海草是生活在温带、热带潮间带和潮下带区域的单子叶植物(黄小平等, 2018),海草床作为海岸带三大生态系统之一,具有极高的生产力和重要的生态系统服务价值(Scott et al, 2018),不仅能净化水质、减缓水流、固定底质(Edgar et al, 1994; Hemming et al, 2000; 高亚平等, 2013; Nordlund et al, 2017),而且是多种海洋生物的食物来源、产卵场和栖息地(Orth et al, 2006; Waycott et al, 2009; 樊敏玲等, 2011; Jiang et al, 2020)。海草生态系统是海洋中重要的碳汇,海草床沉积物有机碳含量是衡量海草床储碳能力的重要指标(Howard et al, 2021)。海草的生长和分布受到各种环境因素的影响,如海水温度、盐度、pH、营养盐、沉积物底质类型、光照和水流流速等(Ogata et al, 2009; Montague et al, 1993; Lee et al, 2007; Touchette et al, 2007; 吴瑞等, 2013; Balestri et al, 2015; 张沛东等, 2020)。近几十年来,由于全球气候变化和人类活动的双重影响,海草床衰退十分严重(Duarte, 2002; UNEP, 2020)。全球气候变化对海草生态系统造成巨大影响,海平面上升、海水温度升高会影响海草生态系统的演化(Short et al, 1999)。人类对沿海区域的过度开发加速了海草床退化,普遍认为引起海草衰退的主要原因为农业化肥的使用和海水养殖引起的营养盐富集(Burkholder et al, 2007; Grech et al, 2012)。营养盐富集造成的大型海藻暴发影响海草光合作用,藻类大量增殖使水体缺氧,造成硫化物积累,从而对海草产生毒害作用(Burkholder et al, 2007)。2008—2018年夏季,山东威海天鹅湖线性硬毛藻(Chaetomorpha linum) [(1 712±780)g DW/m2]和孔石莼(Ulva pertusa)[(1 511±555) g DW/m2]暴发性生长对鳗草海草形态学指标和生理学指标产生了显著的负面影响,导致海草床衰退严重(Zhang et al, 2014; Han et al, 2016)。
泰来草(Thalassia hemprichii)是热带海域普遍存在的海草优势种之一,我国泰来草主要分布在海南周边海域和台湾南部沿海海域(范航清等, 2009; 沈捷等, 2021)。泰来草能增强热带海域中红树林和珊瑚礁生境之间的生态连接,其群落与其他生境之间的联系不仅使它本身成为最具生产力的群落之一,而且提高了邻近生态系统的生产力(Medina-Gόmez et al, 2016)。国外学者对泰来草的研究主要集中在生物量、种群密度和形态特征、遗传多样性和微生物群落变化等方面(Medina-Gόmez et al, 2016; Rotini et al, 2020; Nguyen et al, 2022; Westlake et al, 2022)。例如:Medina-Gόmez等(2016)对墨西哥西部加勒比地区进行了4次季节性抽样调查,研究泰来草的生物量、形态和密度与该地区自然环境因素之间的关系,发现营养盐输入和海水盐度的变化对泰来草生长产生了显著影响。Westlake等(2022)对澳大利亚北部地区的泰来草海草床种群密度、生产力和生物量进行了调查,结果表明该地区泰来草海草床具有较高生产力,支持高水平的放牧率。国内新村湾泰来草海草床研究主要集中在海草床移植修复实践、有性生殖、营养盐对泰来草的影响、泰来草化学成分研究等方面(许战洲等, 2008; Qi et al, 2012; 刘松林等, 2017; 张剑, 2022)。例如:许战洲等(2008)初步研究了新村湾泰来草海草床的有性生殖过程,张剑(2022)分析了新村海域泰来草移植修复效果的影响因素,刘松林等(2017)研究了营养盐负荷对新村湾海草沉积物有机碳的影响,发现富营养化会通过影响沉积物中相关酶活性从而影响泰来草海草床的碳储存能力。新村湾位于海南岛东南部,海草资源非常丰富,其中泰来草为优势种之一(Huang et al, 2006)。截至目前,泰来草海草床长期野外观测数据还很缺乏。因此,本研究在一年内对新村湾泰来草海草床进行了6个月的采样调查,旨在研究各环境因素与海草形态指标和生理指标之间的关系,研究结果对于深入理解环境因素对新村湾泰来草的影响机制、掌握泰来草的种群动态、保护和恢复新村湾泰来草海草床有重要的理论意义和现实意义。
1 材料与方法 1.1 研究区概况新村湾位于海南省陵水县东南部(18°24′~18°27′ N, 109°57′~110°02′ E, 图 1),面积约为21.97 km2,南北长4 km,宽6 km,是一个基本受潮汐控制的天然潟湖,西部有一窄口与陵水湾相通。新村湾为典型的封闭潟湖海湾,湾内无明显地表径流输入,仅有2条小溪注入,水体交换常受潮汐汊道影响(龚文平等, 2004)。湾内水域面积较大,水深条件良好,其地势平坦且水深较浅,底质类型为砂质,港内水质及沉积环境主要受养殖、陆源污染及渔船码头等影响,具有风浪较小、盐度变化大、营养盐充足等特征(陈春华等, 2011; 蔡泽富等, 2017)。湾内网箱养殖渔排,养殖饲料带来的大量营养盐成为新村湾主要的营养负荷来源(黄道建等, 2010; Zhang et al, 2014)。
1.2 采样与测定方法 1.2.1 调查时间与站位布设2019年1月、3月、5月、7月、9月和11月在陵水县新村湾泰来草海草床进行了样品采集,新村湾海草床主要分布在南部和北部地区,北部地区海草床呈块状分布,南部海草床连续且面积较大,具有代表性,因此,如图 1所示,采样点设置在南部。根据《海岸带生态系统现在调查与评价技术第6部分:海草床》,针对分布于潮间带的海草床,垂直于海岸带方向布设3个调查断面,每个断面上沿水深(由浅到深)布设3个采样站位(1 m× 1 m)。
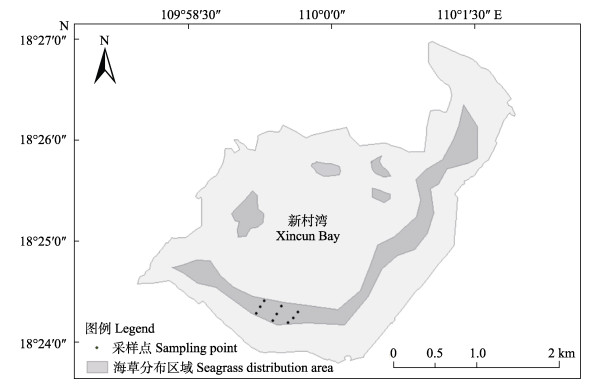
|
图 1 新村湾海草分布和采样站位 Fig.1 Distribution of seagrass beds and sampling sites in Xincun Bay |
采集海水、沉积物以及海草样品,调查方法根据《海岸带生态系统现在调查与评价技术第6部分:海草床》进行采集和预处理。采集的所有样品迅速放入保温箱中冷藏保存;使用美国YSI ProQuatro手持式水质分析仪现场测定海水温度、盐度和pH。实验室内采用45 μm滤膜过滤海水,然后采用锌–镉还原法测定海水样品中硝酸盐浓度,萘乙二胺分光光度法测定亚硝酸盐浓度,靛酚蓝分光光度法测定氨氮浓度,磷钼蓝分光光度法测定活性磷酸盐浓度。溶解性无机氮(DIN)含量为硝酸盐、亚硝酸盐、氨氮之和。
1.2.3 样品分析方法每个样方随机选取5株形态完整的植株测量形态指标和C、N含量,实验室内用清水清洗海草组织,用镊子轻轻刮去海草表面藻类和附生植物,使用游标卡尺测定海草形态指标,包括海草叶长、叶宽、根状茎长、根状茎直径和根长,将地上部分与地下部分分开,放入烘箱烘干并研磨过筛,使用德国ELTRA元素分析仪测试海草地上、地下部分C、N含量。采用麦奇克S3500激光粒径分析仪分析沉积物粒径。沉积物样品自然风干后研磨过筛,用浓度为1 mol/L的盐酸浸泡8~10 h去除无机碳,使用元素分析仪测定沉积物中有机碳含量。所有数据采用平均值±标准差(Mean±SD)表示。
1.3 数据处理采用Excel 2020软件进行数据整理和柱状图绘制,采用SPSS Statistics 26.0软件对数据进行统计分析,先对数据进行分布检验,若符合正态分布和方差齐性,则利用单因素方差分析(one-way ANOVA)和最小显著差异(LSD)比较同一指标不同月份间的差异,利用Pearson相关性分析比较各指标间的相关性。
2 结果与分析 2.1 海水 2.1.1 海水温度、盐度和pH新村湾海水温度各月存在显著差异(F=23.374, P < 0.01) (图 2a),5月最高,为(33.21±0.33) ℃,3月最低,为(27.59±0.03) ℃;盐度各月存在显著差异(F=114.442, P < 0.01) (图 2b),3月最高,为31.60±0.19,9月最低,为23.39±0.79;pH各月份间存在显著差异(F=9.535, P < 0.01) (图 2c),5月最高,为8.48±0.22,9月最低,为8.06±0.09。
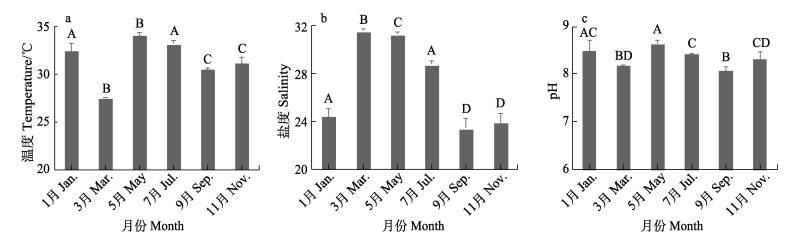
|
图 2 海水温度(a)、盐度(b)和pH(c) Fig.2 Salinity (a), temperature (b) and pH (c) of seawater 不同小写字母代表在0.05水平下差异显著(P < 0.05),不同大写字母代表在0.01水平下差异显著(P < 0.01)。下同。 Different lowercase letters represent significant differences at the 0.05 level (P < 0.05), and different capital letters represent significant differences at the 0.01 level (P < 0.01). The same below. |
新村湾海水氨氮浓度不同月份存在显著差异(F=56.16, P < 0.01) (图 3a),其中,5月最高,为(25.21±3.34) μmol/L,7月最低,为(3.10± 0.59) μmol/L;硝酸盐浓度不同月份间差异不显著(F=1.448, P > 0.05) (图 3b),其中,1月最高,为(4.37± 1.24) μmol/L,5月最低,为(3.30±0.26) μmol/L;各月DIN浓度存在显著差异(F=53.996, P < 0.01) (图 3c),其中,1月最高,为(28.66±2.27) μmol/L,7月最低,为6.75 μmol/L;各月溶解性磷酸盐浓度差异不显著(F=1.955, P > 0.05) (图 3d),其中,9月最高,为(0.29± 0.05) μmol/L,11月最低,为(0.14±0.05) μmol/L。
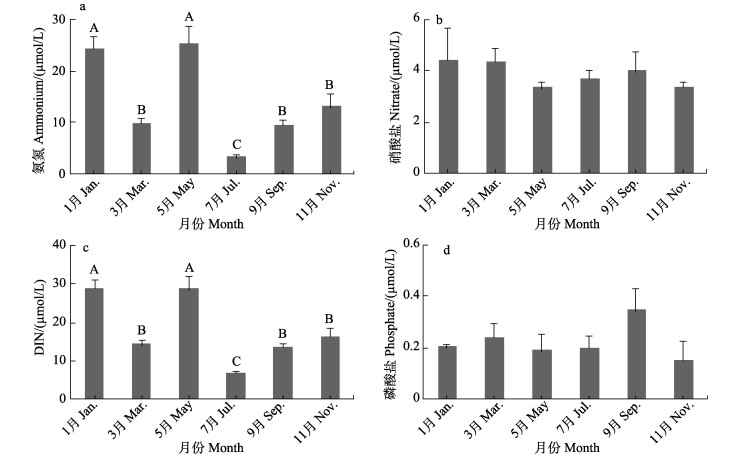
|
图 3 海水氨氮(a)、硝酸盐(b)、DIN(c)和磷酸盐(d)浓度 Fig.3 Ammonium (a), nitrate (b), DIN (c) and phosphate (d) concentration in seawater |
泰来草海草床沉积物粒径不同月份间存在显著差异(F=3.229, P < 0.05) (图 4a),泰来草海草床沉积物粒径7月最高,为(176.80±9.90) μm,1月最低,为(159.06±3.58) μm。泰来草海草床沉积物有机碳含量各月同样存在显著差异(F=5.718, P < 0.01) (图 4b),其中,1月最高,为(0.55±0.11)%,5月最低,为(0.32± 0.05)%。
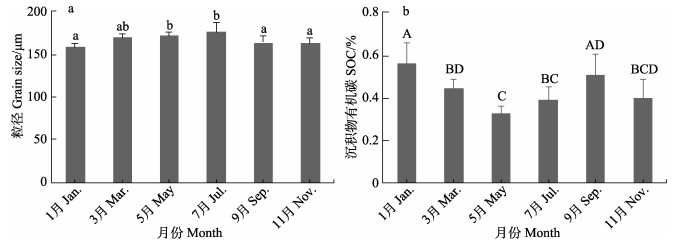
|
图 4 沉积物粒径(a)和有机碳含量(b) Fig.4 Grain size (a) and organic carbon content (b) of sediments |
泰来草叶长各月间存在显著差异(F=9.733, P < 0.01) (图 5a),5月最高,为(15.05± 6.13) cm,9月最低,为(7.19±2.55) cm。泰来草叶宽各月间存在显著差异(F=5.082, P < 0.05) (图 5b),11月最高,为(11.93±1.68) mm,9月最低,为(8.73± 1.96) mm。各月间根状茎长无显著差异(F=1.675, P > 0.05) (图 5c),7月最高,为(6.36±2.69) cm,11月最低,为(4.61±2.36) cm。泰来草根状茎直径各月间有显著差异(F=62.003, P < 0.01) (图 5d),11月最高,为(5.22±1.71) mm,3月最低,为(4.06±0.74) mm。泰来草根长存在显著差异(F=253.968, P < 0.01) (图 5e),3月最高,为(5.91±0.96) cm,5月最低,为(4.16± 3.33) cm。
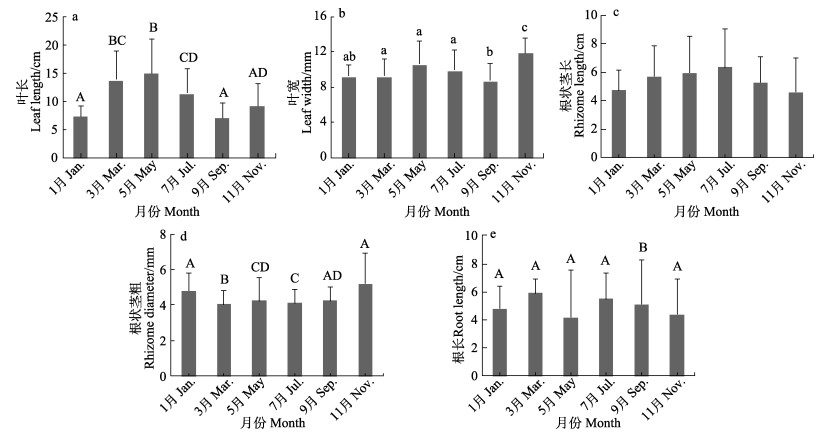
|
图 5 海草叶长(a)、叶宽(b)、根状茎长(c)、根状茎直径(d)和根长(e) Fig.5 Leaf length (a), leaf width (b), rhizome length (c), rhizome diameter (d), and root length (e) of seagrasses |
泰来草地上部分碳含量各月间无显著差异(F=2.841, P > 0.05) (图 6a),11月最高,为(33.49±1.25)%,9月最低,为(30.03±1.51)%。泰来草地上部分氮含量各月间差异不显著(F=0.514, P > 0.05) (图 6b),7月最高,为(3.32±1.21)%,11月最低,为(2.96±0.21)%。泰来草地上部分C/N比值在各月间无显著差异(F=2.171, P > 0.05) (图 6c),11月最高,为11.33±0.57,1月最低,为9.69±0.60。泰来草地下部分碳含量有显著差异(F=9.812, P < 0.01) (图 7a),11月最高,为(31.23±0.94)%,3月最低,为(24.90±3.48)%。泰来草地下部分氮含量在各月间不存在显著差异(F=2.678, P > 0.05) (图 7b),1月最高,为(2.19± 0.15)%,9月最低,为(1.77±0.16)%。泰来草地下部分C/N比值差异不显著(F=1.107, P > 0.05) (图 7c),7月最高,为15.22±3.04,3月最低,为13.30±1.14。

|
图 6 海草地上组织碳含量(a)、氮含量(b)和碳氮比值(c) Fig.6 C content (a), N content (b) and C/N ratio (c) of seagrass aboveground tissues |

|
图 7 海草地下组织碳含量(a)、氮含量(b)和碳氮比值(c) Fig.7 C content (a), N content (b) and C/N ratio (c) of seagrass belowground tissue |
将泰来草生理指标和形态指标与各环境因素指标进行Pearson相关性分析(图 8),结果显示,沉积物有机碳与叶宽和叶长呈极显著负相关,与根状茎长呈显著负相关。沉积物粒径分别与叶长和地下部分氮含量呈显著正相关和负相关。DIN和氨氮均与地下部分氮含量呈极显著正相关。pH与叶长、地下部分碳含量和地下部分氮含量呈显著正相关,与叶宽呈极显著正相关。盐度与叶长和根状茎长呈极显著正相关,与根状茎直径和地下部分C含量呈显著负相关。温度与叶宽呈显著正相关,与地下部分氮含量呈极显著正相关。

|
图 8 形态指标和生理指标与环境因素指标的相关性分析 Fig.8 Correlation analysis between morphological and physiological indicators and environmental factor indicators **表示差异极显著(P < 0.01),*表示差异显著(P < 0.05)。 ** indicates highly significant difference (P < 0.01), and * indicates significant difference (P < 0.05). |
本研究中,新村湾泰来草平均叶长和叶宽在15.05~7.19 cm和11.93~8.73 mm之间,澳大利亚西北部阿什莫尔礁泰来草叶片平均长度和平均宽度分别为37.6 mm和4.5 mm (Westlake et al, 2022),新村湾泰来草叶长高于阿什莫尔礁泰来草,其原因可能是新村湾水动力条件较弱且受人类活动影响较大,大量营养盐流入湾内,泰来草生长受到营养盐富集影响,新村湾泰来草叶长略低于平均水平。新村湾泰来草地上部分碳含量为33.49%~30.03%,氮含量为3.30%~ 2.96%,碳氮比为15.22~13.30,而相比之下,莫桑比克的伊尼亚长岛北湾的泰来草平均地上部分碳含量为29.35%,平均地上部分氮含量为2.28%,平均碳氮比为15 (Martins et al, 2001)。新村湾的泰来草地上部分氮含量比伊尼亚长岛北湾的高,碳氮比较低。原因可能是受新村湾湾内富营养化影响,尤其是海水中氮营养盐富集,地上部分氮含量较高,从而促进海草叶片的生长(Short, 1983; Peterson et al, 2012)。新村湾泰来草叶宽比阿什莫尔礁的窄,可能是由于新村湾较高的沉积物有机碳含量限制海草对磷的吸收和利用,从而对海草叶片宽度产生负面影响(Villazán et al, 2013; Han et al, 2017)。
3.2 新村湾泰来草形态和生理特征的关键影响因素 3.2.1 温度对海草形态指标及生理指标的影响海水温度是影响海草分布和生长的重要因素,海草生长的温度适应范围很广泛,海草栖息地遍布全球大部分纬度地区沿海(Robertson et al, 1984; 江志坚等, 2012; 刘伟妍等, 2017)。全球变暖引起的海水温度升高可能会对海草床造成负面影响(刘伟妍等, 2017)。本研究中,海水温度与泰来草地下组织碳含量呈显著正相关(图 8)。可能由于海草非结构性碳水主要存在于地下组织中,温度升高提高了海草蔗糖磷酸酶的活性,地下组织中的谷氨酰胺合成酶活性下降,从而改变海草地下组织碳含量(Touchette, 2007)。温度对海草的影响通过光合作用和呼吸作用来反映,温度升高将增加光合作用效率和呼吸强度(Short et al, 1999),维持相对较高的呼吸强度所消耗的非结构性碳水化合物将超过其在高温下通过光合作用合成的部分(Liu et al, 2020)。研究表明,不同季节泰来草的生产力和生物量差异较大,夏季高温导致生产力和生物量远远大于冬季(Tussenbroek, 1995)。温度高于或者低于海草最适宜温度都可能降低光合作用,其呼吸速率的增加会打破二者之间的平衡,减少非结构性碳水的合成和储存(Zimmerman et al, 1989)。在适合海草生长的环境中,温度的适度升高会提高泰来草叶片中可溶性淀粉含量,枝茎密度、光合效率和叶片生长速率也会随着温度的升高而增加(Koch et al, 2007; Liu et al, 2020)。
3.2.2 盐度对海草形态指标及生理指标的影响盐度是影响海草群落结构和功能的重要环境因素(Montague et al, 1993),盐度波动会改变植物重要的生长和生理过程,进而影响植物的新陈代谢、生长、发育和繁殖(McMillan et al, 1967; Zieman, 1975; Walker, 1990; Ramage et al, 1998; Vermaat et al, 2000; Torquemada et al, 2005)。海草生活在海陆交接地带,能够承受短期的盐度波动,泰来草可在盐度为60的环境中存活(许战洲等, 2007)。Atkinson等(1983)研究发现,无论海草床处于高盐度或低盐度的条件下,都需要大量的能量来维持细胞内渗透压和细胞膜完整性,从而对海草产生影响。长期低海水盐度会引起诺氏鳗草(Zostera noltii)、约氏喜盐草(Halophila johnsomii)的死亡(Torquemada et al, 2005)。Liman等(2003)发现,低盐度环境显著降低了丝状针叶草(Syringodium filiforme)和莱氏二药草(Halodule wrightii)的叶片生长率,反之,在适宜的范围内,盐度的升高可能提升海草的枝芽密度以及叶片生长效率,这与本研究中海草的叶长、叶宽等形态指标与海水盐度呈显著正相关的结论一致(图 8)。高盐或者低盐环境下水生植物的光合作用和呼吸作用常受到抑制,盐度增加导致植物体内叶绿素含量下降和酶活性降低,影响非结构性碳水化合物的合成(Kahn et al, 2006)。本研究中,盐度与海草地下组织的碳含量呈显著负相关,可能是由于盐胁迫期间海草为调节渗透压将碳水化合物转化为其他化合物,组织内可溶性糖含量降低,地下组织中非结构碳水向上转移,地下组织碳含量降低(Touchette et al, 2007)。
3.2.3 pH对海草形态指标及生理指标的影响pH作为重要的环境因素,会对海草的生长和分布产生影响(Banister et al, 2022)。海草能够承受较大的pH波动,热带海草床的pH变化值可超过1.0,Semesi等(2009)对坦桑尼亚Chwaka湾海草床调查发现,海草能够承受海水pH在7.8~8.9范围内波动。海草光合作用中产生的碳至少50%来自于海水中的CO2,海草的生产力、生长密度、地下组织碳储量都随着海水CO2浓度的增加而增加(Palacios et al, 2007; 韩秋影等, 2008; Andersson et al, 2011; Egea et al, 2018)。在热带以碳酸盐为主体的沉积物中,高CO2分压伴随着碳酸氢盐含量的增加,该现象的产生虽然会增加海草本身的光合作用并且促进海草生长,但可能破坏沉积物及其微生物群落的稳定性,从而对海草造成不利影响(Banister et al, 2022)。pH降低能促进海草的固氮和脱氮作用,削弱硝化作用对海草产生的影响(汪思茹等, 2012)。酚类化合物可作为植物抑制物、消化抑制剂和防污剂,在植物抗病性中发挥重要作用。含有不同类型酚类化合物的鳗草叶片提取物可抑制几种致病微生物的生长(Harrison, 1982; Harrison et al, 1980、1985)。本研究发现,pH与泰来草的叶长、叶宽、海草地下部分碳含量和氮含量呈显著正相关(图 8)。原因可能是随着CO2分压的增加,海洋植物酚类保护物质的浓度下降(Arnold et al, 2012)。在CO2分压较高的地区,酚类物质产量的减少可能导致更高的放牧率以及更大的致病风险,从而对海草造成负面影响(Harrison et al, 1980; Vergeer et al, 1995; Arnold et al, 2002)。
3.2.4 海水营养盐对海草形态指标及生理指标的影响营养盐是限制海草生长的主要因素之一,富营养地区和贫营养地区营养盐对海草的影响不同(Lee et al, 2007)。沉积物中的营养盐浓度通常比海水高10~100倍,贫营养条件下海草可能从沉积物中获得大部分营养盐(Short et al, 1984; Sand-Jensen et al, 1991)。营养盐富集可能引起氨氮对海草的直接毒性(van Katwijk et al, 1997);Zhang等(2014)研究发现,在营养盐富集的区域,泰来草的叶长较短。高浓度的铵态氮会显著降低海草的光合速率和光能利用率,减少非结构性碳水化合物的合成,限制碳库来同化过多的铵态氮,导致海草组织内部碳的失衡(Alcoverro et al, 2001; Christianen et al, 2011; 唐望等, 2012; Alexandre et al, 2015)。营养盐富集会通过影响其他生物生长间接影响海草,高营养盐浓度会导致大型海藻、浮游植物和附生生物等其他海洋生物大量繁殖,通过引发光衰减影响海草光合作用;生物生长和残体分解消耗氧气,导致水体溶解氧不足,影响海草呼吸作用(Koch et al, 2001; Campbell et al, 2003; Castejón-Silvo et al, 2012)。营养盐富集可通过影响微生物以及酶活性对海草床产生深远影响(Jiang et al, 2022; Zhang et al, 2022)。微生物对海草床中碳循环产生重要作用,其分泌的胞外酶可以同时水解沉积物中的有机物,例如:葡萄糖苷酶催化淀粉、蔗糖等寡糖水解,而多酚氧化酶可以分解木质素和芳香族化合物(Sinsabaugh, 2010; Macreadie et al, 2012; Zhang et al, 2017)。营养盐浓度升高会对胞外酶活性产生巨大影响(Liu et al, 2017; Zhang et al, 2017; Spivak et al, 2019)。营养负荷会显著提高微生物胞外酶活性和微生物丰度,从而加速有机物的分解,对海草床产生负面影响(Kearns et al, 2019; Kelleway et al, 2020; Macreadie et al, 2021)。Liu等(2022)研究发现,在高营养条件下,与海草纤维素以及木质素分解有关的胞外酶活性提高。本研究中,海水氨氮以及DIN与泰来草地下部分氮含量呈显著正相关(图 8)。主要原因可能为海草能从沉积物孔隙水和海水中吸收营养盐(Short, 1987),并且由于营养盐的高利用性,海草会吸收和储存多余的营养物质在其地下组织中,从而增加其总氮含量(Touchette et al, 2000; Johnson et al, 2006)。本研究中,硝酸盐与海草地下部分碳氮比值呈显著负相关(图 8)。一方面可能与海草组织氮含量密切相关,另一方面可能是由于营养盐富集会影响溶解有机碳的释放速率,从而降低沉积物有机碳的含量以及海草地下组织中非结构性碳水化合物的含量。Jiang等(2022)研究也发现,海草地下组织的非结构性碳水化合物含量与溶解有机碳的渗出率呈显著正相关,在硝酸盐富集下,沉积物中溶解有机碳浓度呈下降趋势,与地下组织排出溶解有机碳的速率显著正相关。
3.2.5 沉积物性质对海草形态指标及生理指标的影响粒径较小的沉积物有利于海草根的生长,而粒径较大的沉积物会对海草根的生长造成较大的阻力(Balestri et al, 2015)。刘松林等(2021)通过室内模拟实验发现,细砂组海草较长的根有利于植株吸收沉积物中的营养物质,从而促进叶片的生长,表明粒径会影响海草的生长速率。底质类型会影响沉积物中硫化物、有机碳的含量(Terrados et al, 1999)。粒径较小沉积物的养分含量和疏松结构能促进海草根系的生长,海草发达的根系有利于养分的获取和固定能力,而较大粒径的沉积物则会限制海草根生长和养分的获取(Balestri et al, 2006; Schutten et al, 2005; Statton et al, 2014)。Balestri等(1998)移植大洋波喜荡草(Posidonia oceanica)的实验发现,从粒径较小的沉积物转移到较大的沉积物中之后,海草叶片以及侧根的生长加快。本研究发现,沉积物粒径与泰来草叶长呈显著正相关(图 8)。可能是由于沉积物粒径的大小影响营养物质和氧气浓度,从而对海草产生间接影响。较小的沉积物粒径会减弱沉积物与海水的氧气交换,影响海草根部获取氧气,从而间接影响海草叶片生长(Short, 1987; Koch, 2001)。本研究结果中,沉积物粒径与海草地下部分的氮含量呈显著负相关(图 8)。可能是由于沉积物间隙水中营养盐和有机物浓度与沉积物粒径成反比,粒径越小,沉积物中营养盐浓度和有机物浓度越高,海草地下部分组织固氮作用随着营养盐浓度的增高而增高。本研究中,沉积物有机碳含量与泰来草的叶长、叶宽和根状茎长呈显著负相关(图 8)。Terrados等(1999)在实验中也发现,沉积物有机质的添加降低了泰来草的枝密度和叶片生长率。原因可能是沉积物有机碳的富集会导致沉积物缺氧以及硫化物入侵(Olivé et al, 2009; Pérez et al, 2007)。沉积物有机碳含量的升高可能会促使硝酸盐转化为铵态氮,增加铵态氮对海草的毒性,从而对海草的生长产生负面影响(Seitzinger, 1988; Govers et al, 2014)。
4 结论本研究通过对海南新村湾泰来草的形态指标和生理指标研究发现,泰来草受海水温度、盐度和pH影响较大,水体中营养盐增加可能会对泰来草海草床产生负面影响。为了保护新村湾海草床,建议采取以下管理措施:严格控制在新村湾的水产养殖面积,限制生活污水向湾内排放,从而减少新村湾的营养盐输入;加强对新村湾海草床的管理,提高周围居民以及游客保护海草资源的意识;加强海草生态学研究,进行长周期海草床野外观测,通过设计野外操控实验和实验室模拟实验深入理解各环境因素对海草床的影响机制。
ALCOVERRO T, MANZANERA M, ROMERO J. Annual metabolic carbon balance of the seagrass Posidonia oceanica: The importance of carbohydrate reserves. Marine Ecology Progress Series, 2001, 211(8): 105-116 |
ALEXANDRE A, HILL P W, JONES D L, et al. Dissolved organic nitrogen: A relevant, complementary source of nitrogen for the seagrass Zostera marina. Limnology and Oceanography, 2015, 60(5): 1477-1483 DOI:10.1002/lno.10084 |
ANDERSSON A J, MACKENZIE F T, GATTUSO J P. Effects of ocean acidification on benthic processes, organisms, and ecosystems. Ocean Acidification, 2011, 8: 121-153 |
ARNOLD T M, TARGETT N M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. Journal of Chemical Ecology, 2002, 28: 1919-1934 DOI:10.1023/A:1020737609151 |
ARNOLD T, MEALEY C, LEAHEY H, et al. Ocean acidification and the loss of phenolic substances in marine plants. PLoS One, 2012, 7(4): e35107 DOI:10.1371/journal.pone.0035107 |
ATKINSON M J, SMITH S V. C: N: P ratio of benthic marine plants. Limnology and Oceanography, 1983, 28(3): 568-574 DOI:10.4319/lo.1983.28.3.0568 |
BALESTRI E, DE BATTISTI D, VALLERINI F, et al. First evidence of root morphological and architectural variations in young Posidonia oceanica plants colonizing different substrate typologies. Estuarine, Coastal and Shelf Science, 2015, 154: 205-213 DOI:10.1016/j.ecss.2015.01.002 |
BALESTRI E, LARDICCI C. Stimulation of root formation in Posidonia oceanica cuttings by application of auxins (NAA and IBA). Marine Biology, 2006, 149(2): 393-400 DOI:10.1007/s00227-005-0193-0 |
BALESTRI E, PIAZZI L, CINELLI F. Survival and growth of transplanted and natural seedlings of Posidonia oceanica (L.) Delile in a damaged coastal area. Journal of Experimental Marine Biology and Ecology, 1998, 228(2): 209-225 DOI:10.1016/S0022-0981(98)00027-6 |
BANISTER R B, SCHWARZ M T, FINE M, et al. Instability and stasis among the microbiome of seagrass leaves, roots and rhizomes, and nearby sediments within a natural pH gradient. Microbial Ecology, 2022, 83(3): 703-716 |
BURKHOLDER J A M, TOMASKO D A, TOUCHETTE B W. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology, 2007, 350(1/2): 46-72 |
CAI Z F, CHEN S Q, WU Z J, et al. Distribution differences and environmental effects of seagrasses between bays and lagoons of Hainan Island. Transactions of Oceanology and Limnology, 2017, 3: 74-84 [蔡泽富, 陈石泉, 吴钟解, 等. 海南岛海湾与潟湖中海草的分布差异及影响分析. 海洋湖沼通报, 2017, 3: 74-84] |
CAMPBELL S, MILLER C, STEVEN A, et al. Photosynthetic responses of two temperate seagrasses across a water quality gradient using chlorophyll fluorescence. Journal of Experimental Marine Biology and Ecology, 2003, 291(1): 57-78 DOI:10.1016/S0022-0981(03)00090-X |
CASTEJON-SILVO I, DOMINGUEZ M, TERRADOS J, et al. Invertebrate response to nutrient-driven epiphytic load increase in Posidonia oceanica meadows. Estuarine Coastal and Shelf Science, 2012, 112: 225-235 DOI:10.1016/j.ecss.2012.07.028 |
CHEN C H, WU Z J, ZHANG G X. Ecological status and sustainable utilization of seagrass bed in Xincun Port. Ocean Development and Management, 2011, 28(11): 74-78 [陈春华, 吴钟解, 张光星. 新村港海草床的生态状况及可持续利用探讨. 海洋开发与管理, 2011, 28(11): 74-78 DOI:10.3969/j.issn.1005-9857.2011.11.021] |
CHRISTIANEN M J A, VAN DER HEIDE T, BOUMA T J, et al. Limited toxicity of NHx pulses on an early and late successional tropical seagrass species: Interactions with pH and light level. Aquatic Toxicology, 2011, 104(1/2): 73-79 |
DUARTE C M. The future of seagrass meadows. Environmental Conservation, 2002, 19(2): 192-206 |
EDGAR G J, SHAW C, WATSONA G F, et al. Comparisons of species richness size-structure and production of benthos in vegetated and unvegetated habitats in Westem Port Victoria. Journal of Experimental Marine Biology and Ecology, 1994, 176(2): 201-226 DOI:10.1016/0022-0981(94)90185-6 |
EGEA L G, JIMENEZ-RAMOS R, VERGARA J J, et al. Interactive effect of temperature, acidification and ammonium enrichment on the seagrass Cymodocea nodosa. Marine Pollution Bulletin, 2018, 134: 14-26 DOI:10.1016/j.marpolbul.2018.02.029 |
FAN H Q, SHI Y J, QIU G L. Chinese seagrass plants. Beijing: China Ocean Press, 2009 [范航清, 石雅君, 邱广龙. 中国海草植物. 北京: 海洋出版社, 2009]
|
FAN M L, HUANG X P, ZHANG D W, et al. Food sources of fish and macro-invertebrates in a tropical seagrass bed at Xincun Bay, Southern China. Acta Ecologica Sinica, 2011, 31(1): 31-38 [樊敏玲, 黄小平, 张大文, 等. 海南新村湾海草床主要鱼类及大型无脊椎动物的食源. 生态学报, 2011, 31(1): 31-38 DOI:10.3969/j.issn.1674-764x.2011.01.005] |
GAO Y P, FANG J G, TANG W, et al. Seagrass meadow carbon sink and amplification of the carbon sink for eelgrass bed in Sanggou Bay. Progress in Fishery Sciences, 2013, 34(1): 17-21 [高亚平, 方建光, 唐望, 等. 桑沟湾大叶藻海草床生态系统碳汇扩增力的估算. 渔业科学进展, 2013, 34(1): 17-21 DOI:10.3969/j.issn.1000-7075.2013.01.003] |
GONG W P, CHEN M H, WEN X J, et al. Evolution and stability of Xincun tidal inlet, Lingshui County, Hainan Province. Journal of Tropical Oceanography, 2004, 4: 25-32 [龚文平, 陈明和, 温晓骥, 等. 海南陵水新村港潮汐汊道演变及其稳定性分析. 热带海洋学报, 2004, 4: 25-32] |
GOVERS L L, DE BROUWER J H F, SUYKERBUYK W, et al. Toxic effects of increased sediment nutrient and organic matter loading on the seagrass Zostera noltii. Aquatic Toxicology, 2014, 155: 253-260 DOI:10.1016/j.aquatox.2014.07.005 |
GRECH A, CHARTRAND-MILLER K, ERFTEMEIJER P, et al. A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environmental Research Letters, 2012, 7(2): 24006-24013 DOI:10.1088/1748-9326/7/2/024006 |
HAN Q Y, SHI P. Progress in the study of seagrass ecology. Acta Ecologica Sinica, 2008(11): 5561-5570 [韩秋影, 施平. 海草生态学研究进展. 生态学报, 2008(11): 5561-5570 DOI:10.3321/j.issn:1000-0933.2008.11.040] |
HAN Q Y, SOISSONS L M, BOUMA T J, et al. Combined nutrient and macroalgae loads lead to response in seagrass indicator properties. Marine Pollution Bulletin, 2016, 106: 174-182 DOI:10.1016/j.marpolbul.2016.03.004 |
HAN Q Y, SOISSONS L M, LIU D Y, et al. Individual and population indicators of Zostera japonica respond quickly to experimental addition of sediment-nutrient and organic matter. Marine Pollution Bulletin, 2017, 114(1): 201-209 DOI:10.1016/j.marpolbul.2016.08.084 |
HARRISON P G, CHAN A T. Inhibition of the growth of micro-algae and bacteria by extracts of eelgrass (Zostera marina) leaves. Marine Biology, 1980, 61(1): 21-26 DOI:10.1007/BF00410338 |
HARRISON P G, DURANCE C D. Reductions in photosynthetic carbon uptake in epiphytic diatoms by water-soluble extracts of leaves of Zostera marina. Marine Biology, 1985, 90(1): 117-119 DOI:10.1007/BF00428222 |
HARRISON P G. Control of microbial growth and of amphipod grazing by water-soluble compounds from leaves of Zostera marina. Marine Biology, 1982, 67(2): 225-230 DOI:10.1007/BF00401288 |
HEMMING M A, DUARTE C M. Seagrass Ecology. Cambridge: Cambridge University Press, 2000
|
HOWARD J L, LOPES C C, WILSON S S, et al. Decomposition rates of surficial and buried organic matter and the lability of soil carbon stocks across a large tropical seagrass landscape. Estuaries and Coasts, 2021, 44: 846-866 DOI:10.1007/s12237-020-00817-x |
HUANG D J, HUANG X P, HUANG Z G. Spatio temporal variation of TN & TP contents in Enhalus acoroides and response to nutrient load in Xincun Bay, Hainan. Marine Environmental Science, 2010, 29(1): 40-43 [黄道建, 黄小平, 黄正光. 海南新村湾海菖蒲TN和TP含量时空变化及其对营养负荷的响应. 海洋环境科学, 2010, 29(1): 40-43 DOI:10.3969/j.issn.1007-6336.2010.01.009] |
HUANG X P, HUANG L M, LI Y H, et al. Main seagrass beds and threats to their habitats in the coastal sea of South China. Chinese Science Bulletin, 2006, 51: 136-142 DOI:10.1007/s11434-006-9136-5 |
HUANG X P, JIANG Z J, ZHANG J P, et al. The Chinese nomenclatrue of the global seagrasses. Acta Oceanologica Sinica, 2018, 40(4): 127-133 [黄小平, 江志坚, 张景平, 等. 全球海草的中文命名. 海洋学报, 2018, 40(4): 127-133 DOI:10.3969/j.issn.0253-4193.2018.04.012] |
JIANG Z J, HUANG D L, FANG Y, et al. Home for marine species: Seagrass leaves as vital spawning grounds and food source. Frontiers in Marine Science, 2020, 7: 1-9 DOI:10.3389/fmars.2020.00001 |
JIANG Z J, HUANG X P, ZHANG J P. Effect of environmental stress on non-structural carbohydrates reserves and transfer in seagrasses. Acta Ecologica Sinica, 2012, 32(19): 6242-6250 [江志坚, 黄小平, 张景平. 环境胁迫对海草非结构性碳水化合物储存和转移的影响. 生态学报, 2012, 32(19): 6242-6250] |
JIANG Z J, LI L L, FANG Y, et al. Eutrophication reduced the release of dissolved organic carbon from tropical seagrass roots through exudation and decomposition. Marine Environmental Research, 2022, 179: 105703 DOI:10.1016/j.marenvres.2022.105703 |
JOHNSON M, HECK K, FOURQUREAN J. Nutrient content of seagrasses and epiphytes in the northern Gulf of Mexico: Evidence of phosphorus and nitrogen limitation. Aquatic Botany, 2006, 85(2): 103-111 DOI:10.1016/j.aquabot.2006.02.003 |
KAHN A E, DURAKO M J. Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. Journal of Experimental Marine Biology and Ecology, 2006, 335(1): 1-12 DOI:10.1016/j.jembe.2006.02.011 |
KEARNS P J, BULSECO-MCKIM A N, HOYT H, et al. Nutrient enrichment alters salt marsh fungal communities and promotes putative fungal denitrifiers. Microbial Ecology, 2019, 77: 358-369 DOI:10.1007/s00248-018-1223-z |
KELLEWAY J J, SERRANO O, BALDOCK J A, et al. A national approach to greenhouse gas abatement through blue carbon management. Global Environmental Change, 2020, 63: 102083 DOI:10.1016/j.gloenvcha.2020.102083 |
KOCH E W. Beyond light: Physical, geological, and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries, 2001, 24(1): 1-17 DOI:10.2307/1352808 |
KOCH M S, ERSKINE J M. Sulfide as a phytotoxin to the tropical seagrass Thalassia testudinum: interactions with light, salinity and temperature. Journal of Experimental Marine Biology and Ecology, 2001, 266(1): 81-95 DOI:10.1016/S0022-0981(01)00339-2 |
KOCH M S, SCHOPMEYER S, KYHN-HANSEN C, et al. Synergistic effects of high temperature and sulfide on tropical seagrass. Journal of Experimental Marine Biology and Ecology, 2007, 341(1): 91-101 DOI:10.1016/j.jembe.2006.10.004 |
LEE K S, PARK S R, KIM Y K. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. Journal of Experimental Marine Biology and Ecology, 2007, 350(1): 144-175 |
LIMAN D, CROPPER W P. The influence of salinity on seagrass growth, survivorship, and distribution within Biscayne Bay, Florida: Field, experimental, and modeling studies. Estuaries, 2003, 26(1): 131-141 DOI:10.1007/BF02691700 |
LIU J P, ANG S J, MAYFIELD A B, et al. Influence of the seagrass Thalassia hemprichii on coral reef mesocosms exposed to ocean acidification and experimentally elevated temperatures. Science of the Total Environment, 2020, 700: 134464 DOI:10.1016/j.scitotenv.2019.134464 |
LIU S L, JIANG Z J, DENG Y Q, et al. Effects of seagrass leaf litter decomposition on sediment organic carbon composition and the key transformation processes. Scientia Sinica Terrae, 2017, 47(12): 1425-1435 [刘松林, 江志坚, 邓益琴, 等. 海草凋落叶分解对沉积物有机碳组成及其关键转化过程的影响. 中国科学: 地球科学, 2017, 47(12): 1425-1435] |
LIU S L, JIANG Z J, WU Y C, et al. Effects of different sediment types on the seed germination and seedling growth of tropical seagrass, Enhalus acoroides. Journal of Applied Oceanography, 2021, 40(1): 74-81 [刘松林, 江志坚, 吴云超, 等. 底质类型对热带海草海菖蒲种子萌发和幼苗生长的影响. 应用海洋学学报, 2021, 40(1): 74-81] |
LIU S L, JIANG Z J, ZHANG J P, et al. Sediment microbes mediate the impact of nutrient loading on blue carbon sequestration by mixed seagrass meadows. Science of the Total Environment, 2017, 599/600: 1479-1484 DOI:10.1016/j.scitotenv.2017.05.129 |
LIU S L, TREVATHAN-TACKETT S M, JIANG Z J, et al. Nutrient loading decreases blue carbon by mediating fungi activities within seagrass meadows. Environmental Research, 2022, 212: 113280 DOI:10.1016/j.envres.2022.113280 |
LIU W Y, HAN Q Y, TANG Y Q, et al. Review of nutrient enrichment and global warming effects on seagrasses. Chinese Journal of Ecology, 2017, 36(4): 1087-1096 [刘伟妍, 韩秋影, 唐玉琴, 等. 营养盐富集和全球温度升高对海草的影响. 生态学杂志, 2017, 36(4): 1087-1096] |
MACREADIE P I, ALLEN K, KELAHER B P, et al. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Global Change Biology, 2012, 18(3): 891-901 DOI:10.1111/j.1365-2486.2011.02582.x |
MACREADIE P I, COSTA M D, ATWOOD T B, et al. Blue carbon as a natural climate solution. Nature Reviews Earth and Environment, 2021, 2: 826-839 DOI:10.1038/s43017-021-00224-1 |
MARTINS A, BANDERIA S O. Biomass distribution and leaf nutrient concentrations and resorption of Thalassia hemprichii at Inhaca Island, Mozambique. South African Journal of Botany, 2001, 67(3): 439-442 DOI:10.1016/S0254-6299(15)31161-3 |
MCMILLAN C, MOSELEY F N. Salinity tolerances of five marine spermatophytes of Redfish Bay, Texas. Ecolology, 1967, 48(3): 503-506 DOI:10.2307/1932688 |
MEDINA-GOMEZ I, MADDEN C J, HERRERA-SILVEIRA J, et al. Response of Thalassia testudinum morphometry and distribution to environmental drivers in a pristine tropical lagoon. PLoS One, 2016, 11(10): e0164014 DOI:10.1371/journal.pone.0164014 |
MONTAGUE C L, LEY J A. A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in Northeastern Florida Bay. Estuaries, 1993, 16(4): 703-717 DOI:10.2307/1352429 |
NGUYEN X V, NGUYEN-NHAT N T, NGUYEN X T, et al. Microsatellite-based analysis of the genetic diversity and population structure of the seagrass species Thalassia hemprichii from southern Vietnam. Aquatic Botany, 2022, 178: 103497 DOI:10.1016/j.aquabot.2022.103497 |
NORDLUND L M, KOCH E W, BARBIER E B, et al. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS One, 2017, 12(1): e0169942 DOI:10.1371/journal.pone.0169942 |
OGATA E, MATSUI T. Photosynthesis in several marine plants of Japan as affected by salinity, drying and pH, with attention to their growth habitats. Botanica Marina, 2009, 8(2/3/4): 199-217 |
OLIVE I, GARCIA-SANCHEZ M P, BRUN F G, et al. Interactions of light and organic matter under contrasting resource simulated environments: The importance of clonal traits in the seagrass Zostera noltii. Hydrobiologia, 2009, 629: 199-208 DOI:10.1007/s10750-009-9773-1 |
ORTH R J, CARRUTHERS T J, DENNISON W C, et al. A global crisis for seagrass ecosystems. Bioscience, 2006, 56(12): 987-996 DOI:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2 |
PALACIOS S L, ZIMMERMAN R C. Response of eelgrass Zostera marina to CO2 enrichment: Possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series, 2007, 344: 1-13 DOI:10.3354/meps07084 |
PEREZ M, INVER O, RUIZ J M, et al. Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: An experimental assessment. Journal of Experimental Marine Biology and Ecology, 2007, 344(2): 149-160 DOI:10.1016/j.jembe.2006.12.020 |
PETERSON B J, STUBLER A D, WALL C C, GOBLER C J. Nitrogen-rich groundwater intrusion affects productivity, but not herbivory, of the tropical seagrass Thalassia testudinum. Aquatic Biology, 2012, 15: 1-9 DOI:10.3354/ab00413 |
QI S H, HUANG L S, HE F, et al. Phytochemical and chemotaxonomic investigation of seagrass Thalassia hemprichii (Ehrenb.) Aschers (Hydrocharitaceae). Biochemical Systematics and Ecology, 2012, 43: 128-131 DOI:10.1016/j.bse.2012.03.006 |
RAMAGE D L, SCHIEL D R. Reproduction in the seagrass Zostera novazelandica on intertidal platforms in southern New Zealand. Marine Biology, 1998, 130: 479-489 DOI:10.1007/s002270050268 |
ROBERTSON A I, MANN K H. Disturbance by ice and life-history adaptations of the seagrass Zostera marina. Marine Biology, 1984, 80(2): 131-141 DOI:10.1007/BF02180180 |
ROTINI A, CONTE C, SEVESO D, et al. Daily variation of the associated microbial community and the Hsp60 expression in the Maldivian seagrass Thalassia hemprichii. Journal of Sea Research, 2020, 156: 101835 DOI:10.1016/j.seares.2019.101835 |
SAND-JENSEN K, BORUM J. Interactions among phytoplankton, periphyton, and macrophytes in temperate fresh waters and estuaries. Aquatic Botany, 1991, 41(1/2/3): 137-175 |
SCHUTTEN J, DAINTY J, DAVY A J. Root anchorage and its significance for submerged plants in shallow lakes. Journal of Ecology, 2005, 93(3): 556-571 DOI:10.1111/j.1365-2745.2005.00980.x |
SCOTT A L, YORK P H, DUNCAN C, et al. The role of herbivory in structuring tropical seagrass ecosystem service delivery. Frontiers in Plant Science, 2018, 9: 127 DOI:10.3389/fpls.2018.00127 |
SEITZINGER S P. Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnology and Oceanography, 1988, 33(4): 702-724 |
SEMESI I S, BEER S, BJORK M. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Marine Ecology Progress Series, 2009, 382: 41-47 DOI:10.3354/meps07973 |
SHEN J, CHEN S Q, WU Z J, et al. Screening and structural analysis of WOX transcription factors in Thalassia hemprichii. Journal of Applied Oceanography, 2021, 40(4): 597-607 [沈捷, 陈石泉, 吴钟解, 等. 泰来草WOX转录因子的筛选与结构分析. 应用海洋学学报, 2021, 40(4): 597-607 DOI:10.3969/J.ISSN.2095-4972.2021.04.005] |
SHORT F T, MCROY C P. Nitrogen uptake by leaves and roots of the seagrass Zostera marina L. Botanica Marina, 1984, 17: 547-555 |
SHORT F T, NECHLES H A. The effects of global climate change on seagrasses. Aquatic Botany, 1999, 63(3/4): 169-196 |
SHORT F T. Effects of sediment nutrients on seagrasses: Literature review and mesocosm experiment. Aquatic Botany, 1987, 27(1): 41-57 DOI:10.1016/0304-3770(87)90085-4 |
SHORT F T. he seagrass, Zostera marina L.: Plant morphology and bed structure in relation to sediment ammonium in Izembek lagoon, Alaska. Aquatic Botany, 1983, 16: 149-161 DOI:10.1016/0304-3770(83)90090-6 |
SINSABAUGH R L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biology and Biochemistry, 2010, 42: 391-404 DOI:10.1016/j.soilbio.2009.10.014 |
SPIVAK A C, SANDERMAN J, BOWEN J L, et al. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nature Geoscience, 2019, 12: 685-692 DOI:10.1038/s41561-019-0435-2 |
STATTON J, KENDRICK G A, DIXON K W, et al. Inorganic nutrient supplements constrain restoration potential of seedlings of the seagrass, Posidonia australis. Restoration Ecology, 2014, 22(2): 196-203 DOI:10.1111/rec.12072 |
TANG W, FANG J G, GAO Y P, et al. Chlorophll fluorescence characteristics of common temperate seagrass species in Sanggou Bay. Progress in Fishery Sciences, 2012, 33(6): 106-111 [唐望, 方建光, 高亚平, 等. 桑沟湾常见温带海草叶绿素荧光特性的比较. 渔业科学进展, 2012, 33(6): 106-111] |
TERRADOS J, AGAWIN N S R, DUARTE C M, et al. Nutrient limitation of the tropical seagrass Enhalus acoroides (L.) Royle in Cape Bolinao, NW Philippines. Aquatic Botany, 1999, 65: 123-139 DOI:10.1016/S0304-3770(99)00036-4 |
TORQUEMADA Y F, DURAKO M J, LIZASO J L S. Effects of salinity and possible interactions with temperature and pH on growth and photosynthesis of Halophila johnsonii Eiseman. Marine Biology, 2005, 148(2): 251-260 DOI:10.1007/s00227-005-0075-5 |
TOUCHETTE B W, BURKHOLDER J M. Carbon and nitrogen metabolism in the seagrass, Zostera marine L.: Environmental control of enzymes involved in carbon allocation and nitrogen assimilation. Journal of Experimental Marine Biology and Ecology, 2007, 350(1/2): 216-233 |
TOUCHETTE B W, BURKHOLDER J M. Review of nitrogen and phosphorus metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology, 2000, 250: 133-167 |
TOUCHETTE B W. Seagrass-salinity interactions: Physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology, 2007, 350(1/2): 194-215 |
TUSSENBROEK B I. Thalassia testudinum leaf dynamics in a Mexican Caribbean coral reef lagoon. Marine Biology, 1995, 122(1): 33-40 |
UNEP. Out of the blue, the value of seagrasses to the environment and to people. United Nations Environment Programme; GRID-Arendal; United Nations Environment Programme World Conservation Monitoring Centre, 2020
|
VAN KATWIJK M M, VERGEER L H T, SCHMITZ G H W, et al. Ammonium toxicity in eelgrass Zostera marina. Marine Ecology Progress Series, 1997, 157: 159-173 |
VERGEER L H T, AARTS T L, DE KATWIJK J D. The "wasting disease" and the effect of abiotic factors (light intensity, temperature, salinity) and infection with Labyrinthula zosterae on the phenolic content of Zostera marina shoots. Aquatic Botany, 1995, 52: 35-44 |
VERMAAT J E, VERHAGEN F C A, LINDENBURG D. Contrasting responses in two populations of Zostera noltii Hornem to experimental photoperiod manipulation at two salinities. Aquatic Botany, 2000, 67(3): 179-189 |
VILLAZAN B, BRUN F G, JIMENEZ-RAMOS R, et al. Interaction between ammonium and phosphate uptake rates in the seagrass Zostera noltii. Marine Ecology Progress Series, 2013, 488: 133-143 |
WALKER D I, MCCOMB A J. Salinity response of the seagrass Amphibolis antarctica (Labill.) Sonder et Aschers: An experimental validation of field results. Aquatic Botany, 1990, 36(4): 359-366 |
WANG S R, YIN K D, CAI W J, et al. Advances in studies of ecological effects of ocean acidification. Acta Ecologica Sinica, 2012, 32(18): 5859-5869 [汪思茹, 殷克东, 蔡卫君, 等. 海洋酸化生态学研究进展. 生态学报, 2012, 32(18): 5859-5869] |
WAYCOTT M, DUARTE C M, CARRUTHERS T J B, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences, 2009, 106(30): 12377-12381 |
WESTLAKE E L, KEESING J K, HARDIMAN L, et al. Growth, biomass and productivity of the seagrass Thalassia hemprichii at Ashmore Reef, Australia. Aquatic Botany, 2022, 183: 103557 |
WU R, WANG D R. Current status of seagrass beds and ecosystem restoration and reconstruction in Hainan Province. Ocean Development and Management, 2013, 30(6): 69-72 [吴瑞, 王道儒. 海南省海草床现状和生态系统修复与重建. 海洋开发与管理, 2013, 30(6): 69-72] |
XU Z Z, HUANG L M, HUANG X P, et al. A primary study on sexual reproduction of seagrass Thalassia hemprichii at Xincun Bay. Journal of Tropical Oceanography, 2008, 2: 60-63 [许战洲, 黄良民, 黄小平, 等. 新村湾泰来藻有性繁殖的初步研究. 热带海洋学报, 2008, 2: 60-63] |
XU Z Z, HUANG L M, HUANG X P, et al. Review of seagrass biomass and primary production research. Acta Ecologica Sinica, 2007, 6: 2594-2602 [许战洲, 黄良民, 黄小平, 等. 海草生物量和初级生产力研究进展. 生态学报, 2007, 6: 2594-2602] |
ZHANG C, ZHANG X Y, ZOU H T, et al. Contrasting effects of ammonium and nitrate additions on the biomass of soil microbial communities and enzyme activities in subtropical China. Biogeosciences, 2017, 14(20): 4815-4827 |
ZHANG J P, HUANG X P, JIANG Z J. Physiological responses of the seagrass Thalassia hemprichii (Ehrenb.) Aschers as indicators of nutrient loading. Marine Pollution Bulletin, 2014, 83(2): 508-515 |
ZHANG J. Transplant restoration effect of Thalassia hemprichii in Gaolong Bay and Xincun Lagoon of Hainan. Masterxs Thesis of Hainan Tropical Ocean University, 2022 [张剑. 海南高隆湾与新村港泰来草移植修复效果研究. 海南热带海洋学院硕士研究生毕业论文, 2022]
|
ZHANG P D, ZHANG Y H, ZHANG H Y, et al. Research advances in shoot propagation theory and planting technique of seagrasses. Progress in Fishery Sciences, 2020, 41(4): 181-189 [张沛东, 张彦浩, 张宏瑜, 等. 海草植株扩繁理论及其定植效应的研究进展. 渔业科学进展, 2020, 41(4): 181-189] |
ZHANG X M, ZHOU Y, LIU P, et al. Temporal pattern in the bloom-forming macroalgae Chaetomorpha linum and Ulva pertusa in seagrass beds, Swan Lake lagoon, North China. Marine Pollution Bulletin, 2014, 89: 229-238 |
ZHANG X, LIU S, LI J, WU Y, et al. Nutrient enrichment decreases dissolved organic carbon sequestration potential of tropical seagrass meadows by mediating bacterial activity. Ecological Indicators, 2022, 145: 109576 |
ZIEMAN, J C. Seasonal variation of turtle grass, Thalassia testudinum König, with reference to temperature and salinity effects. Aquatic Botany, 1975, 1: 107-123 |
ZIMMERMAN R C, SMITH R D, ALBERTE R S. Thermal acclimation and whole-plant carbon balance in Zostera marina L. (eelgrass). Journal of Experimental Marine Biology and Ecology, 1989, 130(2): 93-109 |


