2. 自然资源部海洋生态保护与修复重点实验室 自然资源部第三海洋研究所 福建 厦门 361005;
3. 厦门海洋职业技术学院 福建 厦门 361100;
4. 广西海洋科学院(广西红树林研究中心) 广西红树林保护与利用重点实验室 广西 北海 536000
2. Key Laboratory of Marine Ecological Conservation and Restoration, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen 361005, China;
3. Faculty of Marine Biology, Xiamen Ocean Vocational College, Xiamen 361100, China;
4. Guangxi Key Laboratory of Mangrove Conservation and Utilization, Guangxi Academy of Marine Sciences Guangxi Mangrove Research Centre), Beihai 536000, China
海草(Seagrass)是一类生活在热带至温带浅海、完全适应海洋环境的沉水被子植物的总称(Unsworth et al, 2022; 张沛东等, 2020)。海草生态系统作为连接珊瑚礁与红树林生态系统的纽带,在改善水质、提供育幼场、保护海岸带、提供蓝碳、缓解气候变化等方面有着重要贡献(Macreadie et al, 2019; Du et al, 2020a、b),海草也为鱼类、海龟、儒艮等海洋生物直接或间接提供食物来源(黄小平等, 2006; Du et al, 2016、2019)。但是,自20世纪80年代以来,由于全球气候变化以及区域性围填海、破坏性挖捕、水体富营养化等人为活动影响,全球海草面积减少约29%,并正以每年1%~7%的速度继续减少(Waycott et al, 2009; Unsworth et al, 2022)。与此同时,我国海草床也面临着不断退化的风险(郑凤英等, 2013; Hu et al, 2021)。截至2020年,我国海草床总面积约为2.6×104 hm2,近岸海域超过80%的海草床已经消失(于硕等, 2022)。以海南岛为例,2009—2019年期间,黎安港海草床面积减少约114 hm2,占黎安港海草床总面积的55% (吴钟解等, 2014; 陈石泉等, 2020);2016—2020年新村港海草床面积减少约79 hm2,约占新村港海草床总面积的16% (Li et al, 2022)。此外,海南岛其他海草床的面积、盖度、密度、生物量也有所降低。分布在中国北方的温带海草床,其面积、种类、生物量等也存在着不同程度的下降(刘伟妍等, 2017)。
为了缓解海草床面临的威胁,了解海草的繁殖特性并积极开展海草床保护修复工作尤为重要(Du et al, 2023)。海草的繁殖方式分为无性繁殖与有性繁殖,其中,无性繁殖主要以克隆生长的方式(张沛东等, 2020),有性繁殖则包括了植株开花、传粉、受精、种子发育等过程,少部分海草还具有胎生和假胎生行为(Kuo et al, 1990; Ballesteros et al, 2005)。当前对海草床的修复大多基于其克隆生长的繁殖模式,虽然这种方式生育成本低、个体存活率高,但易造成海草遗传多样性降低,一旦环境超过某种优势海草的抵抗阈值,便易造成海草床大面积的退化。相比之下,基于海草有性繁殖开展的修复工作能够维持海草床种群遗传多样性、建立当地新的海草斑块,基于此构建的土壤种子库也有利于海草床干扰后快速恢复(邱广龙等, 2022)。成花过程是海草有性繁殖的关键阶段,包括成花诱导、花发育和开花3个过程(周琴等, 2018)。其中,成花诱导是海草从营养生长向生殖生长过渡的重要环节,也是成花过程中最关键的步骤(宋杨等, 2014)。当前对海草成花诱导的机制与影响因素大多停留在野外观测与记录阶段,但基于有性繁殖的海草床修复方式已被公认为是较好的海草床修复方式。本文综述了近年来国内外关于海草成花记录与研究的相关文献,概述了影响海草成花的环境因素,拟为海草床监测、评估与修复提供理论参考。
1 海草有性繁殖特点海草的有性繁殖具有以下几个特点:(1)有性繁殖具有策略性:海草为雌雄同株或雌雄异株(表 1) (Ackerman, 2007)。Cox等(1988)调查发现,龟裂泰来草(Thalassia testudinum)雄花与雌花比例为60∶1,这可能是因为在海洋环境中,花粉易因接收不足而产生花粉限制,以及产生雄花所需能量远低于产生雌花和果实的能量(van Tussenbroek et al, 2010、2016a)。海草的雌雄性器官成熟的先后不同,海草可以通过这种方式促进异交,保证基因的多样性(Ruckelshaus, 1995; Ackerman, 2007; Entrambasaguas et al, 2017)。(2)授粉方式独特:与陆生植物不同,海草的花小且构造上大多退化(柯智仁, 2004)。部分海草产丝状花粉,这种花粉形态更有利于在海水动态流动的状态情况下进行水上或水下授粉(表 1) (Cox, 1983; Ackerman, 2007)。此外,花粉的释放、运输、捕获受到水流与海草冠层之间相互作用的影响(Ackerman, 2002)。之前观点普遍认为,海草授粉仅通过海水流动进行,海洋生物不参与海草的授粉过程,但van Tussenbroek等(2016b)通过室内实验发现,在夜间没有水流的情况下,无脊椎动物在花朵附近觅食时,黏性的花粉粒会附着在无脊椎动物身上,当其靠近雌花柱头时被捕获并成功授粉。(3)海草植株的成熟时间较长:海草具有一年生与多年生的生长形式,不同的生长形式对海草群落的生物多样性与遗传多样性具有重要的贡献(邱广龙等, 2022)。在对菲律宾的海草床调查中发现,圆叶丝粉草(Cymodocea rotundata)与泰来草(Thalassia hemprichii)开花率分别为5.6%和17.0%,且开花株年龄皆为0.5年以上(Duarte et al, 1997),而鳗草(Zostera marina)的开花株年龄均为1年以上(Duarte et al, 1997; Blok et al, 2018)。(4)受多因素影响:海草有性生殖行为不仅受单一因素影响,而是多种因素共同作用的结果。研究表明,温度(McMillan, 1980; Qin et al, 2020a)、光照(Collier et al, 2012; Olesen et al, 2017)、盐度(Fernández et al, 1999; Ankel et al, 2021)、水深(Cox, 1988; Tongkok et al, 2017)、营养盐(Smith et al, 2016; Jackson et al, 2017)等相关因素均会对海草有性生殖造成影响。
|
|
表 1 不同属海草有性生殖特性 Tab.1 Selected reproductive characters in seagrass genera |
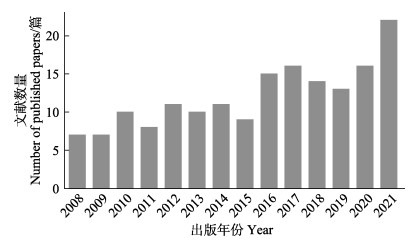
本研究基于文献计量手段对海草成花研究发展趋势进行了分析与归纳总结。研究选择了美国科学信息研究所(Institute for Scientific Information, ISI)的Web of Science核心合集的SCIE数据库为检索源,选择已发表的国际期刊作为研究对象。以“seagrass flowering”作为检索关键词,共检索出相关文献300篇。从2008—2021逐年文献发表数量上看,海草成花相关文献整体呈增长趋势,并在近年来逐渐得到更多的关注(图 1)。
|
图 1 在Web of science检索2008—2021年关键词“seagrass flowering”文献数量 Fig.1 Number of literatures searched for the keyword "seagrass flowering" from 2008 to 2021 in Web of Science |
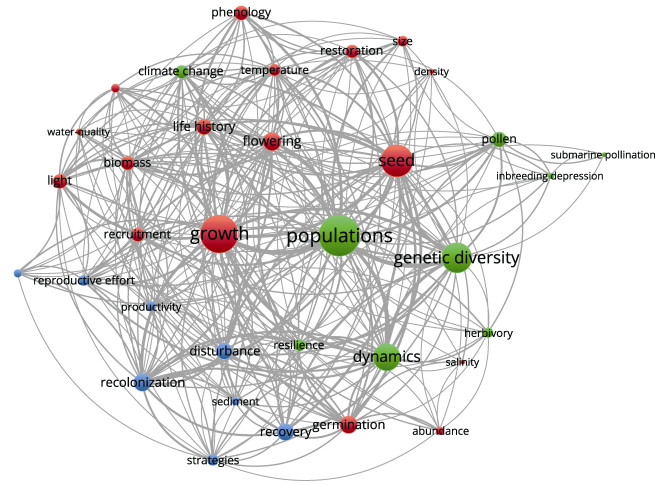
高频关键词反映了该研究领域的热点主题与发展方向。利用VOSviewer (Version 1.6.18)软件中的聚类算法对高频关键词进行统计和共现分析,并构建共现网络(图 2)。结果显示,1991—2022年关于海草有性繁殖研究的高频关键词依次包括种群、发育、种子、遗传多样性、动力学、成花和发芽等。对出现频率 > 3的关键词(共有204个)进行共现分析,通过关键词共现关系聚类,近32年来,海草有性繁殖领域的研究热点主题可归纳为相关因子研究、种群遗传研究、种群保护研究。3个研究主题之间存在交叉联系。
|
图 2 1991—2022年海草有性繁殖研究的主要关键词及其共现关系 Fig.2 Main keywords and their co-occurrence in the seagrass sexual reproduction research in 1991–2022 红色为相关因子研究,绿色为种群遗传研究,蓝色为种群保护研究;字号与圆圈大小表示关键词的共现强度。 Red is the study of relevant factors, green is the study of population genetics, and blue is the study of population protection. Word size and circle size indicate the co-occurrence strength of keyword. |
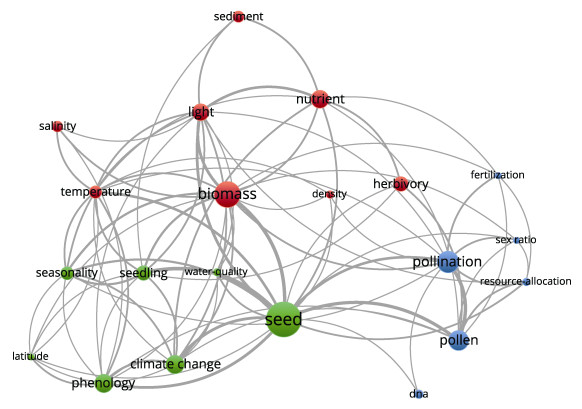
本研究对海草成花研究热点进一步进行筛选,并对高频关键词进行统计和共现分析,并构建共现网络(图 3)。结果显示,从1991—2022年关于海草成花研究的高频关键词依次包括种子、生物量、授粉、花粉、气候变化、物候、营养物、光、食草活动、幼苗、季节性、温度、沉积物和盐度等。对出现频率 > 3的关键词(共有250个)进行共现分析,通过关键词共现关系聚类,近32年来海草成花领域的研究热点主题可归纳为以下3个主题:(1)成花条件研究:主要关键词包括温度、光照、盐度、沉积物、营养盐、密度、生物量等;(2)种子发育研究:主要关键词包括种子、物候学、气候变化、水质、纬度等;(3)海草授粉研究:主要关键词包括授粉、花粉、DNA、雌雄比例、资源分配等。
|
图 3 1991—2022年海草成花研究的主要关键词及其共现关系 Fig.3 Main keywords and their co-occurrence in the seagrass flowering research in 1991-2022 红色为成花条件研究,绿色为种子发育研究,蓝色为海草授粉研究;字号与圆圈大小表示关键词的共现强度。 Red is the study of flowering conditions, green is the study of seed development, and blue is the study of seagrass pollination; word size and circle size indicate the co-occurrence strength of keyword. |
尽管当前关于高等植物的成花诱因的研究多集中于模式生物与经济作物上,对海草的关注度相对较少,但已有相关研究表明,海草中同样存在诱导其成花的相关因子。这些因子主要分为两个方面,一方面主要与生物自身遗传、生理特性等因素相关,另一方面则主要与非生物因素(如温度、盐度、光照等)间接调控有关(Diaz-Almela et al, 2016)。
3.1 温度海草对温度的适应性较强,除了北冰洋沿岸外,全球几乎所有海岸都有海草的分布(Short et al, 2007)。热带海草的最适温度为23~32 ℃,温带海草的最适温度约为12~26 ℃ (林显程等, 2019)。全球气候变化可能通过影响海草成花而影响海草的物候学(Diaz-almela et al, 2007)。当海水温度升高时,海草可能通过减少碳储备、降低有性繁殖的强度,以应对热胁迫造成的生理反应,过高的温度可能导致分生组织缺氧而造成植物死亡,从而影响越冬种群的规模,并对下一年海草种群的开花时间、强度、规模产生影响(Greve et al, 2003; Qin et al, 2020b)。如泰来草的成花与低温表现出显著相关性(McMillan, 1980; 许战洲等, 2008),这种低温促使植物开花的现象成为春化作用,春化途径也是一些植物诱导开花的途径之一(孙昌辉等, 2007; 刘永平等, 2015)。但不同种类的海草对温度的响应不同。对大洋波喜荡草(Posidonia oceanica)研究发现,其开花率、开花强度与全年最高海表温存在显著正相关关系(Diaz-Almela et al, 2007)。Ruiz等(2018)在室内人工环境下对大洋波喜荡草(Posidonia oceanica)进行了热暴露实验,同样表明热暴露是大洋波喜荡草成花的主要诱因。因此,温度的升高或降低都可能诱导海草成花,作者通过收集我国不同种类海草成花温度数据(表 2),推测低温可能是海草受到春化作用而诱导成花,但高温可能是通过环境压力导致海草受迫,从而诱导成花。
|
|
表 2 我国不同种类海草成花温度 Tab.2 Flowering temperature of different seagrass in China |
海草为沉水性植物,除部分浅海海草床在潮汐过程中出现短时间裸露外,大部分种类海草终生沉水生活。光照强度的变化一方面直接影响海草植株的形态特征、元素含量、生物量等(符妙等, 2022);另一方面海草可以通过感知光照时间的周期变化调节自身新陈代谢及生理过程,如植株生长发育、固碳能力、相关基因表达等(Collier et al, 2012; Trevathan-Tackett et al, 2018; Wong et al, 2020; Ruocco et al, 2022)。Wong等(2020)对鳗草海草床进行人为遮阴,遮阴后的鳗草通过改变植株形态、降低密度以维持低光照下的碳平衡。对有性繁殖而言,光照强度不足减少了海草对有性繁殖的投入,限制海草繁殖芽的诱导,导致海草的营养枝与开花枝的密度下降(Olesen et al, 2017; Qin et al, 2020b)。McMillan(1982)曾在实验室连续光照的环境下观察到多个属的海草开花,表明海草可能是长日照植物,即在长日照条件下可以促进开花,短日照则会抑制开花。海草的有性繁殖整体呈季节性,在一年四季中,夏季的日照时间长、温度高,较高温度和较高光照强度是海草进入有性繁殖的诱因,这也与温带海草多集中在夏季开花相符合(Ramage et al, 1998),但同时将温度与光照作为变量对海草有性繁殖的研究较少。因此,未来对海草有性繁殖诱因研究过程中,应将温度与光强、光周期、光质等光照条件综合探讨。
3.3 盐度海草长期生活在近岸海域,盐度的耐受范围为5~35 (Nejrup et al, 2008)。盐度通过影响海草细胞渗透压而影响海草的生理生化结构(杨冉, 2015)。对混合种海草床而言,盐度的升高与降低对不同种类海草的生长动态产生影响,甚至可能会发生物种演替(Qiu et al, 2017)。关于盐度对海草成花的影响,相关文章记载较少且说法不一。McMillan (1980)研究表明,齿叶丝粉草(Cymodocea serrulata)的开花量随着盐度降低至25而增加。Ramage(1998)则认为,盐度下降会导致海草无法繁殖。此外也有研究表明,诺氏鳗草(Zostera noltii)在低盐度与高盐度情况下表现出较为相似的物候学(Ankel et al, 2021)。这表明盐度对海草成花的影响并不直接,可能通过对相关酶活性、光合速率等因子对海草产生影响(Fernández et al, 1999)。对多数海草种类而言,降低盐度可以提高海草种子的萌发率并缩短萌发历期(韦梅球, 2017),故在受到低盐度胁迫时,海草植株可能更倾向于增加有性繁殖的投入。对不同种类、地域的海草在受到盐度胁迫响应是否一致仍然需要进一步研究。
3.4 水深与潮汐海草一般栖息于潮下带6 m以上的近岸浅水区,最深也可分布至90 m,分布深度主要受到光照的影响(Duarte, 1991)。浅层海草与较深层海草的有性繁殖策略相似,但浅层海草开花芽发育早于较深层海草,可能通过这种方式影响种群内的基因交流与遗传结构(von Staats et al, 2020)。潮汐导致的深度变化会影响授粉的成功与否。对部分海草而言,雄花花粉释放后浮于水面,借助水流在水面上漂浮运输,花粉与同样浮于水面雌花柱头完成授粉过程,此时水深若大于雌花花梗长度,雌花将无法浮于水面从而影响授粉过程(Rollón et al, 2003)。Cox等(1988、1991)研究发现,全楔草(Thalassodendron ciliatum)与龟裂泰来草的开花与当地的极低潮有关,这可能是潮间带海草长期适应的结果,通过在低潮时期海草密集开花,更多的花粉与浮于水面的雌花授粉,通过低潮将立体授粉过程转为平面授粉过程,以提高授粉效率(Cox, 1983)。但潮汐在海草有性繁殖过程中可能并不起主导作用,可能通过改变水深影响光照与温度并对海草产生刺激。Tongkok (2017)研究发现,处于花期的泰来草对不同潮位、不同时间段的响应不同,在低潮位的夜间,泰来草的花开得最盛。
3.5 营养盐海草生长于近岸,土地肥料使用与养殖尾水的排放易造成近岸海草床底质有机物富集。相关研究发现,在沉积物营养富集区域的鳗草的开花枝、开花枝高度、子房数量等都随着沉积物营养盐的富集而增加,较高的生殖枝能获得来自更远的花粉,一定程度上避免了近亲杂交,而子房数量则是直接影响海草种子产量(Jackson et al, 2017; Johnson et al, 2017)。营养盐过多富集还可能导致底质缺氧,产生有毒物质,并对海草床系统造成生存压力,通过胁迫诱导海草开花(Smith et al, 2016; Guerrero-Meseguer et al, 2021)。而水体的营养盐富集引发的大型海藻与附生藻类大量繁殖,使得海草可利用光能大幅度减少,从而对有性繁殖产生抑制(黄驰等, 2017; 刘伟妍等, 2017)。
3.6 其他除以上主要环境因素外,地理位置(如纬度)也会对海草成花有一定影响。研究表明,广泛分布于不同纬度的温带海草鳗草,其开花物候与繁殖策略略有不同(Qin et al, 2020b)。未来海洋变暖一方面可能导致温带海草物种北移,另一方面可能导致海草有性繁殖提前,增强高纬度地区海草的有性繁殖能力(Blok et al, 2018; Wang et al, 2022)。此外,近年来一些研究也表明,海草具有逆境诱导开花的现象,即在受到胁迫时提前开花,通过调节自身生长与发育应对外界环境变化(张敏等, 2016)。如日本鳗草(Zostera japonica)在沉积物掩埋的干扰下,有性繁殖率随着掩埋程度的增加呈先上升后下降的趋势(Henderson et al, 2015)。Lekammudiyanse等(2022)研究也表明,修剪至1 cm的牟氏鳗草(Zostera muelleri)的开花率显著高于修剪至3 cm牟氏鳗草的开花率,这些结果均表明,在逆境条件下,海草会将个体更多资源分配给有性繁殖以抵御环境干扰。
4 海草有性繁殖在海草床修复与保护方面的应用海草床修复最早记录于1947年,随着近年来海草床持续退化以及人们对海草床重要性认识的深入,海草床的修复与保护逐渐成为关注的热点(陈石泉等, 2021)。海草床修复方式可分为3种:生境恢复法、种子法和植株移植法(张剑, 2022)。其中,种子法被认为是成本低、破坏少,并能够维持遗传多样性的修复方式(于硕等, 2019)。但现阶段仍然面临种子收集难度大、数量不稳定等问题(韦梅球, 2017)。明确海草有性繁殖的成花诱因、准确判断成花时间,对使用种子法进行海草修复具有重要意义。
水生植物形成新种群更易依赖单一基因型的克隆生殖(Silvertown, 2008)。当一个特定基因型的海草非常适合当地的环境,那么可能会更多选择通过克隆生殖来扩大种群量,减少有性繁殖,降低可能改变关键性状的风险;不适应当地环境的基因型则可能会通过有性繁殖产生新性状以提高适应能力(Henderson et al, 2015)。海草大多为雌雄异株,广泛的无性繁殖可能构成较多单性海草种群,这可能也是众多野外观察中果实与种子数量较少的原因(Rasheed, 2004)。海草在适宜的环境下进行大规模克隆生殖,意味着其面对胁迫时的承受能力较低,对大面积海草床而言,可以将基因型作为一项评估指标。
海草在生长过程中需要将有限的资源分配在生长、繁殖、抵御环境变化等方面,以保证其在当前环境下拥有适应能力(Rasheed, 2004)。海草床有性繁殖情况可能是对当地海草床压力状况的一种反映,长期处于高资源可利用地区的海草个体相较于低资源可利用地区的个体更可能将自身大部分能量用于海草有性繁殖(Johnson et al, 2017)。然而,现阶段关于逆境对海草有性繁殖的研究较少,在海草床长期监测过程中也未将其应用,建议未来在对海草床监测中可将有性繁殖率与一些受迫指标共同分析,更加全面对海草床健康状况进行评估。
5 总结与展望近年来,国外研究者对海草有性繁殖关注度在逐步提升,我国关于海草研究起步较晚,对海草有性繁殖行为关注较低。本文整理了国内外海草成花相关文献,列出6项直接或间接影响海草成花的因素,认为海草响应外界环境刺激与内源信号启动开花途径可能集中在光周期途径、春化途径和自发途径上。但海草不同种类、不同种群间有性繁殖差异较大,现阶段对海草成花诱因与机理的认识仍不充分。
针对目前国内外海草有性繁殖成花诱因研究存在的主要问题,建议在以下几方面开展重点工作:(1)国内外对海草有性繁殖研究较少,野外海草的有性繁殖行为可能比目前认为的更常见,明确主要海草床集中开花时间对研究海草成花诱因、保护海草种子库等有促进作用;(2)开展海草成花诱因室内相关模拟实验,明确海草成花诱因,为室外海草有性生殖行为提供参考依据;(3)海草在逆境环境下进行有性繁殖的行为可用于指示气候与环境的变化,但现有的调查研究对海草有性繁殖的关注度较低,也未将有性繁殖率作为一项长期监测指标,建议在海草床长期监测、评估健康时将有性繁殖率与环境因子相结合,共同评估海草床状况。
ACKERMAN J D. Diffusivity in a marine macrophyte canopy: Implications for submarine pollination and dispersal. American Journal of Botany, 2002, 89(7): 1119-1127 DOI:10.3732/ajb.89.7.1119 |
ACKERMAN J D. Sexual reproduction of seagrasses: Pollination in the marine context. Seagrasses: Biology, Ecology and Conservation, Springer Netherlands, 2007, 89-109 |
ANKEL M, RUBAL M, VEIGA P, et al. Reproductive cycle of the seagrass Zostera noltei in the Ria de Aveiro Lagoon. Plants, 2021, 10(11): 2286 DOI:10.3390/plants10112286 |
BALLESTEROS E, CEBRIAN E, GARCIA-RUBIES A, et al. Pseudovivipary, a new form of asexual reproduction in the seagrass Posidonia oceanica. Botanica Marina, 2005, 48(2): 175-177 |
BLOK S E, OLESEN B, KRAUSE-JENSEN D. Life history events of eelgrass Zostera marina L. populations across gradients of latitude and temperature. Marine Ecology Progress Series, 2018, 590: 79-93 |
CHEN S Q, CAI Z F, SHEN J, et al. Restoration effect and influencing factors of seagrass bed in Gaolong Bay, Hainan. Journal of Applied Oceanography, 2021, 40(1): 65-73 [陈石泉, 蔡泽富, 沈捷, 等. 海南高隆湾海草床修复成效及影响因素. 应用海洋学学报, 2021, 40(1): 65-73] |
CHEN S Q, PANG Q Z, CAI Z F, et al. Analysis of distribution characteristics, health status, and influencing factors of seagrass bed in Li'an Lagoon, Hainan Island. Marine Sciences, 2020, 44(11): 57-64 [陈石泉, 庞巧珠, 蔡泽富, 等. 海南黎安港海草床分布特征、健康状况及影响因素分析. 海洋科学, 2020, 44(11): 57-64] |
COLLIER C J, WAYCOTT M, OSPINA A G. Responses of four Indo-West Pacific seagrass species to shading. Marine Pollution Bulletin, 2012, 65(4/5/6/7/8/9): 342-354 |
COX P A, TOMLINSON P B. Pollination ecology of a seagrass, Thalassia testudinum (Hydrocharitaceae), in St. Croix. American Journal of Botany, 1988, 75(7): 958-965 DOI:10.1002/j.1537-2197.1988.tb08800.x |
COX P A. Hydrophilous pollination of a dioecious seagrass, Thalassodendron ciliatum (Cymodoceaceae) in Kenya. Biotropica, 1991, 159-165 |
COX P A. Search theory, random motion, and the convergent evolution of pollen and spore morphology in aquatic plants. The American Naturalist, 1983, 121(1): 9-31 DOI:10.1086/284037 |
DIAZ-ALMELA E, MARBÀ N, ÁLVAREZ E, et al. Patterns of seagrass (Posidonia oceanica) flowering in the Western Mediterranean. Marine Biology, 2006, 148(4): 723-742 DOI:10.1007/s00227-005-0127-x |
DIAZ-ALMELA E, MARBA N, DUARTE C M. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Global Change Biology, 2007, 13(1): 224-235 DOI:10.1111/j.1365-2486.2006.01260.x |
DU J G, CHEN B, NAGELKERKEN I, et al. Protect seagrass meadows in China's waters. Science, 2023, 379(6631): 447 |
DU J G, CHEN Z H, XIE M L, et al. Analysis of organic carbon sources in tropical seagrass fish: A case study of the east coast of Hainan Province. Marine Biology Research, 2019, 15(8/9): 513-522 |
DU J G, HU W J, NAGELKERKEN I, et al. Seagrass meadows provide multiple benefits to adjacent coral reefs through various microhabitat functions. Ecosystem Health and Sustainability, 2020a, 6(1): 1812433 DOI:10.1080/20964129.2020.1812433 |
DU J G, XIE M L, WANG Y Y, et al. Connectivity of fish assemblages along the mangrove-seagrass-coral reef continuum in Wenchang, China. Acta Oceanologica Sinica, 2020b, 39(8): 43-52 DOI:10.1007/s13131-019-1490-7 |
DU J G, ZHENG X Q, PERISTIWADY T, et al. Food sources and trophic structure of fishes and benthic macroinvertebrates in a tropical seagrass meadow revealed by stable isotope analysis. Marine Biology Research, 2016, 12(7): 748-757 DOI:10.1080/17451000.2016.1183791 |
DUARTE C M, URI J S, AGAWIN N S R, et al. Flowering frequency of Philippine seagrasses. Botanica Marina, 1997, 40: 497-500 |
DUARTE C M. Seagrass depth limits. Aquatic Botany, 1991, 40(4): 363-377 DOI:10.1016/0304-3770(91)90081-F |
ENTRAMBASAGUAS L, JAHNKE M, BIFFALI E, et al. Tissue-specific transcriptomic profiling provides new insights into the reproductive ecology and biology of the iconic seagrass species Posidonia oceanica. Marine Genomics, 2017, 35: 51-61 DOI:10.1016/j.margen.2017.05.006 |
FERNÁNDEZ J A, GARCÍA-SÁNCHEZ M J, FELLE H H. Physiological evidence for a proton pump and sodium exclusion mechanisms at the plasma membrane of the marine angiosperm Zostera marina L. Journal of Experimental Botany, 1999, 50(341): 1763-1768 |
FU M, DENG N, LIAO L G, et al. Effects of shading on morphological characteristics and element content of Enhalus acoroides. Journal of Tropical Biology, 2022, 13(3): 212-219 [符妙, 邓娜, 廖立国, 等. 遮光对海菖蒲形态特征及元素含量的影响. 热带生物学报, 2022, 13(3): 212-219 DOI:10.15886/j.cnki.rdswxb.2022.03.002] |
GREVE T M, BORUM J, PEDERSEN O. Meristematic oxygen variability in eelgrass (Zostera marina). Limnology and Oceanography, 2003, 48(1): 210-216 DOI:10.4319/lo.2003.48.1.0210 |
GUERRERO-MESEGUER L, VEIGA P, SAMPAIO L, et al. Sediment characteristics determine the flowering effort of Zostera noltei meadows inhabiting a human-dominated lagoon. Plants, 2021, 10(7): 1387 DOI:10.3390/plants10071387 |
HENDERSON J, HACKER S D. Buried alive: An invasive seagrass (Zostera japonica) changes its reproductive allocation in response to sediment disturbance. Marine Ecology Progress Series, 2015, 532: 123-136 DOI:10.3354/meps11335 |
HU W J, ZHANG D, CHEN B, et al. Mapping the seagrass conservation and restoration priorities: Coupling habitat suitability and anthropogenic pressures. Ecological Indicators, 2021, 129: 107960 DOI:10.1016/j.ecolind.2021.107960 |
HUANG C, ZHANG J P, JIANG Z J, et al. Nutrients uptake processes of seagrass and its competition with epiphytic algae. Journal of Fisheries Research, 2017, 39(3): 222-228 [黄驰, 张景平, 江志坚, 等. 海草对营养盐的吸收过程及其与附生藻类的竞争机制. 渔业研究, 2017, 39(3): 222-228] |
HUANG X P, HUANG L M, LI Y H, et al. Seagrass beds and its habitat thread in South China coastal areas. Chinese Science Bulletin, 2006, 51(S): 114-119 [黄小平, 黄良民, 李颖虹, 等. 华南沿海主要海草床及其生境威胁. 科学通报, 2006, 51(S): 114-119] |
JACKSON L J, FURMAN B T, PETERSON B J. Morphological response of Zostera marina reproductive shoots to fertilized porewater. Journal of Experimental Marine Biology and Ecology, 2017, 489: 1-6 DOI:10.1016/j.jembe.2017.01.002 |
JOHNSON A J, MOORE K A, ORTH R J. The influence of resource availability on flowering intensity in Zostera marina (L. ). Journal of Experimental Marine Biology and Ecology, 2017, 490: 13-22 DOI:10.1016/j.jembe.2017.02.002 |
KENYON R A, CONACHER C A, POINER I R. Seasonal growth and reproduction of Enhalus acoroides (Lf) Royle in a shallow bay in the western Gulf of Carpentaria, Australia. Marine and Freshwater Research, 1997, 48(4): 335-342 DOI:10.1071/MF96106 |
KO C J. A taxonomic and distributional study of seagrasses in Taiwan. Master´s Thesis of National Sun Yat-Sen University, 2004 [柯智仁. 台湾海草分类与分布之研究. 台湾中山大学硕士研究生学位论文, 2004]
|
KUO J, KIRKMAN H. Anatomy of viviparous seagrasses seedlings of Amphibolis and Thalassodendron and their nutrient supply. Botanica Marina, 1990, 33(1): 117-126 DOI:10.1515/botm.1990.33.1.117 |
LEE S M, LEE S Y, CHOI C I. Reproductive phenology of four Korean seagrasses, Zostera caespitosa, Z caulescens, Z. japonica and Z. marina. Ocean and Polar Research, 2005, 27(2): 125-133 DOI:10.4217/OPR.2005.27.2.125 |
LEKAMMUDIYANSE M U, SAUNDERS M I, FLINT N, et al. Simulated megaherbivore grazing as a driver of seagrass flowering. Marine Environmental Research, 2022, 179: 105698 DOI:10.1016/j.marenvres.2022.105698 |
LI Y Q, BAI J W, ZHANG L, et al. Mapping and spatial variation of seagrasses in Xincun, Hainan Province, China, based on satellite images. Remote Sensing, 2022, 14(10): 2373 DOI:10.3390/rs14102373 |
LIN X C, LING J, ZHANG Y Y, et al. Research progress on affecting factors of seagrass growth and application of omics technology. Biotechnology, 2019, 29(5): 507-511 [林显程, 凌娟, 张燕英, 等. 海草生长的影响因素及组学技术研究进展. 生物技术, 2019, 29(5): 507-511 DOI:10.16519/j.cnki.1004-311x.2019.05.0083] |
LIU W Y, HAN Q Y, TANG Y Q, et al. Review of nutrient enrichment and global warming effects on seagrasses. Chinese Journal of Ecology, 2017, 36(4): 1087-1096 [刘伟妍, 韩秋影, 唐玉琴, 等. 营养盐富集和全球温度升高对海草的影响. 生态学杂志, 2017, 36(4): 1087-1096 DOI:10.13292/j.1000-4890.201704.027] |
LIU Y P, YANG J, YANG M F. Pathways of flowering regulation in plants. Chinese Journal of Biotechnology, 2015, 31(11): 1553-1566 [刘永平, 杨静, 杨明峰. 植物开花调控途径. 生物工程学报, 2015, 31(11): 1553-1566 DOI:10.13345/j.cjb.140626] |
MACREADIE P I, ANTON A, RAVEN J A, et al. The future of blue carbon science. Nature Communications, 2019, 10(1): 1-13 DOI:10.1038/s41467-018-07882-8 |
MCMILLAN C. Flowering under controlled conditions by Cymodocea serrulata, Halophila stipulacea, Syringodium isoetifolium, Zostera capensis and Thalassia hemprichii from Kenya. Aquatic Botany, 1980, 8: 323-336 DOI:10.1016/0304-3770(80)90062-5 |
MCMILLAN C. Reproductive physiology of tropical seagrasses. Aquatic Botany, 1982, 14: 245-258 DOI:10.1016/0304-3770(82)90102-4 |
NEJRUP L B, PEDERSEN M F. Effects of salinity and water temperature on the ecological performance of Zostera marina. Aquatic Botany, 2008, 88(3): 239-246 DOI:10.1016/j.aquabot.2007.10.006 |
OLESEN B, KRAUSE-JENSEN D, CHRISTENSEN P B. Depth-related changes in reproductive strategy of a cold- temperate Zostera marina meadow. Estuaries and Coasts, 2017, 40(2): 553-563 DOI:10.1007/s12237-016-0155-4 |
QIN L Z, KIM S H, SONG H J, et al. Influence of regional water temperature variability on the flowering phenology and sexual reproduction of the seagrass Zostera marina in Korean coastal waters. Estuaries and Coasts, 2020a, 43(3): 449-462 DOI:10.1007/s12237-019-00569-3 |
QIN L Z, KIM S H, SONG H J, et al. Long-term variability in the flowering phenology and intensity of the temperate seagrass Zostera marina in response to regional sea warming. Ecological Indicators, 2020b, 119: 106821 DOI:10.1016/j.ecolind.2020.106821 |
QIU G L, QUAN J H, SU Z N, et al. Characteristics, influence factors, research methods of seagrass seed bank and its significance in seagrass bed recovery. Journal of Applied Oceanography, 2022, 41(2): 193-200 [邱广龙, 权佳惠, 苏治南, 等. 海草土壤种子库: 特征、影响因素、研究方法及其在受损海草场恢复中的作用. 应用海洋学学报, 2022, 41(2): 193-200] |
QIU G L, SHORT F T, FAN H Q, et al. Temporal variation of intertidal seagrass in southern China (2008–2014). Ocean Science Journal, 2017, 52(3): 397-410 DOI:10.1007/s12601-017-0039-y |
RAMAGE D L, SCHIEL D R. Reproduction in the seagrass Zostera novazelandica on intertidal platforms in southern New Zealand. Marine Biology, 1998, 130(3): 479-489 DOI:10.1007/s002270050268 |
RASHEED M A. Recovery and succession in a multi-species tropical seagrass meadow following experimental disturbance: The role of sexual and asexual reproduction. Journal of Experimental Marine Biology and Ecology, 2004, 310(1): 13-45 DOI:10.1016/j.jembe.2004.03.022 |
ROLLÓN R N, VAN STEVENINCK E D D R, VAN VIERSSEN W. Spatio-temporal variation in sexual reproduction of the tropical seagrass Enhalus acoroides (Lf) Royle in Cape Bolinao, NW Philippines. Aquatic Botany, 2003, 76(4): 339-354 DOI:10.1016/S0304-3770(03)00070-6 |
RUCKELSHAUS M H. Estimates of outcrossing rates and of inbreeding depression in a population of the marine angiosperm Zostera marina. Marine Biology, 1995, 123(3): 583-593 DOI:10.1007/BF00349237 |
RUIZ J M, MARÍN-GUIRAO L, GARCÍA-MUÑOZ R, et al. Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Marine Pollution Bulletin, 2018, 134: 49-54 DOI:10.1016/j.marpolbul.2017.10.037 |
RUOCCO M, JAHNKE M, SILVA J, et al. 2b-RAD genotyping of the seagrass Cymodocea nodosa along a latitudinal cline identifies candidate genes for environmental adaptation. Frontiers in Genetics, 2022, 13: 866758 DOI:10.3389/fgene.2022.866758 |
SHORT F, CARRUTHERS T, DENNISON W, et al. Global seagrass distribution and diversity: A bioregional model. Journal of Experimental Marine Biology and Ecology, 2007, 350(1/2): 3-20 |
SILBERHORN G M, DEWING S, MASON P A. Production of reproductive shoots, vegetative shoots, and seeds in populations of Ruppia maritima L. from the Chesapeake Bay, Virginia. Wetlands, 1996, 16(2): 232-239 |
SILVERTOWN J. The evolutionary maintenance of sexual reproduction: Evidence from the ecological distribution of asexual reproduction in clonal plants. International Journal of Plant Sciences, 2008, 169(1): 157-168 DOI:10.1086/523357 |
SMITH T M, YORK P H, MACREADIE P I, et al. Spatial variation in reproductive effort of a southern Australian seagrass. Marine Environmental Research, 2016, 120: 214-224 DOI:10.1016/j.marenvres.2016.08.010 |
SONG Y, DOU L D, ZHANG H J. Molecular and genetic mechanisms of control of floral induction in higher plants. Plant Physiology Journal, 2014, 50(10): 1459-1468 [宋杨, 窦连登, 张红军. 高等植物成花诱导调控的分子和遗传机制. 植物生理学报, 2014, 50(10): 1459-1468] |
SUN C H, DENG X J, FANG J, et al. An overview of flowering transition in higher plants. Hereditas, 2007, 29(10): 1182-1190 [孙昌辉, 邓晓建, 方军, 等. 高等植物开花诱导研究进展. 遗传, 2007, 29(10): 1182-1190] |
TONGKOK P, KAEWSURALIKHIT C, KERMANEE P. Reproductive organ characteristics and phenology of a seagrass Thalassia hemprichii (Ehrenberg) Ascherson in the Andaman Sea, Thailand. Taiwania, 2017, 62(2): 168-174 |
TREVATHAN-TACKETT S M, WESSEL C, CEBRIÁN J, et al. Effects of small-scale, shading-induced seagrass loss on blue carbon storage: Implications for management of degraded seagrass ecosystems. Journal of Applied Ecology, 2018, 55(3): 1351-1359 DOI:10.1111/1365-2664.13081 |
UNSWORTH R K F, CULLEN-UNSWORTH L C, JONES B L H, et al. The planetary role of seagrass conservation. Science, 2022, 377(6606): 609-613 DOI:10.1126/science.abq6923 |
VAN TUSSENBROEK B I, MONTERO M M, WONG R, et al. Pollen limitation in a dioecious seagrass: Evidence from a field experiment. Marine Ecology Progress Series, 2010, 419: 283-288 DOI:10.3354/meps08870 |
VAN TUSSENBROEK B I, SOISSONS L M, BOUMA T J, et al. Pollen limitation may be a common allee effect in marine hydrophilous plants: Implications for decline and recovery in seagrasses. Oecologia, 2016a, 182(2): 595-609 DOI:10.1007/s00442-016-3665-7 |
VAN TUSSENBROEK B I, VILLAMIL N, MÁRQUEZ- GUZMÁN J, et al. Experimental evidence of pollination in marine flowers by invertebrate fauna. Nature Communications, 2016b, 7(1): 1-6 |
VON STAATS D A, HANLEY T C, HAYS C G, et al. Intra- meadow variation in seagrass flowering phenology across depths. Estuaries and Coasts, 2021, 44(2): 325-338 DOI:10.1007/s12237-020-00814-0 |
WANG M, WANG Y, LIU G, et al. Potential distribution of seagrass meadows based on the maxent model in Chinese coastal waters. Journal of Ocean University of China, 2022, 21(5): 1351-1361 DOI:10.1007/s11802-022-5006-2 |
WAYCOTT M, DUARTE C M, CARRUTHERS T J B, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences, 2009, 106(30): 12377-12381 |
WEI M Q. Influencial factors of the storage and germination of seed of intertidal seagrass Halophila beccarii. Master´s Thesis of Guangxi University, 2017 [韦梅球. 潮间带海草贝克喜盐草种子储存与萌发影响因素的研究. 广西大学硕士研究生学位论文, 2017]
|
WILLIAMS S L. Surfgrass (Phyllospadix torreyi) reproduction: Reproductive phenology, resource allocation, and male rarity. Ecology, 1995, 76(6): 1953-1970 |
WONG M C, GRIFFITHS G, VERCAEMER B. Seasonal response and recovery of eelgrass (Zostera marina) to short-term reductions in light availability. Estuaries and Coasts, 2020, 43: 120-134 |
WU Z J, CHEN S Q, WANG D R, et al. The health assessment of the sea grass bed ecosystem in the east coast of Hainan Islands. Marine Sciences, 2014, 38(8): 67-74 [吴钟解, 陈石泉, 王道儒, 等. 海南岛东海岸海草床生态系统健康评价. 海洋科学, 2014, 38(8): 67-74] |
XU Z Z, HUANG L M, HUANG X P, et al. A primary study on sexual reproduction of seagrass Thalassia hemprichii at Xincun Bay. Journal of Tropical Oceanography, 2008, 27(2): 60-63 [许战洲, 黄良民, 黄小平, 等. 新村湾泰来藻有性繁殖的初步研究. 热带海洋学报, 2008, 27(2): 60-63] |
YANG R. The effects of temperature, light intensity and salinity on Halophila ovalis growth, physiological and biochemical characteristics. Master´s Thesis of Guangdong Ocean University, 2015 [杨冉. 温度、光照、盐度对喜盐草生长及生理生化特性的影响. 广东海洋大学硕士研究生学位论文, 2015]
|
YU S, CHEN X Y, RUAN Y G, et al. Atlas of China seagrass plants. Beijing: China Ocean Press, 2022 [于硕, 陈旭阳, 阮迎港, 等. 中国海草植物图鉴. 北京: 海洋出版社, 2022]
|
YU S, ZHANG J P, CUI L J, et al. Preliminary study on seed-based restoration for Enhalus acoroides meadow. Journal of Tropical Oceanography, 2019, 38(1): 49-54 [于硕, 张景平, 崔黎军, 等. 基于种子法的海菖蒲海草床恢复. 热带海洋学报, 2019, 38(1): 49-54] |
ZAKARIA M H, BUJANG J S, ARSHAD A. Flowering, fruiting and seedling of annual Halophila beccarii Aschers in peninsular Malaysia. Bulletin of Marine Science, 2002, 71(3): 1199-1205 |
ZHANG J. Transplant restoration effect of Thalassia hemprichii in Galong Bay and Xincun Lagoon of Hainan. Master´s Thesis of Hainan Tropical Ocean University, 2022 [张剑. 海南高隆湾与新村港泰来草移植修复效果研究. 海南热带海洋学院硕士研究生学位论文, 2022]
|
ZHANG M, ZHU J X, WANG L, et al. Progress of stress- induced flowering in plants. Chinese Journal of Biotechnology, 2016, 32(10): 1301-1308 [张敏, 朱佳旭, 王磊, 等. 逆境诱导植物开花的研究进展. 生物工程学报, 2016, 32(10): 1301-1308] |
ZHANG P D, ZHANG Y H, ZHANG H Y, et al. Research advances in shoot propagation theory and planting technique of seagrasses. Progress in Fishery Sciences, 2020, 41(4): 181-189 [张沛东, 张彦浩, 张宏瑜, 等. 海草植株扩繁理论及其定植效应的研究进展. 渔业科学进展, 2020, 41(4): 181-189] |
ZHENG F Y, QIU G L, FAN H Q, et al. Diversity, distribution and conservation of Chinese seagrass species. Biodiversity Science, 2013, 21(5): 517-526 [郑凤英, 邱广龙, 范航清, 等. 中国海草的多样性、分布及保护. 生物多样性, 2013, 21(5): 517-526] |
ZHOU Q, ZHANG S S, BAO M Z, et al. Advances on molecular mechanism of floral initiation in higher plants. Molecular Plant Breeding, 2018, 16(11): 3681-3692 [周琴, 张思思, 包满珠, 等. 高等植物成花诱导的分子机理研究进展. 分子植物育种, 2018, 16(11): 3681-3692] |



