溶解氧作为鱼类生存的必要条件,是影响鱼类行为、生长和生存的重要环境因子(Wu, 2002)。水体的持续性低氧和短期性低氧是水产养殖中很普遍的现象,在自然因素和人类活动的影响下,水体缺氧现象日益剧增(Diaz, 2001)。大多数鱼类具有适应不同溶解氧水平的能力,若长时间溶氧不足,则会损伤鱼体的组织和器官,导致鱼体生理代谢出现紊乱,影响鱼类的生长发育、繁殖及行为活动,甚至会引起鱼类机体的应激反应,严重时会导致鱼类大量死亡(徐贺等, 2016)。
在低氧环境下,鱼类会发生复杂的生理变化来应对低氧胁迫。低氧胁迫通常会影响鱼类代谢方式,当氧气不足时,鱼类通过快速激活体内的无氧糖酵解来满足缺氧应激期间的能量需求,导致糖酵解底物消耗增加和乳酸(LD)积累(Tripathi et al, 2013)。而乳酸脱氢酶(LDH)是一种糖酵解酶,广泛存在于机体的各个组织中,其中在肾脏和心肌中最为丰富,对机体低氧状态下的乳酸代谢有重要作用(Saavedra et al, 2016)。丙酮酸激酶(PK)是糖酵解过程中主要的限速酶和调节酶,LDH可促进LD和PK之间的相互转化,其活性能够反映糖酵解水平的高低(李金兰等, 2014)。琥珀酸脱氢酶(SDH)是有氧代谢过程中仅存在于线粒体内膜上的酶,催化琥珀酸氧化生成延胡索酸释放ATP。李谣等(2023)、徐宇等(2023)研究表明,低氧胁迫下长吻
鱼类应对低氧胁迫时,通过氧感受器和信号转导途径调控相关基因的表达,从而维持机体的稳态来适应低氧环境。缺氧诱导因子-1α (hypoxia inducible factor-1, HIF-1α)是调节机体氧平衡的关键因子(Mandic et al, 2020)。Soñanez-Organis等(2010)研究发现,低氧胁迫可以增强凡纳对虾(Penaeus vannamei)鳃中LDH和HIF-1α蛋白的表达。HIF-1激活会诱导糖酵解和无氧代谢增强从而促进ATP产生,在一定程度上补偿低氧胁迫下机体对能量的需求(Fulda et al, 2007)。鱼类中HIF-1α蛋白特征首次在虹鳟上被Soitamo等(2001)发现,它与其他HIF-1α蛋白具有高度的进化保守性。HIF-1α主要受脯氨酰羟化酶结构域(PHDs)和肿瘤抑制蛋白(pVHL)等关键酶调控,其活性也受HIF-1α抑制蛋白(FIH-1)和其他转录因子的调节。HIF-1α蛋白可以直接或间接激活多种低氧诱导基因,帮助低氧暴露细胞的代谢和存活。Pei等(2021)和李谣等(2023)研究表明,低氧胁迫使杂交黄颡鱼(Pelteobagrus fulvidraco♀×P.vachelli♂)脑和肝脏及长吻
虹鳟是一种对低氧极其敏感的鱼类,其致死缺氧阈值为3.0 mg/L,是研究鱼类低氧响应机制的良好模型(Wulff et al, 2012; Hou et al, 2020)。近年来,随着养殖密度的增加,水体低氧问题已逐渐成为渔业研究者和养殖者关注的热点。低氧胁迫会严重影响虹鳟的生长和代谢能力,心脏是鱼类应对低氧胁迫的重要器官。然而,目前关于低氧胁迫下虹鳟心脏生化指标和低氧相关基因的分析尚未见报道。因此,本研究通过酶活性测定和实时荧光定量PCR (RT-qPCR)方法分析不同浓度低氧胁迫对虹鳟心脏生化指标和低氧相关基因表达的影响,以期为阐明虹鳟应对低氧胁迫的调控机制提供理论依据。
1 材料与方法 1.1 实验动物本实验虹鳟来源于甘肃省永靖养殖场,平均体重为(130.0±5.0) g。将体表完好无伤、规格基本一致的健康虹鳟放入循环水养殖系统中暂养。溶解氧浓度维持在(8.5±0.1) mg/L,水温控制在(12.0±0.1) ℃。
1.2 实验设计与样品采集暂养结束后,通过氧气和氮气混合营造低氧环境。根据实验设计分为常氧组、中度低氧组和重度低氧组。常氧组溶解氧为(8.5±0.1) mg/L,中度低氧组溶解氧为(4.5±0.1) mg/L,重度低氧组溶解氧为(3.0±0.1) mg/L。在实验期间,水温维持在(12.0±0.1) ℃,pH为7.5±0.1。低氧实验通过调节氧气和氮气的注入量来控制水中的溶解氧浓度,用溶氧仪监测水体溶解氧的变化,使溶解氧浓度快速从(8.5±0.1) mg/L降至(4.5±0.1) mg/L和(3.0±0.1) mg/L,分别持续4、8、12、24 h、中度低氧1个月(TMM)、重度低氧1个月(TMS),常氧恢复实验在低氧胁迫24 h后立即进行,开始往水中大量充入氧气,使溶解氧快速恢复至(8.5±0.1) mg/L后持续12 h和24 h。常氧组作为对照组,在低氧胁迫过程中,分别在低氧胁迫(4、8、12、24 h、TMM和TMS)和复氧12 h和24 h的各个时间点随机选取虹鳟并采集心脏样品,立即放入液氮中速冻,后置于–80 ℃冰箱保存,用于后续生化指标和RNA提取。
1.3 生化指标测定取适量心脏组织,置于1.5 mL无酶离心管,注入9倍体积的生理盐水,用组织研磨仪将组织磨碎,置于低温离心机4 000 r/min离心10 min,吸取上清液置于–80 ℃冰箱用于测定生化指标,生化指标主要包括总胆固醇(total cholesterol, TC/T-CHO)、琥珀酸脱氢酶(succinate dehydrogenase, SDH)、丙酮酸激酶(pyruvate kinase, PK)、三磷酸腺苷酶(adenosine triphosphatase, ATPase)、乳酸(lactic acid, LD)、脂肪酶(lipase, LPS)、谷丙转氨酶(glutamine aminotransferase, GPT)、谷草转氨酶(glutamic transaminase, GOT)和乳酸脱氢酶(lactate dehydrogenase, LDH),试剂购买自南京建成生物工程有限公司,利用多功能微孔板读数仪(Thermo Fisher 3020-1296)测定吸光度。
1.4 总RNA的提取根据TRIzol法提取心脏组织的总RNA,用1%琼脂糖凝胶电泳及NanoDrop超微量紫外分光光度计检测RNA的质量及浓度,使用反转录试剂盒(TaKaRa, 大连)将检测合格的RNA样品进行反转录。
1.5 心脏组织低氧相关基因表达量检测根据本课题组所获得的转录组序列信息,使用Premier 5.0软件设计ldh、pk、sdh、fih-1、hif-1α、egln-1和vhl基因特异性引物,以β-肌动蛋白(β-actin)作为内部参照基因(表 1),以cDNA为模板,通过RT-qPCR检测虹鳟心脏中ldh、pk、sdh、fih-1、hif-1α、egln-1和vhl基因的mRNA水平。按照荧光定量试剂盒说明书设置20 μL体系:ddH2O 7.5 μL、上下游引物(10 μmol/L)各1 μL,SYBR Premix Ex Taq Ⅱ (Tli RNaseH Plus) 10 μL,cDNA 0.5 μL;反应条件:95 ℃预变性30 s;95 ℃ 5 s,60 ℃ 34 s (40个循环),95 ℃ 15 s,60 ℃ 1 min,95 ℃ 15 s。采用2–ΔΔCt法分析样品中ldh、pk、sdh、fih-1、hif-1α、egln-1和vhl基因相对表达量。
|
|
表 1 引物序列信息 Tab.1 Information of primers |
利用SPSS 24.0软件进行数据统计分析,采用单因素方差分析(one-way ANOVA)及多重比较(Duncan)检验数据差异的显著性,当P < 0.05时为差异显著。
2 结果与分析 2.1 低氧胁迫对虹鳟心脏生化指标的影响在中度低氧胁迫下,虹鳟心脏中PK、LDH、TC、LD和GPT水平在8 h时升高,24 h时降低,复氧后显著高于对照水平(P < 0.05)。ATPase和GOT活性在12 h时降低,复氧后恢复至正常水平(P > 0.05)。在重度低氧胁迫下,ATPase和LD水平在24 h时显著升高(P < 0.05),SDH、LPS、GOT和GPT水平在12 h时降低,复氧后恢复至正常水平(P > 0.05)。与对照组相比,TMM和TMS组中PK、TC、LDH和GPT水平显著升高(P < 0.05) (图 1)。

|
图 1 低氧胁迫对虹鳟心脏生化指标的影响 Fig.1 Effects of hypoxia stress on cardiac biochemical indices of rainbow trout 图A、C、E、G、I、K、M、O和Q:中度低氧;图B、D、F、H、J、L、N、P和R:重度低氧。C和CM表示对照;4 h、8 h、12 h和24 h分别表示中度低氧和重度低氧胁迫4、8、12和24 h;R12 h和R24 h分别表示常氧恢复12 h和24 h;TMM表示中度低氧1个月;TMS表示重度低氧1个月。图中不同小写字母表示差异显著(P < 0.05),下同。 Figures A, C, E, G, I, K, M, O, and Q: Moderate hypoxia; Figures B, D, F, H, J, L, N, P, and R: Severe hypoxia. C and CM denote control; 4 h, 8 h, 12 h, and 24 h denote moderate hypoxia and severe hypoxia stress for 4, 8, 12, and 24 h, respectively; R12 h and R24 h denote normoxic recovery for 12 h and 24 h, respectively; TMM: Moderate hypoxia for 1 month; TMS: Severe hypoxia for 1 month. Different lower case letters in the graphs denote significant differences (P < 0.05), and the same below. |
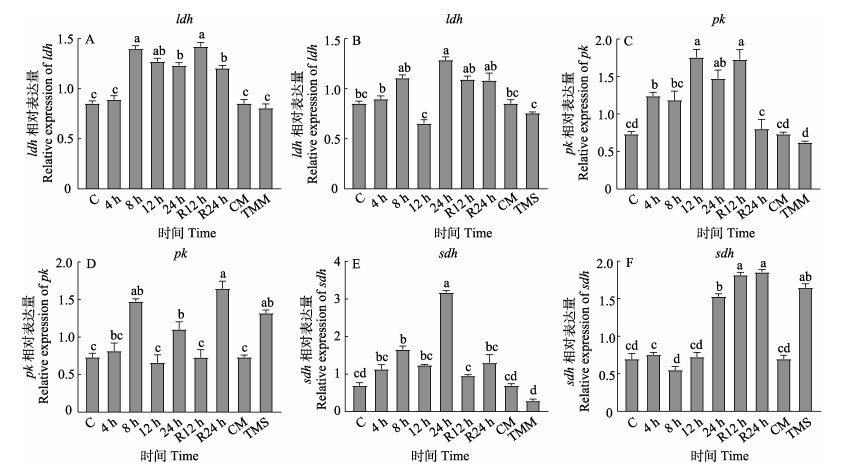
在中度低氧胁迫下,虹鳟心脏中ldh和pk基因表达量在8 h时显著升高,24 h时降低,复氧12 h时仍显著高于对照水平(P < 0.05)。sdh基因表达量在8 h和24 h时升高,复氧12 h恢复至对正常水平(P > 0.05)。在重度低氧胁迫下,ldh、pk和sdh基因表达量在24 h时升高,复氧24 h时其表达量仍显著高于对照水平(P < 0.05)。与对照组相比,TMS组中pk和sdh表达量显著升高(P < 0.05) (图 2)。
|
图 2 低氧胁迫对虹鳟心脏代谢相关基因表达的变化 Fig.2 Effects of hypoxia stress on metabolism related gene expression in rainbow trout 图A、C和E:中度低氧;图B、D和F:重度低氧。 Figure A, C, and E: Moderate hypoxia; Figure B, D, and F: Severe hypoxia. |
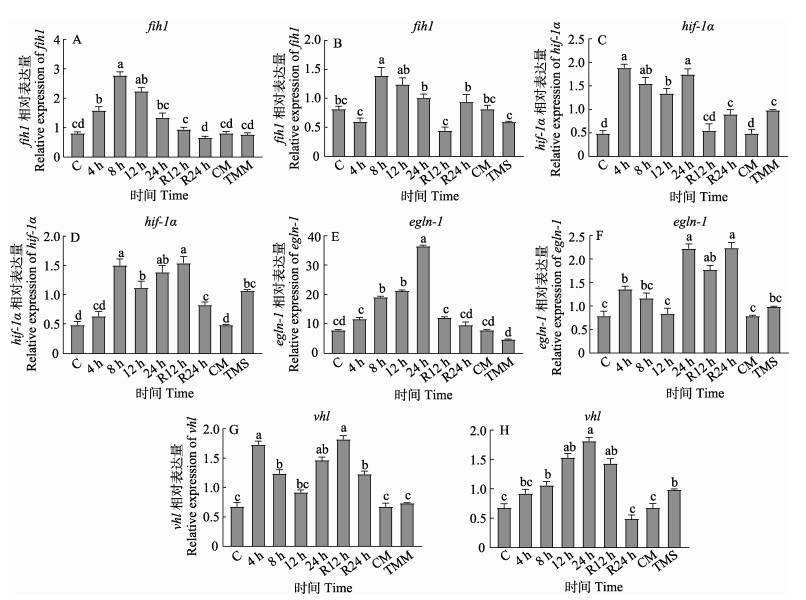
在中度低氧胁迫下,hif-1α和fih1基因表达量在4 h和8 h时显著升高(P < 0.05),12 h时降低;egln-1基因表达量随低氧胁迫时间延长而逐渐升高,在24 h时达到峰值(P < 0.05);vhl基因表达量在4 h和24 h升高,8 h时显著降低(P < 0.05),复氧后均恢复至正常水平。在重度低氧胁迫下,hif-1α和egln-1基因表达量在4 h和24 h时显著升高,在8 h时降低,复氧后仍显著高于对照(P < 0.05)。fih1基因表达量在4 h时降低,在8 h时显著升高(P < 0.05),复氧24 h时恢复至正常水平。vhl基因表达量随低氧随胁迫时间延长而逐渐升高,在24 h时达到峰值(P < 0.05),复氧24 h时恢复至正常水平。与对照组相比,TMM组中fih1、egln-1和vhl基因表达量降低但无显著性差异(P > 0.05),TMS组中vhl基因表达量显著升高(P < 0.05) (图 3)。
|
图 3 低氧胁迫对虹鳟心脏低氧相关基因表达的影响 Fig.3 Effects of hypoxia stress on hypoxia related gene expression in rainbow trout 图A、C、E和G:中度低氧;图B、D、F和H:重度低氧。 Figure A, C, E, and G: Moderate hypoxia; Figure B, D, F, and H: Severe hypoxia. |
溶解氧是影响鱼类生存和生态平衡的重要环境因子,低氧环境会对鱼类的新陈代谢、生长、繁殖及生存等生理过程造成严重影响(Xiao, 2015)。鱼类为适应不同溶解氧水平会产生生化、生理和行为等方面的反应,而许多反应与生化指标的变化以及低氧相关基因的表达有关。本研究用酶活性测定和RT-qPCR法分析了中度低氧和重度低氧对虹鳟心脏生化指标变化和低氧相关基因表达的影响。研究结果表明,虹鳟在不同溶解氧水平的代谢策略不同,中度低氧使机体有氧呼吸减弱,从而降低耗氧量来适应低氧环境的生存;重度低氧使机体有氧呼吸和无氧呼吸同时加强,补充机体对能量的需求。低氧胁迫使虹鳟心脏发生代谢紊乱,影响体内正常的代谢水平,并对虹鳟心脏造成一定损伤。
3.1 低氧胁迫对虹鳟心脏生化指标的影响鱼类在受到低氧胁迫时,通过调节代谢模式和启动自身免疫防御系统来减少低氧胁迫对机体的损伤。本研究发现,低氧胁迫前期,机体通过提高ATPase活性来获取更多能量,以满足新陈代谢需求。在中度低氧胁迫下,ATPase、LDH和LD水平在24 h时降低,复氧后LDH和LD水平显著升高,而ATPase活性恢复至正常水平,机体有氧呼吸代谢无法正常进行,此时无氧代谢过程可能发挥作用。区又君等(2017)研究发现,卵形鲳鲹(Trachinotus ovatus L.)在低氧胁迫下LDH水平显著升高,与本研究结果相似,持续的低氧使机体有氧呼吸代谢被抑制,无氧代谢能力增强。与对照组相比,TMM组中ATPase、LDH和LD水平显著升高,长期中度低氧会快速激活虹鳟体内无氧糖酵解来满足缺氧应激期间的能量需求,导致糖酵解底物消耗增加和乳酸积累(Thoral et al, 2022)。重度低氧胁迫下,ATPase和LD水平在12 h时升高,复氧后仍显著高于对照,机体在重度低氧胁迫下为满足自身能量需求而增强无氧代谢能力。Speers-Roesch等(2010)、Kong等(2023)研究表明,罗非鱼(Oreochromis hybrid sp)和鲤(Cyprinus carpio)在面对低氧胁迫时的代谢方式与虹鳟较为相似,重度低氧使机体无氧代谢产物LD含量积累和ATPase活性升高,从而满足机体对能量的需求。中度低氧使LDH活性升高,并产生大量乳酸,通过无氧代谢糖酵解途径为机体提供能量。与对照组相比,TMS组中LD含量降低,LDH活性显著升高,表明长期无氧呼吸会造成机体内大量乳酸堆积,通过增强一元酸转运来提高乳酸代谢率从而缓解乳酸堆积(Speers-Roesch et al, 2010; 王彦欣等, 2023)。
SDH是三羧酸循环(TCA)的重要组成部分,其活性可反映TCA循环运行程度。在中度低氧胁迫下,SDH活性均显著低于对照,PK活性显著升高。李谣等(2023)在长吻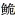
LPS和TC是机体储存能量的主要形式,是机体脂质代谢水平的重要指标。GOT和GPT是氨基酸代谢的关键酶和非特异性免疫酶,可作为组织细胞损伤和坏死的重要指标,而GOT和GPT水平与脂代谢紊乱相关,对非必需氨基酸的合成和蛋白质分解代谢起重要作用。在中度低氧胁迫下,LPS和GOT活性呈先升高后降低的趋势,TC含量和GPT活性显著高于对照。中度低氧胁迫使虹鳟心脏损伤,对机体氨基酸及脂类代谢产生一定的影响,复氧后均恢复至正常水平,对虹鳟心脏的损伤有所减缓。低氧胁迫使大口黑鲈肝脏和军曹鱼幼鱼肠道及血清中LPS和TC指标降低,GPT和GOT显著高于对照水平,与本研究结果相反,不同鱼类在低氧胁迫下其脂质代谢有所差异(郭志雄, 2021; Yang et al, 2021)。低氧胁迫导致机体TC含量不断升高,使虹鳟心肌内脂肪大量堆积,引起脂肪代谢异常。在重度低氧胁迫下,LPS、TC、GOT和GPT水平在12 h时降低,复氧后恢复至正常水平。低氧胁迫12 h后花鲈幼鱼和大口黑鲈肝脏及血浆中LPS、TC、GOT和GPT水平呈先升高后降低的趋势,这可能与机体供能有关(常志成等, 2018; Sun et al, 2020)。重度低氧使虹鳟体内蛋白质和脂质分解加快,以满足机体对能量的需求。与对照组相比,TMM和TMS组中LPS活性无显著性差异,GOT活性降低,TC、LD和GPT水平升高,长期低氧促进了虹鳟的脂质分解,表明长期低氧应激期间的能量需求主要来自脂质分解代谢(Li et al, 2018)。
3.2 低氧胁迫对虹鳟心脏代谢相关基因表达的影响鱼类面对低氧胁迫时的能量代谢会根据水体溶解氧的不同而发生改变。低氧可抑制鱼类有氧代谢,使机体能量供给不足,为保证机体充足的能量供应,机体被迫从有氧代谢转换到糖酵解途径(Ton et al, 2003)。本研究结果显示,在中度低氧胁迫下,虹鳟心脏中ldh、pk和sdh基因表达量在24 h时显著高于对照;复氧后,pk和sdh基因表达量恢复至正常水平。Zeng等(2016)、Han等(2022)研究表明,大黄鱼(Larimichthys crocea)和三倍体虹鳟肝脏中这些基因表达量与本研究结果不同,可能不同鱼类面对低氧胁迫时的代谢水平不同。在中度低氧胁迫下,三羧酸循环酶ldh、pk和sdh基因表达量升高,糖酵解通路增强,表明低氧应激激活了线粒体有氧代谢,将乳酸转化为丙酮酸参与三羧酸循环,在一定程度上补充机体对能量的需求。在重度低氧胁迫下,ldh和pk基因表达量在8 h时显著升高,12 h时恢复至正常水平,sdh基因表达量在24 h时显著升高,复氧后pk和sdh基因表达量在24 h时显著升高,表明有氧代谢水平增强,可补偿机体在低氧胁迫下产生的氧债。低氧胁迫使菲律宾蛤仔(Ruditapes philippinarum)鳃、肝胰和暗纹东方鲀(Takifugu fasciatus)肝脏中pk、sdh和ldh基因表达量显著升高(Hao et al, 2023; Li et al, 2019)。裴雪莹(2021)发现,低氧胁迫使杂交黄颡鱼肝脏中sdh基因表达量在4 h时降低,ldh基因表达量在6.5 h升高,脑中sdh基因表达量与对照无显著性差异,复氧后显著升高,与本研究结果不同。相同基因可能在不同水生动物的不同组织中有不同的表达模式,为了能够在低氧和复氧胁迫下进行正常生命活动来获取能量而做出代谢改变,逐步从有氧代谢转化至无氧代谢。与对照组相比,TMM组中ldh、pk和sdh基因表达量无显著性差异,TMS组中pk和sdh基因表达量升高,虹鳟随着溶解氧浓度的改变而逐步调节自身的代谢水平。长期中度低氧胁迫可能对机体代谢水平影响较小,而长期重度低氧对虹鳟机体内的糖酵解有促进作用(王美琪等, 2023)。
3.3 低氧胁迫对虹鳟心脏低氧相关基因表达的影响hif-1α是重要的调控氧代谢转录因子,也是调控鱼类缺氧应答的主要转录因子。fih-1是hif-1α重要的上游调控基因。在低氧环境中,fih-1可以通过调控hif-1α的表达,进而激活其下游基因的转录。本研究结果显示,在中度低氧胁迫下,hif-1α和fih1基因表达量在4 h和8 h时显著升高,12 h时降低;egln-1基因表达量随胁迫时间延长而逐渐升高,在24 h时达到峰值;vhl基因表达量在4 h和24 h升高,8 h时显著降低;复氧后均恢复至正常水平。在大口黑鲈肝脏(Yang et al, 2017)、暗纹东方鲀心脏(李欣茹, 2019)和杂交黄颡鱼肝脏及心脏(裴雪莹, 2021)中也有相关报道,表明这些差异表达的基因可能与它们特定生理功能的不同有关。在重度低氧胁迫下,hif-1α和vhl基因表达量在24 h时显著升高,8 h时降低;egln-1基因表达量在24 h时显著升高,复氧后显著高于对照水平;fih1基因表达量在4 h时降低,8 h时显著升高。Liu等(2013)发现,低氧胁迫使鲇鲤(Myxocyprinus asiaticus)肝脏和性腺中hif-1α基因表达量较高。长吻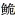
本研究通过采用酶活性测定和RT-qPCR方法,分析了中度低氧和重度低氧胁迫对虹鳟心脏中生化指标变化以及低氧相关基因的表达情况的影响。研究发现,低氧胁迫使虹鳟心脏中PK、LDH、ATPase、SDH、LPS、TC、LD、GOT和GPT水平均有不同程度的升高,使机体发生代谢紊乱,影响体内正常的代谢水平,并对虹鳟心脏造成一定损伤。在低氧胁迫下,虹鳟心脏中ldh、pk、sdh、fih1、hif-1α、egln-1和vhl基因表达量均有不同程度的升高,通过低氧相关基因的高表达模式来进行低氧应激的反馈调节,防止机体心脏一直处于低氧应激状态,使机体快速恢复到稳定状态并发挥正常的生理功能。本研究结果为虹鳟响应低氧胁迫的调控机制研究提供了参考资料。
CHANG Z C, WEN H S, ZHANG M Z, et al. Effects of dissolved oxygen levels on oxidative stress response and energy utilization of juvenile Chinese sea bass (Lateolabrax maculatus) and associate physiological mechanisms. Periodical of Ocean University of China (Natural Science), 2018, 48(7): 20-28 [常志成, 温海深, 张美昭, 等. 溶解氧水平对花鲈幼鱼氧化应激与能量利用的影响及生理机制. 中国海洋大学学报(自然科学版), 2018, 48(7): 20-28] |
DÍAZ R J. Overview of hypoxia around the world. Journal of Environmental Quality, 2001, 30(2): 275-281 DOI:10.2134/jeq2001.302275x |
FULDA S, DEBATIN K M. HIF-1-regulated glucose metabolism a key to apoptosis resistance?. Cell Cycle, 2007, 6(7): 790-792 DOI:10.4161/cc.6.7.4084 |
GUO Z X. Effects of hypoxic environment on biochemical indexes, related gene expression and transcriptome analysis of cobia juveniles. Master´s Thesis of Guangdong Ocean University, 2021 [郭志雄. 低氧环境对军曹鱼幼鱼生化指标、相关基因表达的影响及其转录组学分析. 广东海洋大学硕士研究生学位论文, 2021]
|
HAN B, MENG Y, TIAN H, et al. Effects of acute hypoxic stress on physiological and hepatic metabolic responses of triploid rainbow trout (Oncorhynchus mykiss). Frontiers in Physiology, 2022, 13: 921709 DOI:10.3389/fphys.2022.921709 |
HAO J, LIU Z H, WU B, et al. Physiological and molecular responses to hypoxia stress in Manila clam Ruditapes philippinarum. Aquatic Toxicology, 2023, 257: 106428 DOI:10.1016/j.aquatox.2023.106428 |
HE J H. Effects of hypoxia stress on the physiology, biochemistry, and gene expression of juvenile Acrossocheilus fasciatus fishes. Master´s Thesis of Zhejiang Ocean University, 2023 [何景鸿. 低氧胁迫对光唇鱼幼鱼生理生化以及相关基因表达的影响. 浙江海洋大学硕士研究生学位论文, 2023]
|
HOU Z S, WEN H S, LI J F, et al. Environmental hypoxia causes growth retardation, osteoclast differentiation and calcium dyshomeostasis in juvenile rainbow trout (Oncorhynchus mykiss). Science of the Total Environment, 2020, 705: 135272 DOI:10.1016/j.scitotenv.2019.135272 |
KONG Z, LIU C, OLATUNJI O J. Asperuloside attenuates cadmium-induced toxicity by inhibiting oxidative stress, inflammation, fibrosis, and apoptosis in rats. Scientific Reports, 2023, 13(1): 5698 DOI:10.1038/s41598-023-29504-0 |
LI J L, CHEN G, ZHANG J D, et al. Effects of temperature and salinity on the respiratory metabolism of Derbio (Trachinotus ovatus L). Journal of Guangdong Ocean University, 2014, 34(1): 30-36 [李金兰, 陈刚, 张健东, 等. 温度、盐度对卵形鲳鲹呼吸代谢的影响. 广东海洋大学学报, 2014, 34(1): 30-36] |
LI M, WANG X, QI C, et al. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture, 2018, 495: 187-195 DOI:10.1016/j.aquaculture.2018.05.031 |
LI X R. Effects of hypoxic stress on energy metabolism, blood indices and gene expression in the dark-striped oriental leatherback, Puffinus orientalis. Master´s Thesis of Nanjing Normal University, 2019 [李欣茹. 低氧胁迫对暗纹东方鲀能量代谢、血液指标及基因表达的影响. 南京师范大学硕士研究生学位论文, 2019]
|
LI X, WANG T, YIN S, et al. The improved energy metabolism and blood oxygen-carrying capacity for pufferfish, Takifugu fasciatus, against acute hypoxia under the regulation of oxygen sensors. Fish Physiology and Biochemistry, 2019, 45: 323-340 DOI:10.1007/s10695-018-0565-2 |
LI Y, YANG Z R, CHENG J H, et al. Effects of hypoxia stress and reoxygenation on appetite, hypoxic response genes and physiological and biochemical indexes in the brain tissues of Leiocassis longirostris. Journal of Fisheries of China, 2023, 47(1): 152–164 [李谣, 杨智茹, 程景颢, 等. 低氧胁迫和恢复对长吻 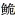 脑组织低氧应答基因、生理生化指标和食欲的影响. 水产学报, 2023, 47(1): 152–164] 脑组织低氧应答基因、生理生化指标和食欲的影响. 水产学报, 2023, 47(1): 152–164]
|
LIU S, ZHU K, CHEN N, et al. Identification of HIF-1α promoter and expression regulation of hif-1α gene by LPS and hypoxia in zebrafish. Fish Physiology and Biochemistry, 2013, 39: 1153-1163 DOI:10.1007/s10695-013-9771-0 |
MALEC V, GOTTSCHALD O R, LI S, et al. HIF-1α signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radical Biology and Medicine, 2010, 48(12): 1626-1635 DOI:10.1016/j.freeradbiomed.2010.03.008 |
MANDIC M, BEST C, PERRY S F. Loss of hypoxia-inducible factor 1α affects hypoxia tolerance in larval and adult zebrafish (Danio rerio). Proceedings of the Royal Society B, 2020, 287(1927): 20200798 DOI:10.1098/rspb.2020.0798 |
OU Y J, CHEN S X, WANG P F, et al. Study on oxidative stress response and physiological metabolism related indices of Trachinotus ovatus under hypoxia stress. South China Fisheries Science, 2017, 13(3): 120-124 [区又君, 陈世喜, 王鹏飞, 等. 低氧环境下卵形鲳鲹的氧化应激响应与生理代谢相关指标的研究. 南方水产科学, 2017, 13(3): 120-124] |
PEI X Y. Physiological response and gene expression of hybrid yellow catfish "Huangyou-1" to hypoxic stress. Master´s Thesis of Nanjing Normal University, 2021 [裴雪莹. 杂交黄颡鱼"黄优1号"应对低氧胁迫的生理响应及基因表达研究. 南京师范大学硕士研究生学位论文, 2021]
|
PEI X, CHU M, TANG P, et al. Effects of acute hypoxia and reoxygenation on oxygen sensors, respiratory metabolism, oxidative stress, and apoptosis in hybrid yellow catfish "Huangyou-1". Fish Physiology and Biochemistry, 2021, 47: 1429-1448 DOI:10.1007/s10695-021-00989-8 |
SAAVEDRA L, QUIÑONES R A, GONZÁLEZ-SALDÍA R R. Aerobic and anaerobic enzyme activity in the hake Merluccius gayi related to the oxygen minimum zone off central- southern Chile. Revista de Biología Marina y Oceanografía, 2016, 51(3): 581-590 DOI:10.4067/S0718-19572016000300009 |
SOITAMO A J, RÅBERGH C M I, GASSMANN M, et al. Characterization of a hypoxia-inducible factor (HIF-1α) from rainbow trout: Accumulation of protein occurs at normal venous oxygen tension. Journal of Biological Chemistry, 2001, 276(23): 19699-19705 DOI:10.1074/jbc.M009057200 |
SOÑANEZ-ORGANIS J G, RACOTTA I S, YEPIZ-PLASCENCIA G. Silencing of the hypoxia inducible factor 1-HIF-1- obliterates the effects of hypoxia on glucose and lactate concentrations in a tissue-specific manner in the shrimp Litopenaeus vannamei. Journal of Experimental Marine Biology and Ecology, 2010, 393(1/2): 51-58 |
SONG Y D, ZHAO L L, MA C X, et al. The effect of diel variation of dissolved oxygen content in a pond on the glucose metabolism and plasma glucose level of Siniperca chuatsi. Progress in Fishery Sciences, 2023, 44(3): 124-132 [宋银都, 赵亮亮, 马晨夕, 等. 池塘溶氧昼夜变化对鳜葡萄糖代谢和血糖含量的影响. 渔业科学进展, 2023, 44(3): 124-132] |
SPEERS-ROESCH B, SANDBLOM E, LAU G Y, et al. Effects of environmental hypoxia on cardiac energy metabolism and performance in tilapia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2010, 298(1): R104-R119 DOI:10.1152/ajpregu.00418.2009 |
SUN J L, ZHAO L L, WU H, et al. Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Science of the Total Environment, 2020, 713: 135157 DOI:10.1016/j.scitotenv.2019.135157 |
THORAL E, FARHAT E, ROUSSEL D, et al. Different patterns of chronic hypoxia led to hierarchical adaptive mechanisms in goldfish metabolism. Journal of Experimental Biology, 2022, 225(1): 243194 DOI:10.1242/jeb.243194 |
TRIPATHI R K, MOHINDRA V, SINGH A, et al. Physiological responses to acute experimental hypoxia in the air-breathing Indian catfish, Clarias batrachus (Linnaeus, 1758). Journal of Biosciences, 2013, 38: 373-383 DOI:10.1007/s12038-013-9304-0 |
TON C, STAMATIOU D, LIEW C C. Gene expression profile of zebrafish exposed to hypoxia during development. Physiological Genomics, 2003, 13(2): 97-106 DOI:10.1152/physiolgenomics.00128.2002 |
WANG M Q, SONG Z D, GUO P, et al. Effects of dietary supplementation with enzymatically hydrolyzed kelp powder on growth, digestion, metabolism, and oxidation resistance of juvenile sea cucumber (Apostichopus japonicus). Progress in Fishery Sciences, 2023, 44(3): 176-187 [王美琪, 宋志东, 郭鹏, 等. 饲料中添加酶解海带粉对刺参幼参生长、体组成、消化代谢和抗氧化能力的影响. 渔业科学进展, 2023, 44(3): 176-187] |
WANG Y X, XIANG M, CHEN L, et al. Effects of long-term acclimation on liver glycolipid metabolism in zebrafish (Danio rerio). Journal of Dalian Ocean University, 2023, 38(3): 429-437 [王彦欣, 向蒙, 陈磊, 等. 长期低氧对斑马鱼肝脏糖脂代谢的影响. 大连海洋大学学报, 2023, 38(3): 429-437] |
WANG W Z, ZENG Z Q, HUANG J S, et al. Hypoxia stress on growth, serum biochemical and non-specific immune indexes of juvenile cobia (Rachycentron canadum). Haiyang Xuebao, 2021, 43(2): 49-58 [王维政, 曾泽乾, 黄建盛, 等. 低氧胁迫对军曹鱼幼鱼生长、血清生化和非特异性免疫指标的影响. 海洋学报, 2021, 43(2): 49-58] |
WANG Y, YOU Y, TIAN Y, et al. Pediococcus pentosaceus PP04 ameliorates high-fat diet-induced hyperlipidemia by regulating lipid metabolism in C57BL/6N mice. Journal of Agricultural and Food Chemistry, 2020, 68(51): 15154-15163 DOI:10.1021/acs.jafc.0c05060 |
WU R S S. Hypoxia: From molecular responses to ecosystem responses. Marine Pollution Bulletin, 2002, 45(1–12): 35-45 |
WULFF T, JOKUMSEN A, HØJRUP P, et al. Time-dependent changes in protein expression in rainbow trout muscle following hypoxia. Journal of Proteomics, 2012, 75(8): 2342-2351 DOI:10.1016/j.jprot.2012.02.010 |
XIAO W H. The hypoxia signaling pathway and hypoxic adaptation in fishes. Science China Life Sciences, 2015, 58: 148-155 DOI:10.1007/s11427-015-4801-z |
XU H, CHEN X M, WANG G Q, et al. Research progress of hypoxia on aquaculture. Feed Industry, 2016, 37(2): 33-37 [徐贺, 陈秀梅, 王桂芹, 等. 低氧胁迫在水产养殖中的研究进展. 饲料工业, 2016, 37(2): 33-37] |
XU Y, XU Z Q, YAN W H, et al. Acute hypoxia/reoxygenation stress affect antioxidant and energy metabolism of Procambarus clarkii. Acta Hydrobiologica Sinica, 2023, 47(4): 594-601 [徐宇, 许志强, 严维辉, 等. 急性低氧/复氧胁迫对克氏原螯虾抗氧化–能量代谢的影响. 水生生物学报, 2023, 47(4): 594-601] |
YANG E J, ZHANG J D, YANG L T, et al. Effects of hypoxia stress on digestive enzyme activities, intestinal structure, and the expression of tight junction proteins coding genes in juvenile cobia (Rachycentron canadum). Aquaculture Research, 2021, 52(11): 5630-5641 DOI:10.1111/are.15438 |
YANG S, YAN T, WU H, et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of hif-1a and glut-1 genes in largemouth bass (Micropterus salmoides). Fish and Shellfish Immunology, 2017, 67: 449-458 DOI:10.1016/j.fsi.2017.06.035 |
ZENG L, WANG Y H, AI C X, et al. Effects of β-glucan on ROS production and energy metabolism in yellow croaker (Pseudosciaena crocea) under acute hypoxic stress. Fish Physiology and Biochemistry, 2016, 42: 1395-1405 DOI:10.1007/s10695-016-0227-1 |



