 犬甲杓肌肌纤维型及其运动终板分布的研究. 中国医科大学学报, 2002, 31(6): 426-427 DOI:10.3969/j.issn.0258-4646.2002.06.009] |
BEAMISH F W H. Swimming capacity. Fish Physiology, 1978(7): 101-187 |
BONE Q. 6-Locomotor muscle. Fish Physiology, 1978(7): 361-424 |
BONE Q. On the function of the two types of myotomal muscle fibre in elasmobranch fish. Journal of the Marine Biological Association of the United Kingdom, 1966, 46(2): 321-349 DOI:10.1017/S0025315400027168 |
BRETT J R. The respiratory metabolism and swimming performance of young sockeye salmon. Journal of the Fisheries Board of Canada, 1964, 21(5): 1183-1226 DOI:10.1139/f64-103 |
BRIAN LANGERHANS R, REZNICK D N. Ecology and evolution of swimming performance in fishes: Predicting evolution with biomechanics. In: DOMENICI P, KAPOOR B G. Fish locomotion: An eco-ethological perspective. CRC Press, 2010, 212–260
|
CEDIEL R A, BLOB R W, SCHRANK G D, et al. Muscle fiber type distribution in climbing Hawaiian gobioid fishes: Ontogeny and correlations with locomotor performance. Zoology, 2008, 111(2): 114-122 DOI:10.1016/j.zool.2007.06.004 |
CHEN K W, LI H F, ZHANG X Y, et al. Study on the relation between muscle fiber and meat quality in Broilers. Chinese Journal of Animal Science, 2002, 38(6): 6-7 [陈宽维, 李慧芳, 张学余, 等. 肉鸡肌纤维与肉质关系研究. 中国畜牧杂志, 2002, 38(6): 6-7] |
CHEN L P. Expression of MRFs and muscle growth during starvation and refeeding in Megalobrama amblycephala. Masterxs Thesis of Huazhong Agricultural University, 2013 [陈丽萍. 饥饿再投喂对团头鲂幼鱼生肌调节因子表达和肌肉特性的影响. 华中农业大学硕士研究生学位论文, 2013]
|
CUI C Y. A comparative study on the histological characteristics of muscle fibers of Du Chang Bai outside the ternary pigs with different age. Masterxs Thesis of Yanbian University, 2017 [崔长艳. 不同日龄杜长白外三元猪肌纤维组织学特性比较研究. 延边大学硕士研究生学位论文, 2017]
|
DRAZEN J C, DUGAN B, FRIEDMAN J R. Red muscle proportions and enzyme activities in deep-sea demersal fishes. Journal of Fish Biology, 2013, 83(6): 1592-1612 DOI:10.1111/jfb.12268 |
GUAN W J, ZHU Y F, CHEN Z D. Muscle quality in fish related to characteristics of muscular fibers. Fisheries Science, 2008, 27(2): 101-104 [关文静, 朱艺峰, 陈芝丹. 鱼类肌纤维特性与鱼肉品质关系. 水产科学, 2008, 27(2): 101-104 DOI:10.16378/j.cnki.1003-1111.2008.02.002] |
HANDELSMAN C, CLAIREAUX G, NELSON J A. Swimming ability and ecological performance of cultured and wild European sea bass (Dicentrarchus labrax) in coastal tidal ponds. Physiological and Biochemical Zoology, 2010, 83(3): 435-445 DOI:10.1086/651099 |
HISTAND M B. Mathematical biofluiddynamics (James Lighthill). SIAM Review, 1977, 19(3): 576-577 DOI:10.1137/1019092 |
JACKSON H E, INGHAM P W. Control of muscle fibre-type diversity during embryonic development: The zebrafish paradigm. Mechanisms of Development, 2013, 130(9/10): 447-457 |
JIAO S, TAN X G, SUI Y L, et al. Muscle fibre type composition in the lateral muscle of olive flounder Paralichthys olivaceus. Acta Histochemica, 2019, 121(1): 1-6 DOI:10.1016/j.acthis.2018.10.002 |
JOBLING M, JØRGENSEN E H, ARNESEN A M, et al. Feeding, growth and environmental requirements of Arctic charr: A review of aquaculture potential. Aquaculture International, 1993, 1(1): 20-46 DOI:10.1007/BF00692662 |
JOHNSTON I A, ALDERSON R, SANDHAM C, et al. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture, 2000, 189(3/4): 335-349 |
KIESSLING A, RUOHONEN K, BJØRNEVIK M. Muscle fibre growth and quality in fish. Archiv fur Tierzucht, 2006, 49 |
KIESSLING A, STOREBAKKEN T, ÅSGÅRD T, et al. Changes in the structure and function of the epaxial muscle of rainbow trout (Oncorhynchus mykiss) in relation to ration and age: Ⅰ. Growth dynamics. Aquaculture, 1991, 93(4): 335-356 |
LI B X, WEI Y L, LIANG M Q, et al. The effects of fish protein hydrolysate on the growth, body composition and morphological structure of muscle fiber of turbot (Scophthalmus maximus L.). Progress in Fishery Sciences, 2019, 40(5): 155-165 [李本相, 卫育良, 梁萌青, 等. 水解鱼蛋白对大菱鲆生长、体组成及肌纤维组织形态结构的影响. 渔业科学进展, 2019, 40(5): 155-165] |
LI X M. The effect and mechanism of exercise training on growth performance in juvenile Spinibarbus sinensis. Doctoral Dissertation of Southwest University, 2013 [李秀明. 运动训练对中华倒刺鲃幼鱼生长的影响及其机理研究. 西南大学博士研究生学位论文, 2013]
|
LIANG T Y, WU J P, LIU T, et al. Recent progress in classification and transformation mechanism of muscle fiber types. Meat Research, 2018, 32(9): 55-61 [梁婷玉, 吴建平, 刘婷, 等. 肌纤维类型分类及转化机理研究进展. 肉类研究, 2018, 32(9): 55-61] |
LIANG X Y. The comparative study on the muscle fibre in different ploidy fishes. Masterxs Thesis of Hunan Normal University, 2011 [梁向阳. 不同倍性鱼骨骼肌肌纤维的比较研究. 湖南师范大学硕士研究生学位论文, 2011]
|
LIU X L. Classification of muscle fiber and molecular cloning and expression of the sMyHC1 gene from Siniperca chuatsi. Masterxs Thesis of Guangxi Normal University, 2013 [刘希良. 翘嘴鳜肌纤维初步分型及其红肌sMyHC1基因克隆、表达分析. 广西师范大学硕士研究生学位论文, 2013]
|
MARTINEZ M, GUDERLEY H, DUTIL J D, et al. Condition, prolonged swimming performance and muscle metabolic capacities of cod Gadus morhua. Journal of Experimental Biology, 2003, 206(3): 503-511 DOI:10.1242/jeb.00098 |
MOSS F P, LEBLOND C P. Satellite cells as the source of nuclei in muscles of growing rats. Anatomical Record, 1971, 170(4): 421-435 DOI:10.1002/ar.1091700405 |
PERIAGO M J, AYALA M D, LóPEZ-ALBORS O, et al. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture, 2005, 249 |
PINTE, N, COUBRIS C, JONES E, et al. Red and white muscle proportions and enzyme activities in mesopelagic sharks. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2021, 256: 110649 |
ROWLERSON A, VEGGETTI A. Cellular mechanisms of post- embryonic muscle growth in aquaculture species. Fish Physiology, 2001, 18: 103-140 |
SHEN Y X, XU J C. The relationship between the muscle tissue characteristics of Jinhua swine and its hybrid and its meat quality. Acta Agriculturae Universitatis Zhejiangensis, 1984, 10(3): 265-272 [沈元新, 徐继初. 金华猪及其杂种肌肉组织学特性与肉质的关系. 浙江大学学报, 1984, 10(3): 265-272] |
SUN W B, ZHOU M R, HOU M D, et al. Comparing the effects of pond and rice field culture methods on muscle quality of rice flower carp. Progress in Fishery Sciences, 2023, 44(2): 196-204 [孙文波, 周明瑞, 侯梦丹, 等. 稻田和池塘养殖禾花鲤肌肉营养与品质分析. 渔业科学进展, 2023, 44(2): 196-204] |
WANG L. Effects and regulatory mechanisms of dietary selenium on fish muscle growth. Doctoral Dissertation of Huazhong Agricultural University, 2020 [王力. 日粮硒对鱼类肌肉生长的调控作用及其机制研究. 华中农业大学博士研究生学位论文, 2020]
|
WU M P, CHANG N C, CHUNG C L, et al. Analysis of titin in red and white muscles: Crucial role on muscle contractions using a fish model. BioMed Research International, 2018, 2018: 5816875 |
XIE X X, MENG Q X, REN L P, et al. Study on characteristics of muscle fiber from six breeds of cattle in China. Journal of China Agricultural University, 2011, 16(1): 66-72 [解祥学, 孟庆翔, 任丽萍, 等. 我国6个肉牛品种肌肉纤维特征研究. 中国农业大学学报, 2011, 16(1): 66-72] |
YIN L Q, SU L, JIN Y. Relationship between fish muscle fiber characteristics and meat quality and its regulation technology. The Food Industry, 2015, 36(2): 231-234 [尹丽卿, 苏琳, 靳烨. 肌纤维特性与鱼肉品质的关系及其调控技术的研究进展. 食品工业, 2015, 36(2): 231-234] |
ZHANG G Q, CHU W Y, HU S N, et al. Identification and analysis of muscle-related protein isoforms expressed in the white muscle of the mandarin fish (Siniperca chuatsi). Marine Biotechnology, 2011, 13: 151-162 |
ZHANG L, ZHAO L K, GAO J, et al. Effects of training with different loading on the muscle fiber types and moter end-plates of gastrocnemius muscle in mouse. Journal of China Medical University, 1992, 21(1): 1-4 [张霖, 赵连科, 高杰, 等. 不同负荷训练对小白鼠腓肠肌内侧头肌纤维型和运动终板的影响. 中国医科大学学报, 1992, 21(1): 1-4] |
ZHU Q, ZHAO J L, CHANG J J, et al. Comparison on characterization of fast-twitch and slow-twitch skeletal muscles of mandarin fish Siniperca chuatsi. Journal of Shanghai Ocean University, 2011, 20(4): 488-493 [朱琼, 赵金良, 苌建菊, 等. 鳜骨骼肌快肌和慢肌的组成特征比较. 上海海洋大学学报, 2011, 20(4): 488-493] |



