2. 上海海洋大学水产与生命学院 上海 201306;
3. 邦普种业科技有限公司 山东 潍坊 261312
2. College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai 201306, China;
3. BLUP Aquabreed Co., Ltd., Weifang 261312, China
凡纳对虾(Penaeus vannamei)又称南美白对虾,是我国和世界养殖产量最高的对虾种类,于1988年引入我国,因生长速度快、易于养殖、抗逆和抗病能力强、出肉率高等特点,其产量逐年增加(刘峻宇等, 2021)。2021年,我国凡纳对虾养殖产量为197.74万t (农业农村部渔业渔政管理局等, 2022),占世界总产量的三分之一。优质种苗是凡纳对虾产业发展的基础(汪春玲等, 2018)。国内对于凡纳对虾种苗的需求量逐年增加,目前,我国每年种虾需求量高达100万对,虾苗总量突破1.3万亿尾(农业农村部渔业渔政管理局等, 2022)。然而,在凡纳对虾实际生产中发现,进行促熟的雌虾产卵频次差异巨大(Arcos et al, 2003、2004)。Arcos等(2003)对106尾摘除单侧眼柄的凡纳对虾雌虾36 d内的产卵频次进行统计,发现48%的雌虾没有产卵,18%的雌虾产卵1次,15%的雌虾产卵2次,11%的雌虾产卵3次,8%的雌虾产卵4次或更多。樊云鹏等(2021)对371尾摘除单侧眼柄的凡纳对虾雌虾进行为期30 d的产卵频次记录,发现51%的雌虾不产卵,26%的雌虾产卵1次,只有23%的雌虾产卵2次或2次以上。我国每年需要从国外引进30万对凡纳对虾种虾,每对种虾价格约为200美元,需要花费人民币约4.2亿元,假使40%的雌虾存在不产卵的情况,每年仅引进亲虾的损失就超过8 000万元。通过遗传改良提高亲虾的繁殖性能,培育高繁殖力凡纳对虾,是降低育种育苗单位成本和提高经济效益的有效途径。
卵巢发育是甲壳动物繁殖过程的重要环节,直接影响到雌性个体的产卵量、后代受精率和存活率等,是甲壳动物育种及商业化养殖过程中人们极为关注的问题。甲壳类动物卵巢的发育受多种激素和基因的控制,研究人员从多个层面对影响甲壳动物卵巢发育的因素展开研究,发现卵母细胞中卵周隙内含物、内质网和线粒体等多种细胞组分参与了卵黄粒的形成(姜永华等, 2005a),同时,发现vasa基因(何雪莹, 2021)、组织蛋白酶(cathepsin)家族基因(Cheng et al, 2022)等多种基因在卵巢发育过程中发挥重要功能。眼柄中的X器官–窦腺复合体(XO-SG)作为甲壳动物重要的神经内分泌器官,在卵巢的发育和成熟的过程中起着重要作用。由XO-SG合成和释放的甲壳动物高血糖激素(crustacean hyperglycemic hormone, CHH) (Chen et al, 2020)、蜕皮抑制激素(molt-inhibiting hormone, MIH)(Chen et al, 2020)、性腺抑制激素(vitellogenesis/gonad-inhibiting hormone, VIH/GIH) (Jayasankar et al, 2020)、大颚器官抑制激素(mandibular organ-inhibiting hormone, MOIH) (Jayasankar et al, 2020)等在甲壳动物生殖方面发挥重要作用。然而,在相同养殖环境下凡纳对虾雌虾繁殖力产生巨大差异的分子调控机制至今仍不清楚。
研究团队前期在繁殖力分离群体的基因组选择清除区域中发现了大量差异候选基因,其中包括在其他物种卵母细胞成熟、细胞增殖、早期发育等过程中发挥重要作用的硫氧还蛋白2基因(TRX2)、分离缺陷基因3 (PARD3)、磷脂酶Cβ4基因(PLCβ4)和精氨酸谷氨酸二肽重复基因(RERE) (Sui et al, 2022)。其中,硫氧还蛋白(Thioredoxin, Trx)广泛分布于微生物、植物和动物体内,是细胞内一种重要的氧化还原蛋白,参与体内氧化还原等反应,具有调控细胞凋亡、机体代谢和免疫反应等作用(马宇光等, 2011);PARD3是细胞极性的重要调控因子,参与胚胎发育、神经分化、上皮形态和细胞迁移过程中的许多极化事件(Li et al, 2017);PLCβ4是磷脂酶C基因家族的一员,该基因编码的蛋白质催化磷脂酰肌醇4, 5-二磷酸形成肌醇-1, 4, 5-三磷酸和二酰甘油(陈徐, 2021);RERE (精氨酸-谷氨酸二肽重复序列)是一种蛋白质编码基因,与RERE相关的疾病包括伴有或不伴有大脑、眼睛或心脏异常的神经发育障碍和染色体1p36缺失综合征(Jordan et al, 2018)。上述基因的研究多在模式生物中展开,甲壳动物中的功能研究尚未见报道。本研究以一个生产周期中雌虾产卵频次为繁殖力指标性状,以高繁殖力和低繁殖力雌虾为研究对象,对雌虾繁殖期不同卵巢发育阶段(增殖期、小生长期、大生长期和成熟期)进行组织学观察,采用实时荧光定量PCR方法(qRT-PCR)分析比较上述繁殖候选基因在高、低繁殖力雌虾不同卵巢发育阶段卵巢和眼柄的表达模式,以期为凡纳对虾雌虾繁殖力产生差异的分子机制研究提供参考。
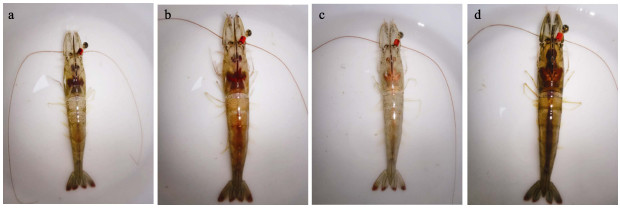
1 材料与方法 1.1 实验材料实验用凡纳对虾雌虾来自邦普种业科技有限公司(山东潍坊)于2021年6月培育的439尾亲虾。从外部对雌虾卵巢进行观察,按照卵巢的颜色、大小、形状和结构等发育情况,将卵巢发育分为4个时期(Tinikul et al, 2011):增殖期(Ⅰ期)雌虾的卵巢在体外进行观察时无法辨别其形态与颜色;小生长期(Ⅱ期)雌虾卵巢的体积增大,体外观察依稀可辨;大生长期(Ⅲ期)雌虾的卵巢体积持续增大,从头胸甲背侧可以清楚地观察到卵巢;成熟期(Ⅳ期)雌虾的卵巢发育达到最大饱满度,在整个头胸甲及背部都能观察到卵巢,雌虾即将产卵。凡纳对虾卵巢Ⅰ~Ⅳ期发育变化的外观见图 1。
|
图 1 凡纳对虾卵巢Ⅰ~Ⅳ期发育变化 Fig.1 Developmental changes of the ovaries of P. vannamei from stageⅠto Ⅳ a:Ⅰ期;b:Ⅱ期;c:Ⅲ期;d:Ⅳ期。 a: StageⅠ; b: StageⅡ; c: Stage Ⅲ; d: Stage Ⅳ. |
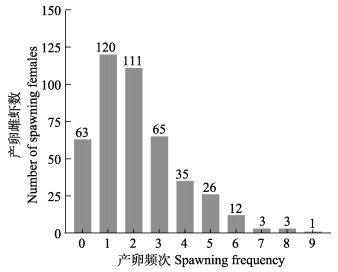
实验前,对凡纳对虾7月龄亲虾投喂商品亲虾强化饲料、促熟饲料和鱿鱼进行营养强化。促熟1个月后,使用镊烫法剪去雌虾单侧眼柄,恢复15~20 d后开始下一代家系构建。每天上午9点观察雌虾的卵巢发育情况,将卵巢发育饱满且色泽为红褐色或者黄褐色的Ⅳ期成熟雌虾挑出,按照配种方案放入雄虾池进行自然交尾。家系构建时间持续30 d,每天记录性腺成熟雌虾的眼标号。将繁殖期内的439尾雌虾的产卵频次进行统计,统计结果见图 2。
|
图 2 凡纳对虾一个生产周期内(30 d)439尾雌虾的产卵频次 Fig.2 Spawning frequency map of 439 female shrimp in a production cycle (30 d) of P. vannamei |
产卵频次为0的定为低繁殖力组,大于3次的定为高繁殖力组。在高繁殖力组中,卵巢能够发育到Ⅳ期即成熟期;而在低繁殖力组中,卵巢只能发育到Ⅲ期,很少观察到卵巢发育到Ⅳ期。
取高繁殖力组Ⅰ~Ⅳ期的雌虾和低繁殖力组Ⅰ~Ⅲ期雌虾相同部位的卵巢组织,迅速浸没于通用型组织固定液(赛维尔, G1101)中,组织与固定液比例不小于1∶10。取样完成后置于4 ℃冰箱保存,用于石蜡切片制作。
将高繁殖力组Ⅰ~Ⅳ期的雌虾和低繁殖力组Ⅰ~Ⅲ期的雌虾,每个时期各挑选3尾置于冰上,每尾虾取卵巢和眼柄组织,液氮保存,用于后续RNA的提取和基因表达量验证。
1.2 基因选择根据课题组前期在凡纳对虾繁殖力分离群体中得到的基因组选择清除分析结果,以Fst和πRatio确定选择清除指标阈值(Fst > 0.162, πRatio > 1.2),筛选得到121个差异候选基因(Sui et al, 2022)。在候选基因中选择可能在卵巢发育中发挥重要功能的TRX2、PARD3、PLCβ4和RERE基因,分析其在凡纳对虾繁殖力分离群体卵巢不同发育阶段卵巢及眼柄组织中的相对表达量。
1.3 实验方法 1.3.1 石蜡切片制作将凡纳对虾高繁殖力组(Ⅰ~Ⅳ期)和低繁殖力组(Ⅰ~Ⅲ期)卵巢组织从固定液中取出,用手术刀将卵巢组织修平整,经脱水浸蜡、包埋、切片、石蜡切片脱蜡复水、苏木素–伊红染色、脱水封片等步骤后,显微镜镜检,采集图像并进行分析。
1.3.2 qRT-PCR首先,使用RNA-easyTM Isolation试剂盒(诺唯赞)分别对凡纳对虾卵巢和眼柄组织进行RNA提取。将获得的RNA样本采用琼脂糖凝胶电泳法检测其完整性,采用NanoDrop检测法检测其浓度和OD值。质量检测合格后,使用HiScript® Ⅲ RT SuperMix for qPCR (+gDNA wiper)试剂盒(诺唯赞)对获得的RNA样本进行反转录获得cDNA,实验操作流程按照试剂盒说明书进行。
根据凡纳对虾TRX2、PARD3、PLCβ4和RERE基因的mRNA序列,采用生工生物工程(上海)股份有限公司引物在线设计软件设计qRT-PCR所用的引物。眼柄组织使用18S作为内参(根据预实验结果,18S在眼柄中的表达更稳定),卵巢组织使用β-actin作为内参。4个功能基因和内参基因所需要的引物序列如表 1所示。
|
|
表 1 qRT-PCR所用引物序列 Tab.1 Primer sequences used in qRT-PCR |
使用美国应用生物系统公司7300/7500实时定量PCR仪,按照ChamQ Universal SYBR qPCR Master Mix试剂盒(诺唯赞)说明书进行实验操作。
1.4 数据处理与统计分析基于2–ΔΔCT法计算目的基因在样品中的相对表达量,实验数据使用SPSS 26软件进行显著性检验分析,P < 0.05为差异显著,P < 0.01为差异极显著。
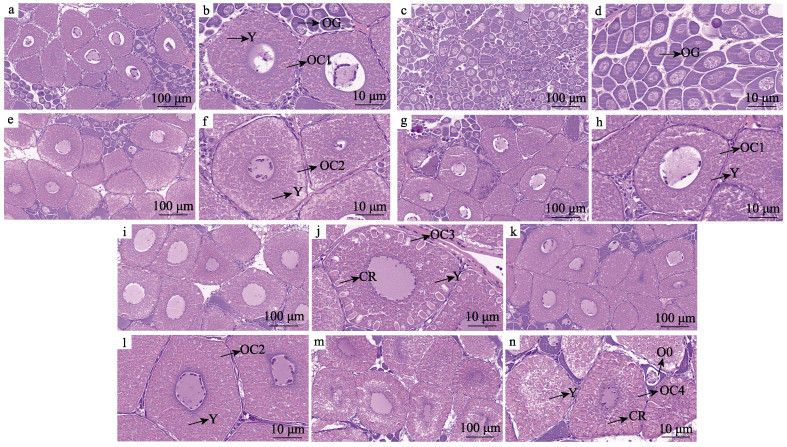
2 结果与分析 2.1 石蜡切片 2.1.1 Ⅰ期卵巢高繁殖力组(图 3a):主要由卵原细胞和卵黄合成初期卵母细胞组成。卵原细胞呈圆形或椭圆形,少数为梨形及不规则形状,体积小,卵原细胞聚集在一起形成“增殖中心”。卵黄合成初期,与卵原细胞相比,卵母细胞体积较大,且卵母细胞胞质中开始形成小球形的卵黄颗粒(图 3b)。
|
图 3 凡纳对虾高繁殖力组(Ⅰ~Ⅳ期)和低繁殖力组(Ⅰ~Ⅲ期)卵巢组织切片 Fig.3 Ovarian tissue sections of P. vannamei in high-fecundity group (stageⅠ~Ⅳ) and low-fecundity group (stageⅠ~Ⅲ) a和b:高繁殖力组Ⅰ期卵巢组织切片;c和d:低繁殖力组Ⅰ期卵巢组织切片;e和f:高繁殖力组Ⅱ期卵巢组织切片;g和h:低繁殖力组Ⅱ期卵巢组织切片;i和j:高繁殖力组Ⅲ期卵巢组织切片;k和l:低繁殖力组Ⅲ期卵巢组织切片;m和n:高繁殖力组Ⅳ期卵巢组织切片。OG:卵原细胞;O0:初级卵母细胞;OC1:卵黄合成初期卵母细胞;Y:卵黄颗粒;OC2:卵黄合成中的卵母细胞;OC3:卵黄合成后期的卵母细胞;CR:皮质棒;OC4:成熟卵母细胞。 a and b: Ovarian tissue section of high-fecundity group in stageⅠ; c and d: Ovarian tissue section of low-fecundity group in stageⅠ; e and f: Ovarian tissue section of high-fecundity group in stageⅡ; g and h: Ovarian tissue section of low-fecundity group in stageⅡ; i and j: Ovarian tissue section of high-fecundity group in stageⅢ; k and l: Ovarian tissue section of low-fecundity group in stageⅢ; m and n: Ovarian tissue section of high-fecundity group in stage Ⅳ. OG: Oogonia; O0: Primary oocytes; OC1: Oocyte in the early stage of vitellogenin synthesis; Y: Yolk granules; OC2: Oocyte in vitellogenin synthesis; OC3: Oocytes in the late stage of vitellogenin synthesis; CR: Cortical rod; OC4: Mature oocyte. |
低繁殖力组(图 3c):卵巢在视野中观察到的细胞数量多,卵巢中绝大多数为卵原细胞,且细胞大小差异较大,细胞排列整齐紧密,观察不到卵黄颗粒的形成(图 3d)。
2.1.2 Ⅱ期卵巢高繁殖力组(图 3e):主要由卵黄合成中的卵母细胞构成,与高繁殖力组Ⅰ期(图 3a)相比,卵母细胞开始变大,排列比较整齐紧密,卵黄颗粒变大且数量增多(图 3f、b)。
低繁殖力组(图 3g):卵巢主要由卵黄合成初期的卵母细胞组成,与低繁殖力组Ⅰ期相比,卵黄颗粒开始形成,低繁殖力组Ⅱ期卵巢组织发育情况与高繁殖力组Ⅰ期的卵巢发育情况相似(图 3h)。
2.1.3 Ⅲ期卵巢高繁殖力组(图 3i):主要由卵黄合成后期卵母细胞组成,相较于Ⅰ期卵巢(图 3a),卵原细胞数量明显减少,卵母细胞体积增大,细胞质中形成大量球形卵黄颗粒(图 3j),且相较于Ⅰ期(图 3a),卵母细胞内卵黄颗粒明显增多。相较于Ⅱ期(图 3f),Ⅲ期的卵母细胞开始出现皮质棒(图 3j),但此时皮质棒长度较短。
低繁殖力组(图 3k):卵巢由卵黄合成中的卵母细胞组成,胞质中充满了球形的卵黄颗粒,但在低繁殖力组Ⅲ期卵巢的卵母细胞中,并未有皮质棒的出现(图 3l)。
2.1.4 Ⅳ期卵巢高繁殖力组(图 3m):卵巢被成熟的卵母细胞所充满,体积达到最大,且排列整齐,大颗的卵黄颗粒充满了整个胞质,卵母细胞内形成粗大的皮质棒,有的卵母细胞已无法准确分辨核的位置,成熟卵母细胞间还夹杂部分初级卵母细胞,为下一次发育做准备(图 3n)。
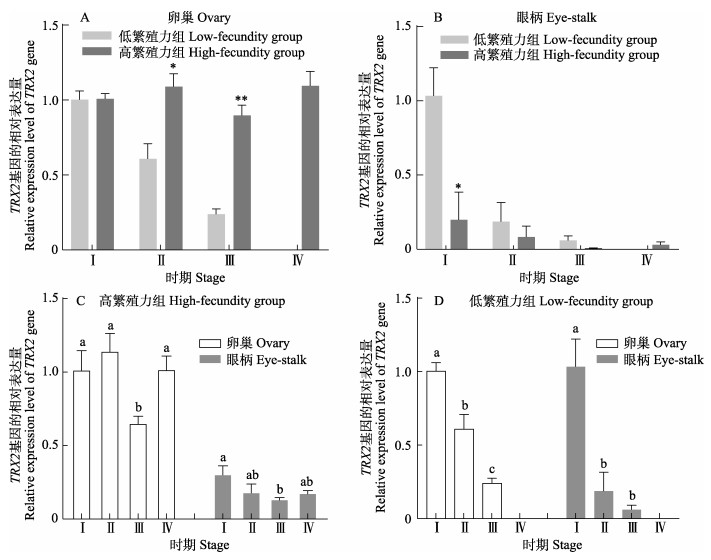
2.2 qRT-PCR结果 2.2.1 TRX2基因的表达与分析卵巢中,高繁殖力组Ⅰ~Ⅲ期TRX2基因的表达量均高于低繁殖力组,且在Ⅱ、Ⅲ期组间表达量呈显著或极显著差异(P < 0.05或P < 0.01)(图 4a);眼柄中,高繁殖力组TRX2基因的表达量都低于低繁殖力组,Ⅰ期的表达量在组间差异显著(P < 0.05)(图 4b);高繁殖力组中,卵巢和眼柄中TRX2基因的表达量都在Ⅲ期达到最低水平,在Ⅳ期又有所升高(图 4c);低繁殖力组中,卵巢和眼柄中TRX2基因的表达量均呈逐渐下降的趋势,且Ⅰ期与Ⅲ期表达量差异显著(P < 0.05)(图 4d)。
|
图 4 凡纳对虾TRX2基因的表达 Fig.4 Expression of TRX2 gene in P. vannamei *表示同时期组间差异显著(P < 0.05),**表示同时期组间差异极显著(P < 0.01);不同字母表示不同时期组内差异显著(P < 0.05)。下同。 * and ** indicate that the difference within the same stage is significant (P < 0.05) and extremely significant (P < 0.01) respectively; Different letters indicate significant difference between groups at different stages (P < 0.05). The same below. |
卵巢中,Ⅰ期高繁殖力组PARD3基因的表达量极显著低于低繁殖力组(P < 0.01),Ⅱ期和Ⅲ期略高于低繁殖力组(图 5a);眼柄中,高繁殖力组PARD3基因的表达量都显著或极显著低于低繁殖力组(P < 0.05或P < 0.01) (图 5b);高繁殖力组中,PARD3基因的表达量呈先下降后升高的趋势,在Ⅳ期达到最高水平,与Ⅱ期和Ⅲ期相比差异显著(P < 0.05)(图 5c);低繁殖力组中,PARD3基因的表达量卵巢和眼柄组织中都呈现先下降后上升的趋势(图 5d)。
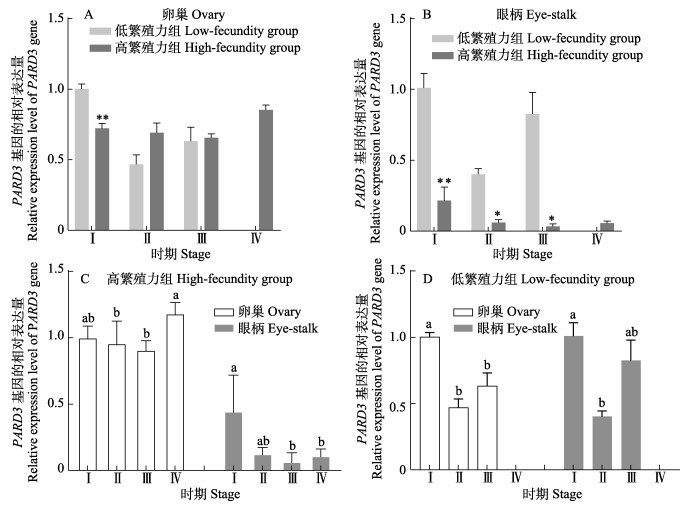
|
图 5 凡纳对虾PARD3基因的表达 Fig.5 Expression of PARD3 gene in P. vannamei |
卵巢中,PLCβ4基因在高繁殖力组Ⅰ~Ⅲ期的表达量均高于低繁殖力组,且Ⅱ期和Ⅲ期之间显著差异(P < 0.05)(图 6a);眼柄中,PLCβ4基因在高繁殖力组的表达量都低于低繁殖力组,且Ⅰ~Ⅱ期之间差异显著(图 6b);高繁殖力组中,PLCβ4基因的表达量在卵巢中呈逐渐升高的趋势,眼柄组织中呈先降低后升高的趋势(图 6c);低繁殖力组中,PLCβ4基因的表达在Ⅰ~Ⅲ期的卵巢呈先下降后升高的趋势,眼柄中呈下降的趋势(图 6d)。
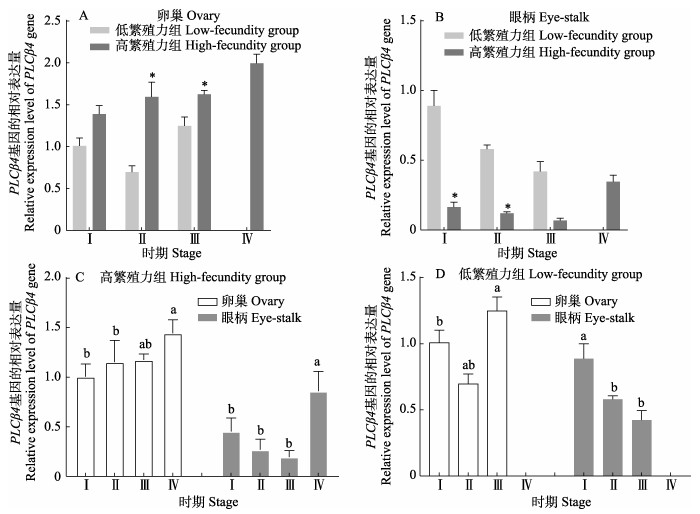
|
图 6 凡纳对虾PLCβ4基因的表达 Fig.6 Expression of PLCβ4 gene in P. vannamei |
卵巢中,高繁殖力组RERE基因的表达量极显著高于低繁殖力组(P < 0.01) (图 7a);眼柄中,高繁殖力组RERE基因的表达量显著高于低繁殖力组(P < 0.05)(图 7b);高繁殖力组中,RERE基因的表达量在Ⅰ~Ⅳ期的卵巢和眼柄中无显著差异(P > 0.05)(图 7c);低繁殖力组中,RERE基因的表达量在卵巢中呈先下降后上升的趋势,眼柄中RERE的表达量呈逐渐下降的趋势(图 7d)。
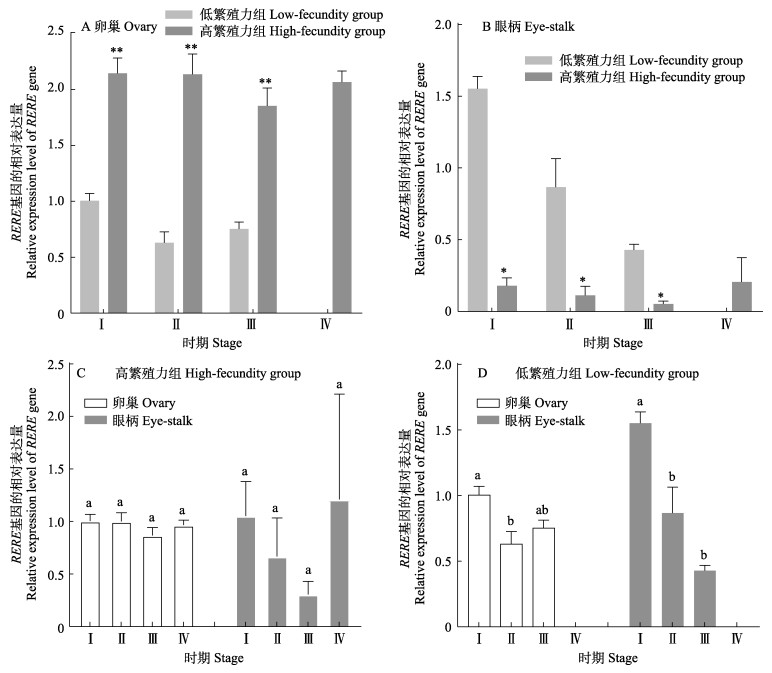
|
图 7 凡纳对虾RERE基因的表达 Fig.7 Expression of RERE gene in P. vannamei |
研究人员根据划分依据将凡纳对虾卵巢发育划分为不同时期。颜素芬等(2004)通过对凡纳对虾的卵巢进行组织学观察,将凡纳对虾卵巢分为6期,分别是增殖期、小生长期、大生长期早期、将成熟期、成熟期和恢复期。姜永华等(2005b)根据卵细胞形态、内部胞器和卵细胞发育情况等,将凡纳对虾划分为3个时期:卵原细胞、卵黄发生前的卵母细胞和卵黄发生的卵母细胞。Tinikul等(2011)根据凡纳对虾卵巢发育过程中卵巢的体积、颜色、大小、形状和结构等发育情况,将卵巢划分为4个时期,本研究采用了这种卵巢分期划分方式。
卵黄发生是甲壳动物卵巢成熟过程中的关键环节,是甲壳动物雌性生殖活动的良好指标。在卵母细胞的发育过程中,蛋白质、脂肪和其他营养物质逐渐积累,对于日后成熟卵母细胞的生成和胚胎发育起着重要作用。皮质棒的生成是对虾卵巢发育过程中卵黄积累完成的标志(Kruevaisayawan et al, 2007),参与精子入卵时的顶体反应。高、低繁殖力雌虾在各发育期卵巢外部形态相似的情况下,石蜡切片结果显示,低繁殖力组卵巢中卵母细胞卵黄颗粒的生成和积累速度(Ⅰ期)以及皮质棒的出现(Ⅲ期)均慢于高繁殖力组。由于雌虾个体间卵黄积累速度不一,雌虾卵巢发育至成熟期所需时间也不一致,导致在特定生产周期内,雌虾呈现不同的产卵频次。
卵母细胞的成熟不仅与卵黄等营养物质的积累有关,也受到相关分子机制的调控。硫氧还蛋白-2 (Trx2)是一种线粒体蛋白二硫氧化还原酶,对哺乳动物在胚胎发育过程中控制细胞存活至关重要(Patenaude et al, 2004)。孙业清等(2017)研究发现,TRX2在牛卵母细胞生发泡期至胚胎卵裂期都保持较高的表达水平。在可以有效诱导海参卵母细胞成熟的棘皮动物卵原始提取物(REES)中,发现了硫氧还蛋白-2,并被命名为Trx-REES (Léonet et al, 2019)。海参卵巢中的卵母细胞停滞在减数分裂的前期Ⅰ阶段,硫氧还蛋白作为一种氧化还原活性蛋白,被认为可以减少卵母细胞膜内的靶蛋白,并启动细胞内级联反应,从而解除卵母细胞减数分裂的阻滞(Delroisse et al, 2021)。本研究中,TRX2基因在高繁殖力组凡纳对虾卵巢发育的Ⅰ~Ⅳ期一直处于较高的表达水平,且在Ⅱ期和Ⅲ期表达量显著或极显著(P < 0.05或P < 0.01)高于低繁殖力组。PARD3是一种细胞极性蛋白,能够使细胞间紧密连接,同时也影响着细胞的增殖和代谢。在小鼠胚胎发育的过程中,靶向破坏PARD3基因会使上皮细胞发育异常,导致胚胎死亡(Hong et al, 2010)。董玉婷等(2021)在使用黄体生成素(luteinizing hormone)干预大鼠卵巢颗粒细胞后发现,PARD3基因表达水平上调,提高了卵巢颗粒细胞的细胞极性,促进卵巢细胞的增殖和生长。本研究中,PARD3基因的表达量在凡纳对虾高繁殖力组Ⅳ期卵巢中最高,推测TRX2基因和PARD3基因可能在凡纳对虾卵母细胞的增殖、生长等过程发挥重要作用。
PLCβ3与PLCβ4为同工酶,成熟的小鼠卵母细胞具有编码PLCβ3的mRNA (Dupont et al, 1996),且PLCβ3在小鼠未受精卵、第3细胞时期和卵圆筒期的胚胎中表达,PLCβ3缺陷使小鼠胚胎在第4细胞期处于发育停滞状态(Wang et al, 1998)。甄辉等(2020)研究发现,PLCβ4基因在东亚三角涡虫(Dugesia japonica)胚胎发育的第3阶段开始表达,参与胚胎神经原基的形成和分化。本研究中,高繁殖力组PLCβ4基因在卵巢的表达量均高于低繁殖力组,Ⅱ期与Ⅲ期差异显著(P < 0.05),且在高繁殖力雌虾的Ⅳ期卵巢中表达量达到最高。Kim等(2014)研究发现,RERE基因缺失会导致小鼠出生前小脑发育过程中主要裂隙的发育延迟和浦肯野细胞的成熟和迁移延迟,以及出生后小脑发育期间的小脑叶状结构异常和浦肯野细胞成熟。RERE基因的缺失也会导致小鼠出生后小眼症、生长缺陷和听力下降等缺陷,这些结果表明,RERE基因在包括眼、脑和内耳在内的多个器官的发育和功能中起关键作用(Kim et al, 2013)。本研究结果显示,高繁力组Ⅰ~Ⅲ期卵巢组织RERE基因的表达量极显著高于低繁殖力组(P < 0.01)。推测PLCβ4基因和RERE基因可能在凡纳对虾后代早期胚胎发育及各组织器官发生过程中发挥重要作用。
位于眼柄中神经内分泌组织XO-SG分泌的多种重要的神经肽类激素在卵巢发育和成熟过程中发挥至关重要的作用(García et al, 1998)。XO-SG中合成分泌的卵黄抑制激素(vitellogenesis-inhibiting hormone, VIH)对虾蟹类卵巢的发育和成熟具有直接的抑制作用(Jayasankar et al, 2020)。XO-SG中产生的大颚器官抑制激素(MOIH)可抑制大颚器官中甲基法尼酯(methyl farnesoate)的合成,而后者对虾蟹类卵巢的发育具有促进作用(Jayasankar et al, 2020; Pamuru, 2019)。眼柄分泌的甲壳动物高血糖素(crustacean hyperglycemic hormone, CHH)家族的其他一些成员,包括CHH-A、CHH-B、蜕皮抑制激素等神经肽激素也在虾蟹类卵巢发育过程中发挥重要作用(Pamuru, 2019)。本研究中,高繁殖力组4个基因的表达量在眼柄中均低于低繁殖力组,但在卵巢组织中,除了PARD3基因在Ⅰ期卵巢组织中高繁殖力组低于低繁殖力组外,在其他时期,4个基因的表达量均为高繁殖力组高于低繁殖力组,与它们在眼柄中的表达趋势相反。因此,推测这些基因在眼柄中也具有促进细胞增殖发育及靶基因表达的作用,而它们在低繁殖力组眼柄中的高表达可能加强了卵巢发育抑制因子的分泌,进而对卵巢发育产生了抑制作用,还需要进一步研究以揭示其功能机制。
4 结论本研究利用石蜡切片术对凡纳对虾高繁殖力组和低繁殖力组不同时期的卵巢组织进行组织学观察,利用实时荧光定量PCR技术首次对凡纳对虾不同时期的卵巢和眼柄组织中的TRX2、PARD3、PLCβ4和RERE基因的表达量进行分析。结果显示,高繁殖力组卵巢发育速度要快于低繁殖力组,可能与卵黄颗粒的形成和积累、皮质棒形成等关键过程有关;4个基因在高繁殖力组成熟期卵巢中均维持较高的表达水平,在小生长期、大生长期卵巢组织中,4个基因在高繁殖力组卵巢的表达量均高于低繁殖力组,推测4个基因可能与凡纳对虾卵巢成熟有关;在不同发育时期的眼柄组织中,低繁殖力组中TRX2、PARD3、PLCβ4和RERE基因的表达量均高于高繁殖力组,推测这些基因在低繁殖力组眼柄中的高表达可能加强了卵巢发育抑制因子的分泌,进而对卵巢发育产生了抑制作用,但仍需要进一步研究。研究表明,上述4种基因可能在凡纳对虾卵巢发育过程中发挥重要作用。研究结果为凡纳对虾卵巢发育调控机理研究提供了依据。
ARCOS F G, IBARRA A M, PALACIOS E, et al. Feasible predictive criteria for reproductive performance of white shrimp Litopenaeus vannamei: Egg quality and female physiological condition. Aquaculture, 2003, 228(1): 335-349 |
ARCOS F G, RACOTTA I S, IBARRA A M. Genetic parameter estimates for reproductive traits and egg composition in Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture, 2004, 236(1): 151-165 |
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook in 2022. Beijing: China Agriculture Press, 2022 [农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2022中国渔业统计年鉴. 北京: 中国农业出版社, 2022]
|
CHEN H Y, TOULLEC J Y, LEE C Y. The crustacean hyperglycemic hormone superfamily: Progress made in the past decade. Frontiers in Endocrinology (Lausanne), 2020, 11: 578958 DOI:10.3389/fendo.2020.578958 |
CHEN X. The SENP2-PLCβ4 signal axis regulates mouse neurons calcium homeostasis affects neurogenesis in hippocampus. Doctoral Dissertation of Shaanxi Normal University, 2021 [陈徐. SENP2-PLCβ4信号轴通过调节小鼠神经元钙稳态影响海马的神经发生. 陕西师范大学博士研究生学位论文, 2021]
|
CHENG D, ZHANG W, JIANG S, et al. Cathepsin D plays a vital role in Macrobrachium nipponense of ovary maturation: Identification, characterization, and function analysis. Genes (Basel), 2022, 13(8): 1495 DOI:10.3390/genes13081495 |
DELROISSE J, LÉONET A, ALEXANDRE H, et al. Intracellular pathways of holothuroid oocyte maturation induced by the thioredoxin Trx-REES. Antioxidants, 2021, 10(8): 1201 DOI:10.3390/antiox10081201 |
DONG Y T, CHEN T R, YE X F, et al. Proteomic analysis of the effects of luteinizing hormone on rat primary ovarian granulosa cells. Journal of Ningxia Medical University, 2021, 43(6): 569-576 [董玉婷, 陈泰任, 叶晓锋, 等. 黄体生成素干预大鼠卵巢颗粒细胞的蛋白质组学分析. 宁夏医科大学学报, 2021, 43(6): 569-576] |
DUPONT G, MCGUINNESS O M, JOHNSON M H, et al. Phospholipase C in mouse oocytes: characterization of beta and gamma isoforms and their possible involvement in sperm-induced Ca2+ spiking. Biochemical Journal, 1996, 316(Pt 2): 583–591
|
FAN Y P, TAN J, LUAN S, et al. Comparative analysis of breeding characteristics of different strains of Litopenaeus vannamei. Journal of Fishery Sciences of China, 2021, 28(9): 1141-1151 [樊云鹏, 谭建, 栾生, 等. 凡纳滨对虾不同品系繁育性状的比较分析. 中国水产科学, 2021, 28(9): 1141-1151] |
GARCÍA U, ARÉCHIGA H. Regulation of crustacean neurosecretory cell activity. Cellular and Molecular Neurobiology, 1998, 18(1): 81-99 DOI:10.1023/A:1022527210808 |
HE X Y. Identification and characterization of a new germline-specific marker vasa gene and its promoter in the giant freshwater prawn Macrobrachium rosenbergii. Master´s Thesis of Shanghai Ocean University, 2021 [何雪莹. 罗氏沼虾生殖细胞特异分子标记vasa基因及其启动子的分离和鉴定. 上海海洋大学硕士研究生学位论文, 2021]
|
HONG E, JAYACHANDRAN P, BREWSTER R. The polarity protein Pard3 is required for centrosome positioning during neurulation. Developmental Biology, 2010, 341(2): 335-345 DOI:10.1016/j.ydbio.2010.01.034 |
JAYASANKAR V, TOMY S, WILDER M N. Insights on molecular mechanisms of ovarian development in decapod crustacea: Focus on vitellogenesis-stimulating factors and pathways. Frontiers in Endocrinology (Lausanne), 2020, 11: 577925 DOI:10.3389/fendo.2020.577925 |
JIANG Y H, YAN S F, SONG Z R. Ultrastructure of vitellogenesis in the oocytes of Litopenaeus vannamei. Acta Zoologica Sinica, 2005a, 51(1): 133-141 [姜永华, 颜素芬, 宋振荣. 凡纳滨对虾卵母细胞卵黄发生的超微结构. 动物学报, 2005a, 51(1): 133-141] |
JIANG Y H, YAN S F, WANG C G, et al. Ultrastructure on oogenesis of Litopenaeus vannamei. Journal of Fisheries of China, 2005b, 29(4): 454-460 [姜永华, 颜素芬, 王重刚, 等. 凡纳滨对虾卵子发生的超微结构. 水产学报, 2005b, 29(4): 454-460] |
JORDAN V K, FREGEAU B, GE X. Genotype-phenotype correlations in individuals with pathogenic RERE variants. 2018, 39(5): 666–675
|
KIM B J, SCOTT D A. Mouse model reveals the role of RERE in cerebellar foliation and the migration and maturation of Purkinje cells. PLoS One, 2014, 9(1): e87518 DOI:10.1371/journal.pone.0087518 |
KIM B J, ZAVERI H P, SHCHELOCHKOV O A, et al. An allelic series of mice reveals a role for RERE in the development of multiple organs affected in chromosome 1p36 deletions. PLoS One, 2013, 8(2): e57460 DOI:10.1371/journal.pone.0057460 |
KRUEVAISAYAWAN H, VANICHVIRIYAKIT R, WEERACHATYANUKUL W, et al. Biochemical characterization and physiological role of cortical rods in black tiger shrimp, Penaeus monodon. Aquaculture, 2007, 270(1): 289-298 |
LÉONET A, DELROISSE J, SCHUDDINCK C, et al. Thioredoxins induce oocyte maturation in holothuroids (Echinodermata). Aquaculture, 2019, 510: 293-301 DOI:10.1016/j.aquaculture.2018.12.090 |
LI T, LIU X, JIANG Q, et al. High expression of partitioning defective 3-like protein is associated with malignancy in colorectal cancer. Tumor Biology, 2017, 39(4): 568835857 |
LIU J Y, LIU J H, KONG J, et al. Development of SNP markers and verification analysis of relationship on family in Litopenaeus vannamei. Progress in Fishery Sciences, 2021, 42(1): 108-116 [刘峻宇, 刘均辉, 孔杰, 等. 凡纳滨对虾SNP标记开发与家系亲缘关系验证分析. 渔业科学进展, 2021, 42(1): 108-116] |
MA Y G, YANG F, YANG W J. The structure of thioredoxin and its role in the biological resistance of oxidation stress. Chemistry of Life, 2011, 31(3): 429-433 [马宇光, 杨帆, 杨卫军. 硫氧还蛋白的结构及在生物抗氧化中的功能. 生命的化学, 2011, 31(3): 429-433] |
PAMURU R R. Endocrinology of reproduction in crustaceans. Narayan E. Comparative Endocrinology of Animals. Rijeka: IntechOpen, 2019
|
PATENAUDE A, MURTHY M R V, MIRAULT M. Mitochondrial thioredoxin system. Journal of Biological Chemistry, 2004, 279(26): 27302-27314 DOI:10.1074/jbc.M402496200 |
SUI J, LUAN S, CAO J, et al. Genomic signatures of artificial selection in fecundity of Pacific white shrimp, Penaeus vannamei. Frontiers in Genetics, 2022, 13: 929889 DOI:10.3389/fgene.2022.929889 |
SUN Y Q, PANG Y W, HAO H S, et al. Transcriptional expression of Trx and its inhibitor TXNIP genes during different developmental stages of bovine embryos produced in vitro. Journal of Gansu Agricultural University, 2017, 52(1): 7-12 [孙业清, 庞云渭, 郝海生, 等. Trx及其抑制因子TXNIP基因在发育不同阶段牛体外胚胎中的转录与表达. 甘肃农业大学学报, 2017, 52(1): 7-12] |
TINIKUL Y, POLJAROEN J, KORNTHONG N, et al. Distribution and changes of serotonin and dopamine levels in the central nervous system and ovary of the Pacific white shrimp, Litopenaeus vannamei, during ovarian maturation cycle. Cell and Tissue Research, 2011, 345(1): 103-124 DOI:10.1007/s00441-011-1176-8 |
WANG C L, LIANG M Q, XU H G. Optimum feeding frequency for Litopenaeus vannamei during the breeding period. Progress in Fishery Sciences, 2018, 39(4): 74-82 [汪春玲, 梁萌青, 徐后国. 凡纳滨对虾亲虾繁殖期间适宜投喂频率的研究. 渔业科学进展, 2018, 39(4): 74-82] |
WANG S, GEBRE-MEDHIN S, BETSHOLTZ C, et al. Targeted disruption of the mouse phospholipase C beta3 gene results in early embryonic lethality. FEBS Letters, 1998, 441(2): 261-265 DOI:10.1016/S0014-5793(98)01518-X |
YAN S F, JIANG Y H. Histology of the ovarian structure and development of Penaeus vannamei. Transactions of Oceanology and Limnology, 2004(2): 52-58 [颜素芬, 姜永华. 南美白对虾卵巢结构及发育的组织学研究. 海洋湖沼通报, 2004(2): 52-58] |
ZHEN H, ZHAO B S. Molecular cloning and expression pattern of the DjPLCβ4 gene in Dugesia japonica during embryonic development. Sichuan Journal of Zoology, 2020, 39(3): 249-257 [甄辉, 赵博生. 东亚三角涡虫DjPLCβ4基因克隆及在胚胎发育中的组织特异性表达. 四川动物, 2020, 39(3): 249-257] |



