2. 中国科学院烟台海岸带研究所 牟平海岸带环境综合试验站 山东 烟台 264117;
3. 中国科学院大学 北京 100049
2. Muping Coastal Environmental Research Station, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264117, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
微塑料是指粒径小于5 mm的塑料碎片,是世界上最受关注的新兴污染物之一。直接加工形成的微塑料,通过自然和人为因素进入海洋环境,称为初级微塑料;塑料碎片还能经过光氧化、生物降解、热降解等方式,分解成为更小的塑料碎片,称为次级微塑料。很多研究发现,微塑料暴露能够导致海洋生物的组织损伤,并能影响其能量代谢、免疫和发育等过程(Bakir et al, 2014; Qiao et al, 2019; Bringer et al, 2020; Teng et al, 2021; 夏斌等, 2019)。例如,Teng等(2021)研究发现,微塑料暴露可以改变长牡蛎(Crassostrea gigas)的能量代谢并引发长牡蛎的炎症反应。Opitz等(2020)研究发现,环境相关浓度微塑料对贻贝(Choromytilus chorus)的能量平衡和生理指标的影响最小。小粒径微塑料暴露会导致菲律宾蛤仔(Ruditapes philippinarum)血淋巴细胞凋亡率升高(柳佳佳等, 2021)。此外,不同粒径大小的微塑料粒径对翡翠贻贝(Perna viridis)具有不同的毒性效应,大粒径(300~1 000 μm)的聚苯乙烯(PS)、聚丙烯(PP)和聚丁二酸丁二醇酯(PBS)微塑料与中等粒径(30~300 μm)和小粒径(<30 μm)微塑料相比,更可能导致翡翠贻贝死亡率升高(Phothakwanpracha et al, 2021)。
全球变暖使得海洋生物生活在更高的海水温度下。联合国政府间气候变化专门委员会(IPCC)预测,到21世纪末,温度将上升1.4~3.1 ℃ (Pachauri et al, 2014)。海水温度升高会影响贝类的免疫反应、发育和能量代谢等多种生理过程(Rahman et al, 2019; Rahman et al, 2021; Wu et al, 2021; Zhang et al, 2023; 吕旭宁等, 2018)。Coppola等(2017)研究发现,温度升高会对紫贻贝(Mytilus galloprovincialis)产生更高的氧化损伤。也有研究表明,温度升高能够显著增加牡蛎(Crassostrea virginica)的细胞凋亡,并能引起热休克蛋白(heat shock protein 70, HSP70)基因mRNA表达量的升高(Rahman et al, 2021)。
在海洋和河口环境中,海洋生物经常暴露于高温、污染物等多种环境应激源中(Abe, 2021; Andrady, 2015; 高云涛等, 2022; 孔祥辉等, 2022)。以往的研究大多开展微塑料或温度变化对海洋生物的单一暴露实验(Paul-Pont et al, 2016; Pei et al, 2022; Rahman et al, 2021; 高振锟等, 2017)。升温和微塑料复合暴露的研究多集中在淡水生物(Kratina et al, 2019; Weber et al, 2020; Wen et al, 2018)。例如,聚苯乙烯微塑料和热刺激对淡水贻贝(Dreissena polymorpha)的复合暴露研究发现,热刺激对贻贝的影响大于微塑料(Weber et al, 2020)。Kratina等(2019)研究表明,温度能够改变微塑料对蚤状钩虾(Gammarus pulex)代谢率的影响,在低温条件下代谢率随着微塑料浓度的增加而增加,而在较高温度条件下代谢率随着微塑料浓度的增加反而降低。Wen等(2018)在探究升温和微塑料复合暴露对丽鱼(Symphysodon aequifasciatus)的研究中发现,升温与微塑料复合暴露对淀粉酶活性具有拮抗作用,而对脂肪酶活性无显著影响。有关升温和微塑料复合暴露对海洋生物的研究较少(Ferreira et al, 2016; Fonte et al, 2016)。例如,Ferreira等(2016)在探究升温、金纳米颗粒(Au-NP)和微塑料复合暴露对海水虾虎鱼(Pomatoschistus microps)的研究中发现,在高温条件下,Au-NP暴露对虾虎鱼个体和种群适应性产生不利影响的风险增加。因此,升温和微塑料复合暴露对海洋生物毒性效应研究亟待开展。本研究采用3个微塑料水平[无微塑料、小粒径聚苯乙烯微塑料(SPS-MPs, 6 μm)和大粒径聚苯乙烯微塑料(LPS-MPs, 50~60 μm)]和2个温度水平(20 ℃和25 ℃),探究升温和微塑料对长牡蛎血细胞功能、能量代谢和免疫基因表达的影响,以期为评估全球变暖背景下污染物对海洋生物的毒性效应提供数据支撑。
1 材料与方法 1.1 实验材料2020年5月于山东威海乳山长牡蛎养殖场购买410只长牡蛎(Crassostrea gigas) (壳长6~8 cm)用于复合暴露实验。实验开始前,将长牡蛎在40 L的养殖缸中暂养2周[盐度为32±0.4;温度为(20±0.2) ℃;pH为8.1±0.2]。暂养期间,每天用小球藻(Chlorella) (1×105 cells/mL)喂养牡蛎,养殖海水每2 d更换一次。
1.2 微塑料工作液制备SPS-MPs (6 μm, 2.5% w/v, 10 mL)和LPS-MPs (50~ 60 μm, 2.5% w/v, 10 mL)均购买于天津市倍思乐色谱技术开发中心。采用0.22 μm滤膜过滤的Milli-Q超纯水配制SPS-MPs工作液(浓度为4 × 105个/mL)。每次使用前均对原液和工作液进行超声处理,使其分散均匀。通过扫描电子显微镜(SEM, 日立S-4800)检查微塑料的粒径和形态(图 1)。
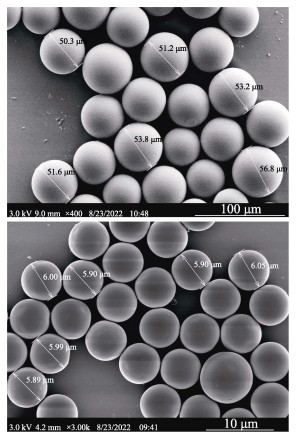
|
图 1 LPS-MPs(A)和SPS-MPs(B)的扫描电子显微镜图 Fig.1 The SEM image of LPS-MPs (A) and SPS-MPs (B) |
长牡蛎暂养后,随机分为6组,分别采用3个微塑料水平[无微塑料、SPS-MPs (6 μm)和LPS-MPs (50~60 μm)]和2个温度水平(20 ℃和25 ℃),共计6个处理组合,探究升温和PS-MPs复合暴露对长牡蛎的影响,暴露实验持续21 d。每个处理组设3个水箱(40 L)作为重复,每个水箱养殖20只牡蛎。考虑到长牡蛎的物种适应性和环境最高水温(Sun et al, 2022),25 ℃作为升温条件,20 ℃为实验期间环境实际水温。实验开始前,升温组的海水温度由环境温度(20 ℃)每天升高2 ℃逐渐升高至25 ℃,使长牡蛎逐渐适应25 ℃的水温。微塑料暴露浓度设置为1×104个/L。各组别海水每天更换1次,并在微塑料暴露组中添加微塑料。于暴露第21天采样,收集各组长牡蛎的消化腺和鳃组织,液氮冷冻后,–80 ℃保存。
1.4 血淋巴细胞相关免疫指标采用一次性注射器抽取长牡蛎血淋巴,经300目筛绢过滤后,迅速与等量的抗凝剂混合,将血淋巴样本分装2份各500 μL用于活性氧(reactive oxygen species,ROS)和吞噬活性的检测,采用台式冷冻高速离心机4 ℃、2000×g离心10 min,弃上清液,加入等量500 μL的PBS,再以4 ℃、2000×g离心10 min后,弃上清液,加入相应的缓冲溶液进行各指标的检测。
采用2′, 7′-二氯二氢荧光素二乙酸酯(2′, 7′-dichlorofluorescein diacetate, DCFH-DA)荧光探针(Sigma)对血淋巴组织中的活性氧进行检测。向血淋巴细胞(500 μL)中加入5 μL荧光探针DCFH-DA (0.01 mmol/L),避光,在18 ℃混合孵育30 min。在激发波长为488 nm、发射波长为530 nm的条件下,用流式细胞仪(BD Accuri™ C6 flow cytometer)对样本进行检测。上机前,采用300目筛绢过滤,根据FL-1通道的荧光强度的几何平均值,来表征血淋巴细胞ROS的含量。
采用荧光微球(YG 2.0 μm,Polysciences, 德国)对血细胞的吞噬活性进行测定。将250 μL的长牡蛎血淋巴与2.3%的荧光微球进行混合,并避光放置60 min,然后向混合液中加入福尔马林(15 μL)终止反应,经过300目筛绢过滤,采用流式细胞仪FL-1通道检测,采用摄入3个或更多荧光微球的血细胞占总的血细胞数目的百分比来估算血细胞吞噬活性。
1.5 糖原含量测定长牡蛎消化腺组织中的糖原含量采用蒽酮显色法,并用肝/肌糖原检测试剂盒进行检测,购买自南京建成生物工程研究所。按照说明书的方法进行检测,单位为mg/g组织。
1.6 免疫和应激相关基因的mRNA表达采集各实验组和对照组长牡蛎(n=6)的消化腺和鳃组织进行基因的mRNA表达检测,于–80 ℃保存。用TRIzol试剂(Invitrogen)分离提取总RNA,Nanodrop检测总RNA浓度。cDNA用逆转录酶M-MLV (Promega, 美国)合成。核因子κB抑制蛋白(inhibitor of NF-κB, IκB)基因、p53基因和HSP90基因的mRNA表达量采用StepOne Plus实时荧光定量PCR仪(ABI公司,美国)进行检测。荧光定量PCR所用引物信息见表 1。选择转录延伸因子1α (EF1α)作为内参基因。
|
|
表 1 荧光定量PCR引物序列 Tab.1 Primers used in real-time PCR |
为了观察长牡蛎是否摄入微塑料,采用显微镜进行镜检,由于SPS-MPs在体式显微镜下较难识别,只对LPS-MPs进行了镜检。首先,在复合暴露实验过程中收集长牡蛎粪便,并在显微镜下观察。然后,为了方便观察,将LPS-MPs采用Shim等(2016)的方法进行尼罗红染色,在20 ℃和25 ℃对长牡蛎进行复合暴露后,采集长牡蛎的鳃和消化腺组织,并加入180 mL 10% KOH和20 mL 30% H2O2进行消解,60 ℃放置24 h,采用8 μm滤膜(上海兴亚, 中国)进行真空抽滤,采用体式显微镜(奥林巴斯SZX10, 日本)对LPS-MPs进行镜检(Munno et al, 2018)。
1.8 数据分析结果均以平均值±标准误(Mean±SEM)表示。血细胞指标的数据通过FlowJo软件进行分析。数据的正态性检验采用Shapiro-Wilk检验,方差齐性检验采用Levene检验。对于不符合正态分布或方差齐性的数据,进行以10为底的对数变换(lg)。采用SPSS 22.0软件进行双因素方差分析(two-way ANOVA),P<0.05被认为具有显著性。采用LSD检验(LSD test)进行多重比较分析。
2 结果 2.1 血细胞免疫指标升温和微塑料复合暴露21 d后,各组别长牡蛎的血淋巴免疫指标如图 2所示。ANOVA分析表明,升温和微塑料复合暴露对长牡蛎血淋巴细胞中的ROS含量和吞噬活性无显著的交互作用(P>0.05)(表 2)。总体而言,在各温度水平下,SPS-MPs均可抑制长牡蛎血淋巴细胞吞噬活性,增加ROS产量。
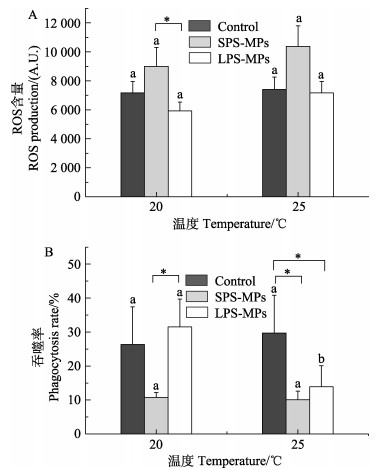
|
图 2 升温和微塑料暴露对长牡蛎血淋巴免疫指标的影响 Fig.2 Immune-related parameters in hemocytes of C. gigas exposed to elevated temperature and MPs A:呼吸爆发(n=5);B:吞噬活性(n=4~6)。不同字母表示相同微塑料水平下不同温度水平之间存在显著差异(P<0.05);星号(*)表示相同温度水平下不同微塑料水平之间存在显著差异(P<0.05)。下同。 A: ROS (n=5); B: Phagocytosis (n=4~6). Different letters indicate significant differences between different temperatures within the same MPs level (P < 0.05); asterisks indicate significant differences between different MPs levels within the same temperature (P < 0.05). The same below. |
|
|
表 2 升温和微塑料暴露对长牡蛎血细胞功能、糖原含量和免疫基因表达的影响(双因素方差分析) Tab.2 Effects of elevated temperature and MPs on hemocytes function, glycogen content, and the expression of immune related genes of C. gigas (two-way ANOVA) |
升温和微塑料复合暴露21 d后,各处理组长牡蛎消化腺组织中糖原含量如图 3所示。ANOVA分析表明,升温与微塑料复合暴露对消化腺组织中糖原含量具有显著的交互作用(P<0.05)(表 2)。升温能够增强微塑料对糖原含量的诱导作用,25 ℃+LPS-MPs复合暴露组中长牡蛎消化腺组织中糖原的含量相比于升温和LPS-MPs单独暴露组显著增加(P<0.05)(图 3)。
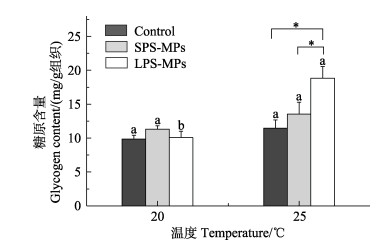
|
图 3 升温和微塑料暴露对长牡蛎消化腺组织中糖原含量的影响(n=4) Fig.3 Glycogen content in digestive glands of C. gigas exposed to elevated temperature and MPs (n=4) |
升温和微塑料复合暴露21 d后,各处理组长牡蛎消化腺组织中免疫相关基因mRNA的表达量如图 4所示。ANOVA分析表明,升温与微塑料复合暴露对长牡蛎消化腺组织中HSP90、p53和IκB基因的表达量具有显著的交互作用(P<0.05)(表 2)。25 ℃+SPS-MPs复合暴露组长牡蛎消化腺中HSP90、p53和IκB基因表达量相较于SPS-MPs和升温单独暴露组均显著升高(P<0.05)。微塑料单独暴露能够引起HSP90和IκB基因表达量相较于对照组上调。此外,25 ℃+LPS-MPs复合暴露相较于LPS-MPs单独暴露能够显著降低IκB基因的表达量(P<0.05)(图 4E)。

|
图 4 升温和微塑料暴露对长牡蛎消化腺和鳃组织中免疫相关基因mRNA表达量的影响(n=6) Fig.4 The mRNA expression of immune related genes in digestive glands and gills of C. gigas exposed to elevated temperature and MPs (n=6) |
升温和微塑料复合暴露21 d后,各处理组长牡蛎鳃组织中免疫相关基因mRNA的表达量如图 4所示。ANOVA分析表明,微塑料与升温复合暴露对长牡蛎鳃组织中p53、IκB和HSP90基因的表达表现出显著交互作用(P<0.05)(表 2)。25 ℃+SPS-MPs复合暴露组长牡蛎鳃组织HSP90基因的表达相较于SPS-MPs单独暴露组显著降低(P<0.05)(图 4B)。此外,在20 ℃条件下,微塑料暴露会抑制p53基因的表达量;而在25 ℃条件下,微塑料暴露会诱导p53基因的表达量(图 4D)。微塑料和升温单独暴露相较于对照组能够显著增加IκB基因的表达量(P<0.05)(图 4F)。
2.4 微塑料镜检显微镜视野下,长牡蛎粪便中和组织消解后滤膜上的LPS-MPs如图 5所示。镜检结果发现,在长牡蛎的粪便以及消化腺和鳃组织消解后的滤膜上均发现LPS-MPs。
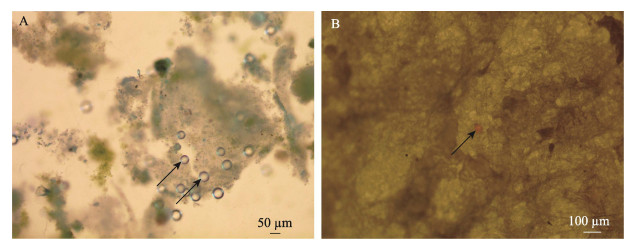
|
图 5 显微镜视野下长牡蛎粪便中(A)和组织消解后(B)的LPS-MPs Fig.5 Microscopic view of LPS-MPs in feces (A) and after tissue digestion (B) of C. gigas |
很多研究表明,微塑料暴露能够诱发海洋生物体内ROS的产生。ROS包括过氧化氢(hydrogen peroxide,H2O2)、羟自由基(hydroxyl radical, ·OH)和超氧阴离子(superoxide anion,O2–)等,其作为细胞氧化代谢的有毒副产物,会破坏细胞结构,导致细胞膜系统损坏(Landis et al, 2005)。有研究表明,聚苯乙烯微塑料暴露可导致贻贝(Mytilus spp.)血细胞活性氧的积累,增强抗氧化酶活性(Paul-Pont et al, 2016)。金头鲷鱼(Sparus aurata)在聚甲基丙烯酸甲酯(PMMA)纳米塑料暴露后,能够诱发机体产生抗氧化反应(Brandts et al, 2021)。本研究中,SPS-MPs单独暴露能够显著增加长牡蛎血淋巴组织中ROS含量,这可能是由于SPS-MPs引起长牡蛎血淋巴组织发生氧化应激所导致,而LPS-MPs暴露对长牡蛎血淋巴细胞ROS含量无显著影响,说明微塑料尺寸越小,对长牡蛎血细胞ROS含量的影响越大。与之类似,SPS-MPs暴露能够抑制长牡蛎血淋巴细胞的吞噬活性,影响其细胞免疫功能,而LPS-MPs对长牡蛎血淋巴细胞的吞噬活性无影响。同样,厚壳贻贝(Mytilus coruscus)暴露于聚苯乙烯微塑料21 d后,其血淋巴细胞的吞噬活性受到抑制(Huang et al, 2022)。Pavičić-Hamer等(2022)研究也表明,PMMA微塑料暴露能够诱发紫贻贝血淋巴细胞的免疫反应,引起血细胞总数的增加,并抑制细胞活力。此外,Phothakwanpracha等(2021)研究也表明,小粒径微塑料具有更强的毒性作用。
前期研究表明,生物在受到热应激胁迫时会产生ROS,从而诱发氧化应激反应(Banh et al, 2016)。然而,本研究发现,升温对长牡蛎血淋巴细胞中ROS的产量无影响,但在25 ℃条件下,升温组长牡蛎血淋巴细胞ROS整体有升高趋势,推测未发现显著性差异的原因可能与牡蛎的个体差异有关。与之类似,升温对大马蹄螺(Trochus niloticus)血淋巴细胞中ROS含量无显著性影响(Zhang et al, 2021)。本研究中,25 ℃+LPS-MPs复合暴露组长牡蛎血淋巴细胞吞噬活性相较于LPS-MPs单独暴露组显著抑制,提示复合暴露组血淋巴组织免疫功能受到抑制。尽管升温组血淋巴的吞噬活性有升高趋势,但升温单独暴露组血淋巴细胞吞噬活性相较于对照组无显著性差异,这可能是由于牡蛎的个体差异所致。Rahman等(2019)研究表明,升温(25 ℃)显著提高了长牡蛎、紫贻贝和蛤蜊(Katelysia rhytiphora)血淋巴细胞的吞噬活性。然而,Monari等(2007)研究表明,升温(30 ℃)降低了蛤蜊(Chamelea gallina)血淋巴细胞的吞噬活性。
3.2 能量代谢能量代谢相关标志物能够用于指示细胞能量水平的状态和环境压力的强度(Dong et al, 2016)。有研究发现,糖原在能量储备中发挥重要的作用(Smolders et al, 2003; Sokolova, 2013)。以往的研究表明,贝类体内糖原的储备情况能够直接反映贝类应对环境胁迫的能力,并且其含量受到自身的生理过程以及外界环境的影响(Cordeiro et al, 2016; 梅丽敏等, 2023)。本研究中,升温单独暴露对长牡蛎消化腺糖原含量无影响,可能是由于糖原被大量利用,因而没有表现出积累的趋势。与此相似,热应激对日本鼓虾(Alpheus japonicus Miers)肌肉组织中的糖原含量没有显著影响(李笑等, 2020)。然而,Zhang等(2021)报道,海水升温能够导致大马蹄螺肌肉组织中糖原含量下降。这可能是由于长牡蛎与大马蹄螺具有不同的能量代谢机制。
升温和微塑料复合暴露对长牡蛎消化腺组织糖原含量的协同作用增加了糖原储备,可能是由于复合暴露组的长牡蛎具有更高的能量需求。这可能是由于海洋生物在复合压力条件下需要增加能量储备,其体内的氧化应激反应需要更高的能量来维持(Gagné et al, 2010)。
3.3 免疫相关基因表达HSP90基因是一种重要的分子伴侣蛋白基因,在生物体中能够被广泛诱导,在应对环境胁迫过程中起到重要的调节作用(Schopf et al, 2017)。IκB基因是核因子NF-κB的抑制蛋白基因,NF-κB是细胞免疫、促炎反应、凋亡和生长等基因转录激活的重要调节因子,IκB基因mRNA的表达能够影响NF-κB等免疫炎症信号通路的调控作用,从而对环境胁迫产生免疫应答(Baeuerle, 1998; Jobin et al, 2000)。肿瘤抑制因子p53是一种重要的转录因子,在应对各种细胞应激(如DNA损伤)中发挥重要的作用(Lowe et al, 2013)。
在本研究中,升温和微塑料对长牡蛎消化腺组织HSP90和IκB基因mRNA表达的交互作用具有粒径依赖性:SPS-MPs与升温表现为协同作用,mRNA表达水平较高;LPS-MPs与升温则表现为拮抗作用。这些结果提示,SPS-MPs与升温复合暴露会引起长牡蛎消化腺组织较强的免疫反应,这可能是由于SPS-MPs相较于LPS-MPs对长牡蛎具有更强的毒性作用所致。与之相似,本研究发现,SPS-MPs单独暴露相较于LPS-MPs单独暴露能够引起长牡蛎鳃组织HSP90基因表达量显著升高。柳佳佳等(2021)研究也表明,小粒径微塑料比大粒径微塑料对菲律宾蛤仔具有更强的毒性作用。在消化腺和鳃组织中,微塑料单独暴露能引起长牡蛎IκB基因表达量的上调,说明IκB基因在长牡蛎应对微塑料暴露的免疫应答中发挥重要的调控作用。同样,聚乙烯微塑料能够增加鲤鱼鳃组织中NF-κB通路的IκB激酶复合物(IKKα和IKKβ)基因和NF-κB p65基因的表达量(Cao et al, 2023)。升温和微塑料对长牡蛎鳃组织IκB基因的mRNA表达具有显著的拮抗作用,与消化腺组织表现出不同的调控模式,说明IκB基因的调控作用具有组织特异性,这可能是长牡蛎鳃和消化腺组织受到胁迫刺激后发挥免疫防御功能的调节机制不同所导致。此外,升温和微塑料对消化腺和鳃中p53基因表达均有拮抗作用,在20 ℃,微塑料暴露能够降低p53基因的表达量,而在25 ℃,微塑料暴露能够升高p53基因的表达量,说明复合暴露能够启动p53基因相关免疫信号通路,从而引起机体产生免疫应答。
4 结论本研究以长牡蛎为研究对象,探究了升温与聚苯乙烯微塑料对长牡蛎免疫和能量代谢的复合毒性效应。结果发现,复合暴露会增强长牡蛎消化腺组织糖原储备;SPS-MPs与升温复合暴露会引起长牡蛎消化腺组织IκB和HSP90基因表达上调,表明升温和SPS-MPs复合暴露会引起较强的免疫反应,且SPS-MPs相较于LPS-MPs毒性作用更强;升温和微塑料的拮抗作用导致消化腺和鳃组织中p53基因的表达量上调,说明p53基因参与了升温和微塑料复合暴露的免疫应答。此外,SPS-MPs能够引起长牡蛎血淋巴细胞ROS积累,抑制吞噬活性。因此,升温与微塑料复合暴露能够诱导免疫反应,增加糖原储备,诱发血淋巴细胞产生氧化应激,提示SPS-MPs与升温长期复合暴露可能会对长牡蛎种群维持造成潜在威胁。
ABE H. Climate warming promotes Pacific oyster (Magallana gigas) production in a subarctic lagoon and bay, Japan: Projection of future trends using a three dimensional physical-ecosystem coupled model. Regional Studies in Marine Science, 2021, 47: 101968 DOI:10.1016/j.rsma.2021.101968 |
ANDRADY A L. Persistence of plastic litter in the oceans. Marine Anthropogenic Litter, 2015, 57-72 |
BAEUERLE P A. IκB-NF-κB structures: At the interface of inflammation control. Cell, 1998, 95(6): 729-731 DOI:10.1016/S0092-8674(00)81694-3 |
BAKIR A, ROWLAND S J, THOMPSON R C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuarine, Coastal and Shelf Science, 2014, 140: 14-21 DOI:10.1016/j.ecss.2014.01.004 |
BANH S, WIENS L, SOTIRI E, et al. Mitochondrial reactive oxygen species production by fish muscle mitochondria: Potential role in acute heat-induced oxidative stress. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2016, 191: 99-107 DOI:10.1016/j.cbpb.2015.10.001 |
BRANDTS I, BARRÍA C, MARTINS M A, et al. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. Journal of Hazardous Materials, 2021, 403: 123590 DOI:10.1016/j.jhazmat.2020.123590 |
BRINGER A, THOMAS H, PRUNIER G, et al. High density polyethylene (HDPE) microplastics impair development and swimming activity of Pacific oyster D-larvae, Crassostrea gigas, depending on particle size. Environmental Pollution, 2020, 260: 113978 DOI:10.1016/j.envpol.2020.113978 |
CAO J W, XU R, WANG F H, et al. Polyethylene microplastics trigger cell apoptosis and inflammation via inducing oxidative stress and activation of the NLRP3 inflammasome in carp gills. Fish and Shellfish Immunology, 2023, 132: 108470 DOI:10.1016/j.fsi.2022.108470 |
CAO R W, LIU Y L, WANG Q, et al. The impact of ocean acidification and cadmium on the immune responses of Pacific oyster, Crassostrea gigas. Fish and Shellfish Immunology, 2018, 81: 456-462 DOI:10.1016/j.fsi.2018.07.055 |
COPPOLA F, ALMEIDA Â, HENRIQUES B, et al. Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Science of the Total Environment, 2017, 601/602: 1129-1138 DOI:10.1016/j.scitotenv.2017.05.201 |
CORDEIRO N I S, ANDRADE J T M, MONTRESOR L C, et al. Physiological response of invasive mussel Limnoperna fortunei (Dunker, 1857)(Bivalvia: Mytilidae) submitted to transport and experimental conditions. Brazilian Journal of Biology, 2016, 77: 191-198 |
DONG Y W, ZHANG S. Ecological relevance of energy metabolism: transcriptional responses in energy sensing and expenditure to thermal and osmotic stresses in an intertidal limpet. Functional Ecology, 2016, 30(9): 1539-1548 DOI:10.1111/1365-2435.12625 |
FARCY E, FLEURY C, LELONG C, et al. Molecular cloning of a new member of the p53 family from the Pacific oyster Crassostrea gigas and seasonal pattern of its transcriptional expression level. Marine Environmental Research, 2008, 66(2): 300-308 DOI:10.1016/j.marenvres.2008.04.006 |
FERREIRA P, FONTE E, SOARES M E, et al. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: Gold nanoparticles, microplastics and temperature. Aquatic Toxicology, 2016, 170: 89-103 DOI:10.1016/j.aquatox.2015.11.011 |
FONTE E, FERREIRA P, GUILHERMINO L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquatic Toxicology, 2016, 180: 173-185 DOI:10.1016/j.aquatox.2016.09.015 |
GAGNÉ F, GÉLINAS M, GAGNON C, et al. Change in metallothionein phosphorylation state in Mya arenaria clams: Implication in metal metabolism and oxidative stress. Invertebrate Survival Journal, 2010, 7(1): 22-31 |
GAO Y T, GAO Y H, LI M Y, et al. Hypoxia tolerance and alternation of blood physiological and biochemical indexes in spotted knifejaw Oplegnathus punctatus. Progress in Fishery Sciences, 2022, 43(6): 79-88 [高云涛, 高云红, 李明月, 等. 斑石鲷低氧耐受能力及血液生理生化指标变化研究. 渔业科学进展, 2022, 43(6): 79-88] |
GAO Z K, ZHANG J H, LI M, et al. Effects of temperature fluctuation on physiological and immune parameters of scallop (Patinopecten yessoensis). Progress in Fishery Sciences, 2017, 38(3): 148-154 [高振锟, 张继红, 李敏, 等. 温度波动对不同规格虾夷扇贝(Patinopecten yessoensis)生理和免疫指标的影响. 渔业科学进展, 2017, 38(3): 148-154] |
HUANG X Z, LEUNG J Y S, HU M H, et al. Microplastics can aggravate the impact of ocean acidification on the health of mussels: Insights from physiological performance, immunity and byssus properties. Environmental Pollution, 2022, 308: 119701 DOI:10.1016/j.envpol.2022.119701 |
JOBIN C, SARTOR R B. The IκB/NF-κB system: A key determinant of mucosal inflammation and protection. American Journal of Physiology-Cell Physiology, 2000, 278(3): C451-C462 DOI:10.1152/ajpcell.2000.278.3.C451 |
KONG X H, WANG S S, DONG Y H, et al. Analysis of expression characteristics of related genes in response to acute thermal stress in the razor clam Sinonovacula constricta. Progress in Fishery Sciences, 2022, 43(2): 194-203 [孔祥辉, 王莎莎, 董迎辉, 等. 缢蛏急性高温胁迫应答主要候选基因的表达特征分析. 渔业科学进展, 2022, 43(2): 194-203] |
KRATINA P, WATTS T J, GREEN D S, et al. Interactive effects of warming and microplastics on metabolism but not feeding rates of a key freshwater detritivore. Environmental Pollution, 2019, 255: 113259 DOI:10.1016/j.envpol.2019.113259 |
LANDIS G N, TOWER J. Superoxide dismutase evolution and life span regulation. Mechanisms of Ageing and Development, 2005, 126: 365-379 DOI:10.1016/j.mad.2004.08.012 |
LI X, QU Y, ZHANG Q Q, et al. Effects of seawater acidification and thermal stress on the antioxidant responses and energy metabolism of Alpheus japonicus Miers. Oceanologia et Limnologia Sinica, 2020, 51(6): 1412-1421 [李笑, 曲艺, 张倩倩, 等. 海水酸化和热应激对日本鼓虾氧化应激和能量代谢的影响. 海洋与湖沼, 2020, 51(6): 1412-1421] |
LIU J J, ZHU X P, TENG J, et al. Toxic effects of polystyrene microplastics and pyrene on Ruditapes philippinarum. Marine Science Bulletin, 2021, 40(6): 644-656 [柳佳佳, 朱效鹏, 滕佳, 等. 微塑料和芘对菲律宾蛤仔的毒性效应研究. 海洋通报, 2021, 40(6): 644-656 DOI:10.11840/j.issn.1001-6392.2021.06.005] |
LOWE J, SHATZ M, RESNICK M A, et al. Modulation of immune responses by the tumor suppressor p53. BioDiscovery, 2013, 8: 1-12 |
LÜ X N, WANG X Q, WU Y L, et al. Effect of temperature on the energy budget of Arcuatula senhousei. Progress in Fishery Sciences, 2018, 39(4): 119-125 [吕旭宁, 王晓芹, 吴亚林, 等. 温度对凸壳肌蛤能量收支的影响. 渔业科学进展, 2018, 39(4): 119-125] |
MEI L M, ZHOU C X. A review: Research progress on storage, transport and utilization of glycogen in bivalves. Fisheries Science, 2023, 42(1): 167-174 [梅丽敏, 周成旭. 糖原在双壳贝类中的储存、转运和利用研究进展. 水产科学, 2023, 42(1): 167-174] |
MONARI M, MATOZZO V, FOSCHI J, et al. Effects of high temperatures on functional responses of haemocytes in the clam Chamelea gallina. Fish and shellfish immunology, 2007, 22(1/2): 98-114 |
MUNNO K, HELM P A, JACKSON D A, et al. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environmental Toxicology and Chemistry, 2018, 37(1): 91-98 DOI:10.1002/etc.3935 |
OPITZ T, BENÍTEZ S, FERNÁNDEZ C, et al. Minimal impact at current environmental concentrations of microplastics on energy balance and physiological rates of the giant mussel Choromytilus chorus. Marine Pollution Bulletin, 2020, 162(1/2): 111834 |
PACHAURI R K, ALLEN M R (eds.). Climate change 2014: Synthesis report. Contribution of working groups Ⅰ, Ⅱ and Ⅲ to the fifth assessment report of the intergovernmental panel on Climate Change. IPCC, Geneva, Switzerland, 2014, 151
|
PAUL-PONT I, LACROIX C, FERNÁNDEZ C G, et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environmental Pollution, 2016, 216: 724-737 |
PAVIČIĆ-HAMER D, KOVAČIĆ I, SOVIĆ T, et al. Exposure to polymethylmethacrylate microplastics induces a particle size-dependent immune response in Mediterranean mussel Mytilus galloprovincialis. Fishes, 2022, 7(6): 307 DOI:10.3390/fishes7060307 |
PEI X, HENG X, CHU W H. Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microbial Pathogenesis, 2022, 163: 105387 DOI:10.1016/j.micpath.2021.105387 |
PHOTHAKWANPRACHA J, LIRDWITAYAPRASIT T, PAIROHAKUL S. Effects of sizes and concentrations of different types of microplastics on bioaccumulation and lethality rate in the green mussel, Perna viridis. Marine Pollution Bulletin, 2021, 173: 112954 |
QIAO R X, DENG Y F, ZHANG S H, et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere, 2019, 236: 124334 |
RAHMAN M A, HENDERSON S, MILLER-EZZY P, et al. Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora. Fish and Shellfish Immunology, 2019, 86: 868-874 |
RAHMAN M S, RAHMAN M S. Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: Signaling pathways of cellular apoptosis during heat stress. Environmental Research, 2021, 196: 110428 |
SCHOPF F H, BIEBL M M, BUCHNER J. The HSP90 chaperone machinery. Nature Reviews Molecular Cell Biology, 2017, 18(6): 345-360 |
SHIM W J, SONG Y K, HONG S H, et al. Identification and quantification of microplastics using Nile Red staining. Marine Pollution Bulletin, 2016, 113(1/2): 469-476 |
SMOLDERS R, DE BOECK G, BLUST R. Changes in cellular energy budget as a measure of whole effluent toxicity in zebrafish (Danio rerio). Environmental Toxicology and Chemistry, 2003, 22(4): 890-899 |
SOKOLOVA I M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integrative and Comparative Biology, 2013, 53(4): 597-608 |
SUN X Y, DONG Z J, ZHANG W J, et al. Seasonal and spatial variations in nutrients under the influence of natural and anthropogenic factors in coastal waters of the northern Yellow Sea, China. Marine Pollution Bulletin, 2022, 175: 113171 |
SUSSARELLU R, FABIOUX C, SANCHEZ M C, et al. Molecular and cellular response to short-term oxygen variations in the Pacific oyster Crassostrea gigas. Journal of Experimental Marine Biology and Ecology, 2012, 412: 87-95 |
TENG J, ZHAO J M, ZHU X P, et al. Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: Accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environmental Pollution, 2021, 269: 116169 |
WEBER A, JECKEL N, WAGNER M. Combined effects of polystyrene microplastics and thermal stress on the freshwater mussel Dreissena polymorpha. Science of the Total Environment, 2020, 718: 137253 |
WEN B, ZHANG N, JIN S R, et al. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquatic Toxicology, 2018, 195: 67-76 |
WU F L, SOKOLOV E P, DELLWIG O, et al. Season-dependent effects of ZnO nanoparticles and elevated temperature on bioenergetics of the blue mussel Mytilus edulis. Chemosphere, 2021, 263: 127780 |
XIA B, DU Y S, ZHAO X G, et al. Research progress on microplastics pollution in marine fishery water and their biological effects. Progress in Fishery Sciences, 2019, 40(3): 178-190 [夏斌, 杜雨珊, 赵信国, 等. 微塑料在海洋渔业水域中的污染现状及其生物效应研究进展. 渔业科学进展, 2019, 40(3): 178-190] |
ZHANG T Y, QU Y, ZHANG Q Q, et al. Risks to the stability of coral reefs in the South China Sea: An integrated biomarker approach to assess the physiological responses of Trochus niloticus to ocean acidification and warming. Science of the Total Environment, 2021, 782: 146876 |
ZHANG Y M, NIE H T, YAN X W. Metabolomic analysis provides new insights into the heat-hardening response of Manila clam (Ruditapes philippinarum) to high temperature stress. Science of the Total Environment, 2023, 857: 159430 |
ZHANG Y, HE X C, YU Z N. Two homologues of inhibitor of NF-kappa B (IκB) are involved in the immune defense of the Pacific oyster, Crassostrea gigas. Fish and Shellfish Immunology, 2011, 30(6): 1354-1361 |



