2. 中国海洋大学海洋生命学院 海洋生物遗传学与育种教育部重点实验室 山东 青岛 266003;
3. 上海海洋大学水产与生命学院 上海 201306;
4. 中国水产科学研究院长岛增殖实验站山东 烟台 265800;
5. 国家海洋局北海信息中心 山东 青岛 266061
2. Ministry of Education, Key Laboratory of Marine Genetics and Breeding, College of Marine Life Sciences, Ocean University of China, Qingdao, Shandong 266003, China;
3. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China;
4. Changdao Enhancement and Experiment Station, Chinese Academy of Fishery Sciences, Yantai, Shandong 265800, China;
5. North China Sea Data and Information Service of SOA, Qingdao, Shandong 266061, China
鼠尾藻(Sargassum thunbergii)隶属于褐藻门(Phaeophyta)、圆子纲(Cyclospreae)、墨角藻目(Fucales)、马尾藻科(Sargassaceae)、马尾藻属(Sargassum)(曾呈奎, 2009),是北太平洋西部特有的暖温带海藻,在我国北至辽东半岛、南至雷州半岛均有分布。鼠尾藻可与增殖型鱼礁结合进行海洋生态修复(吴海一等, 2010a),对水体中的营养盐如N、P等的吸收能力较强(王翔宇等, 2011; 包杰等, 2008),并对重金属Pb、Cu、Zn、Cd和As等具有富集和吸附效应(崔大练等, 2013; 范彩彩, 2013; 吴海一等, 2010b; 杨承虎, 2012),是藻场建设、生态修复的优良藻种。鼠尾藻中甘氨酸和丙氨酸含量较高,被认为是贝类、海参及鲍鱼养殖的优质饵料(谭杰等, 2020; 韩晓弟等, 2005; 何平等, 2011; 王吉桥等, 2008; 周玮等, 2010)。另外,在医药(抗菌、抗肿瘤)和工业原料等方面具有重要的应用潜力(郭奇等, 2010; Park et al, 2005; 李敬等, 2007)。
研究表明,低潮露空下的干露胁迫是影响鼠尾藻生存的关键因素(Chu et al, 2012a、b)。不同环境胁迫条件下,海藻受到的胁迫程度及应答机制也不同。目前,关于鼠尾藻在强光、高温及失水环境下的胁迫及应答的研究较多。如高温、强光和干露失水叠加胁迫对海藻光合器官(PSⅡ反应中心)造成严重的氧化胁迫,导致其光合活性下降(Takahashi et al, 2011)。鼠尾藻遭受失水和强光胁迫时,分别通过抗氧化系统和依赖叶黄素循环的热耗散机制发挥主导作用(Nan et al, 2016)。干露胁迫引起的失水是影响鼠尾藻光合活性的主要胁迫因子(Yu et al, 2013),是仅次于强光胁迫的第二大影响因素(盛子彤等, 2017)。另外,鼠尾藻通过上调热休克蛋白家族、超氧化物歧化酶(SOD)、抗坏血酸过氧化物酶(POD)、过氧化氢酶(CAT)、泛素连接酶、谷氧还蛋白、谷硫还蛋白等基因表达,下调光捕获复合蛋白、叶绿素a结合蛋白基因表达来应对干露胁迫(刘福利等, 2014)。但关于鼠尾藻在不同干露时间胁迫下的保水能力及恢复不同时间下的生理生化变化未见研究。本研究通过分析不同干露胁迫时间和不同恢复时间条件下藻体重量、叶绿素荧光和生理指标的变化差异,研究鼠尾藻保水能力,解析在干露胁迫条件下鼠尾藻的抗胁迫及恢复能力,为鼠尾藻资源的保护、开发和利用,藻场建设评估提供理论支持。
1 材料与方法 1.1 实验材料在山东省青岛太平角海边礁石上采集鼠尾藻样品,以藻体长度约为3~5 cm的幼苗为实验材料,用灭菌过滤海水充分清洗后暂养于温度为15℃、光强约为40 μmol photons/(m2·s)的低温室中。
1.2 实验方法将暂养后的鼠尾藻藻体从海水中取出至无连续滴水,测定初始鲜重,然后进行自然干露胁迫。实验条件:温度为20℃,光强为200 μmol photons/(m2·s),平均湿度为45%。设置4个干露处理时间,分别为0 (对照组)、1、3和6 h,每组处理均进行3次平行实验,分别进行如下实验。
1.2.1 干露处理后鼠尾藻的失水情况按照单株藻体鲜重将鼠尾藻分为3组,分别为0.5~0.9 g组、1~ 1.4 g组、1.5~2.5 g组。干露处理结束后,立即测定重量,计算相对失水率,并拍照观察表观形态的变化,评价干露胁迫条件下的保水能力,失水率公式如下:
| $ D= (W_{0}–W_{t})/(W_{0}–W_{d}) × 100 $ |
式中,D为失水率(%),W0为初始藻体鲜重(g),Wt为经过一段失水时间后的藻体鲜重(g),Wd为干重(即实验结束后,于75℃烘箱中烘干24 h后的重量)。t为实验持续时间(d)。
1.2.2 干露处理后鼠尾藻光合生理指标的变化干露处理结束后,立即进行暗处理0.5 h,将暗处理后的样品放在调制叶绿素荧光成像系统(Imaging-Milti Color Pam,WALZ,德国) MAX探头下10 cm × 13 cm的黑盒子中,进行光系统Ⅱ最大荧光产量(maximal photochemical efficiency of PSⅡ, Fv/Fm)、荧光诱导动力学曲线(chlorophyll a fluorescence transient)和快速光曲线(rapid light curve, RLC)的第1次测定;分别恢复培养1、3和6 h后,对藻体进行第2次Fv/Fm、荧光诱导动力学曲线、RLC的测定;根据藻体情况进行恢复24 h的第3次测定,测定结束后保存所有的图片和数据。
1.2.3 干露处理后鼠尾藻生化参数的变化每组藻体鲜重约为0.3 g,干露处理结束后,立刻用液氮把藻体冷冻并于–80℃保存。测定指标包括总蛋白(total protein, TP)浓度,丙二醛(MDA)、叶绿素a、可溶性糖、维生素C、脯氨酸含量,抗超氧阴离子自由基(anti-superoxide anion free radical, ASAFR)活性,CAT、POD、SOD的比活力。按照王丽梅等(2011)和Chouhan等(2011)的方法测定叶绿素a和可溶性糖含量,其他生化成分均采用酶联免疫吸附分析法(ELISA)测定,试剂盒购于上海酶联生物科技有限公司,主要试剂盒为植物总蛋白ELISA试剂盒、植物丙二醛ELISA试剂盒、植物维生素C ELISA试剂盒、植物脯氨酸ELISA试剂盒、植物抗超氧阴离子ELISA试剂盒、植物过氧化氢酶ELISA试剂盒、植物过氧化物酶ELISA试剂盒、植物超氧化物歧化酶ELISA试剂盒。主要仪器为352型酶标仪(Labsystems Multiskan MS,芬兰)、AC8洗板机(Thermo Labsystems,芬兰),按照试剂盒说明书进行测定。SOD、POD和CAT的比活力单位为酶单位每毫克蛋白(U/mg prot)。
1.3 数据处理采用SPSS 19.0数据统计软件进行单因素方差分析(one-way ANOVA)、Duncan多重比较及Pearson相关性分析,P < 0.05为差异显著。根据荧光诱导动力学曲线数据分析光系统Ⅱ调节性能量耗散的量子产量[Y(NPQ)]、光系统Ⅱ非调节性能量耗散的量子产量[Y(NO)]和光系统Ⅱ的实际量子产量[Y(Ⅱ)]。利用RLC数据和叶子飘等(Ye et al, 2013)机理模型拟合光合–光响应曲线,求出幼苗的最大电子传递速率(maximum electron transport rate, Jmax)、饱和光强(saturation irradiance, Isat)和光响应曲线初始斜率α。
2 结果 2.1 干露处理不同时间下鼠尾藻的失水情况从图 1可以看出,干露1 h时,藻体叶片表面少量失水,叶片萎靡;恢复1 h后,叶片伸展。干露3 h时,叶片颜色加深,为深褐色,叶片萎靡、不伸展、围绕于柄、失水严重;恢复3 h后,叶片为深褐色、叶片饱满且向外伸张。干露6 h时,叶片为黑褐色,叶片干燥且严重缩小,枝条干燥且直径明显变小,放入水中后为绿褐色,叶片舒展、直径变大;恢复24 h后,藻体基部和枝条较粗,叶片较多的部位颜色恢复正常,鼠尾藻的小枝条和较细部分的颜色未恢复,仍为黑褐色,且叶片韧性较低、较软。

|
图 1 不同干露和恢复时间下鼠尾藻的外观形态变化 Fig.1 Morphology of S. thunbergii under different dehydration and rehydration durations A:干露0 h;B:干露1 h,B1:干露1 h后恢复1 h;C:干露3 h,C1:干露3 h后恢复3 h;D:干露6 h,D1:干露6 h后恢复24 h A: Control; B: Dehydration for 1 h; B1: Dehydration for 1 h followed by rehydration for 1 h; C: Dehydration for 3 h; C1: Dehydration for 3 h followed by rehydration for 3 h; D: Dehydration for 6 h; D1: Dehydration for 6 h followed by rehydration for 24 h |
不同鲜重的鼠尾藻干露胁迫不同时间下的失水率变化显著(图 2)。干露1 h时,不同鲜重鼠尾藻的失水率无明显变化,此时,水分的减少多为藻体叶片外部的水分。干露3 h和6 h时,鲜重低于0.9 g的小藻体的失水率最高,分别达到55%和89%,且随着藻体鲜重的增大,失水率显著降低,即随着胁迫时间的延长,大藻体鼠尾藻的保水能力高于小藻体。

|
图 2 不同干露时间下鼠尾藻的失水率 Fig.2 Water loss rate of S. thunbergii under different dehydration durations 不同小写字母表示组间差异显著,下同。 Different lowercase letters indicated significant different, the same as below. |
由图 3和图 4可知,同株鼠尾藻不同部位对干露胁迫的耐受程度显著不同。图 3为干露胁迫不同时间条件下鼠尾藻Fv/Fm值的变化,颜色越蓝,表明藻体状态越好。干露1、3和6 h时(分别为图 3B1、C1、D1),藻体梢部分别为蓝绿、绿色和红褐色,表明胁迫时间越长藻体生理状态越差。干露处理过程中,藻体基部颜色变化较小,即生理状态变化较小。较小的鼠尾藻干露耐受能力差,同株鼠尾藻中梢部干露耐受能力最差,基部耐受能力最强。1 h干露对鼠尾藻基本无影响,3 h干露对鼠尾藻的梢部和中部影响较大,但正常条件下培养3 h后有一定程度的恢复;6 h干露对鼠尾藻的影响最大,鼠尾藻的小枝条和梢部的荧光值最低,接近死亡,且24 h后不能恢复正常,鼠尾藻的大枝条可恢复。
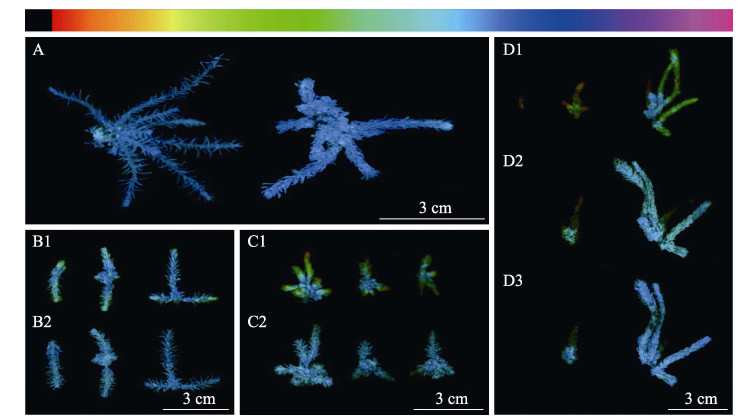
|
图 3 干露处理及恢复不同时间后鼠尾藻的光合生理状态(颜色条越往右表示Fv/Fm值越高) Fig.3 Photosynthetic physiological state of S. thunbergii after dehydration and rehydration (the color bar toward the right indicates the higher Fv/Fm value) A:干露0 h;B1:干露1 h,B2:干露1 h恢复1 h;C1:干露3 h,C2:干露3 h恢复3 h;D1:干露6 h;D2:干露6 h恢复3 h;D3:干露6 h恢复24 h A: Control; B1: Dehydration for 1 h; B2: Dehydration for 1 h followed by rehydration for 1 h; C1: Dehydration for 3 h; C2: Dehydration for 3 h followed by rehydration for 3 h; D1: Dehydration for 6 h; D2: Dehydration for 6 h followed by rehydration for 3 h; D3: Dehydration for 6 h followed by rehydration for 24 h |
|
图 4 干露处理及恢复不同时间后鼠尾藻的Fv/Fm Fig.4 Fv/Fm of S. thunbergii after dehydration and rehydration A:干露0 h (蓝色);B:干露1 h,B1:顶部和叶片(荧绿色),B2:基部(蓝色);C:干露1 h恢复1 h,C1:顶部和叶片(蓝绿色),C2:基部和柄(蓝色);D:干露3 h,D1:梢部(橙黄色),D2:中部(绿色),D3:基部(蓝色);E:干露3 h恢复3 h,E1:边缘(蓝绿色),E2:基部(蓝色);F:干露6 h,F1:梢部(橙红色),F2:中部(黄绿色),F3:中部(绿色),F4:基部(蓝绿色),F5:基部(蓝色);G:干露6 h恢复3 h,G1:梢部(橙黄色),G2:中部(蓝绿色),G3:基部(蓝色);H:干露6 h恢复24 h,H1:中部(浅蓝绿色),H2:基部(蓝色)横坐标中不同颜色指的是图 3干露处理及恢复后鼠尾藻的光合生理状态所呈现的颜色,下同。 A: Control (blue); B: Dehydration for 1h, B1: Top and leaf (fluorescent green), B2: Base (blue); C: Dehydration for 1 h followed by rehydration for 1 h, C1: Top and leaf (blue-green), C2: Base and handle (blue); D: Dehydration for 3 h, D1: Slightly (orange-yellow), D2: Central (green), D3: Base (blue); E: Dehydration for 3 h followed by rehydration for 3 h, E1: Edge (blue-green), E2: Base (blue); F: Dehydration for 6 h, F1: Slightly (orange red), F2: Central (yellow-green), F3: Central (green), F4: Base (blue-green), F5: Base (blue); G: Dehydration for 6 h followed by rehydration for 3 h, G1: Slightly (orange-yellow), G2: Central (blue-green), G3: Base (blue); H: Dehydration for 6 h followed by rehydration for 24 h, H1: Middle (light blue-green), H2: Base (blue). The different colors in the abscissa refer to the colors of the photosynthetic physiological state of S. thunbergii after dehydration and rehydration in Fig.3, the same as below. |
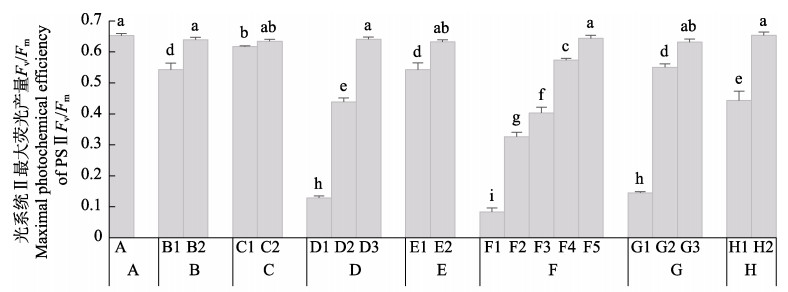
由图 5可知,干露胁迫对鼠尾藻的叶绿素荧光参数有显著影响。干露胁迫后,鼠尾藻藻体的Y(NPQ)显著降低,恢复正常条件培养后逐渐升高。胁迫后藻体的Y(NO)值显著升高,恢复培养后数值降低。胁迫后藻体的实际量子产率Y(Ⅱ)降低,恢复培养后有所增高。结果表明,干露胁迫时,鼠尾藻以非调节性能量耗散机制为主,主动光保护能力降低,说明干露胁迫会降低鼠尾藻对过剩光强的适应能力。
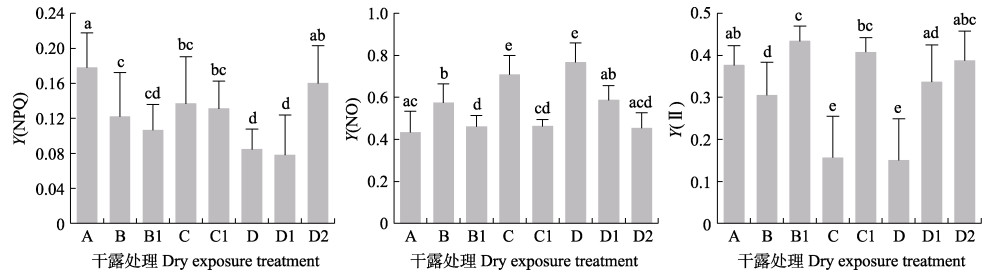
|
图 5 干露处理及恢复后鼠尾藻的Y(NPQ)、Y(NO)和Y(Ⅱ) Fig.5 Y(NPQ), Y(NO) and Y(Ⅱ) of S. thunbergii after dehydration and rehydration A:干露0 h;B:干露1 h,B1:干露1 h恢复1 h;C:干露3 h,C1:干露3 h恢复3 h;D:干露6 h恢复3 h,D1:干露6 h恢复3 h,D2:干露6 h恢复24 h A: Control; B: Dehydration for 1 h, B1: Dehydration for 1 h followed by rehydration for 1 h; C: Dehydration for 3 h, C1: Dehydration for 3 h followed by rehydration for 3 h; D: Dehydration for 6 h, D1: Dehydration for 6 h followed by rehydration for 3 h, D2: Dehydration for 6 h followed by rehydration for 24 h |
由图 6可知,干露胁迫对鼠尾藻不同部位藻体的饱和光强(Isat)影响显著。干露1 h后,鼠尾藻的Isat与对照组相比无显著变化;干露3 h后,鼠尾藻的梢部和中部Isat显著降低,对基部影响较小,3 h后可恢复;干露6 h后,鼠尾藻的Isat显著低于对照组,干露对小藻体伤害较大,不能恢复正常,大藻体可恢复正常。
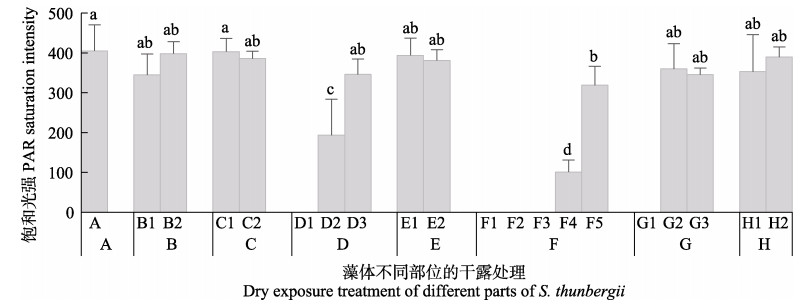
|
图 6 不同干露时间对鼠尾藻饱和光强(Isat)的影响 Fig.6 Effect of dehydration on the saturated light intensity of S. thunbergii A:干露0 h (蓝色);B:干露1 h,B1:顶部和叶片(荧绿色),B2:基部(蓝色);C:干露1 h恢复1 h,C1:顶部和叶片(蓝绿色),C2:基部和柄(蓝色);D:干露3 h,D1:梢部(橙黄色),D2:中部(绿色),D3:基部(蓝色);E:干露3 h恢复3 h,E1:边缘(蓝绿色),E2:基部(蓝色);F:干露6 h,F1:梢部(橙红色),F2:中部(黄绿色),F3:中部(绿色),F4:基部(蓝绿色),F5:基部(蓝色);G:干露6 h恢复3 h,G1:梢部(橙黄色),G2:中部(蓝绿色),G3:基部(蓝色);H:干露6 h恢复24 h,H1:中部(浅蓝绿色),H2:基部(蓝色) A: Control (blue); B: Dehydration for 1h, B1: Top and leaf (fluorescent green), B2: Base (blue); C: Dehydration for 1 h followed by rehydration for 1 h, C1: Top and leaf (blue-green), C2: Base and handle (blue); D: Dehydration for 3 h, D1: Slightly (orange-yellow), D2: Central (green), D3: Base (blue); E: Dehydration for 3 h followed by rehydration for 3 h, E1: Edge (blue-green), E2: Base (blue); F: Dehydration for 6 h, F1: Slightly (orange red), F2: Central (yellow-green), F3: Central (green), F4: Base (blue-green), F5: Base (blue); G: Dehydration for 6 h followed by rehydration for 3 h, G1: Slightly (orange-yellow), G2: Central (blue-green), G3: Base (blue); H: Dehydration for 6 h followed by rehydration for 24 h, H1: Middle (light blue-green), H2: Base (blue) |
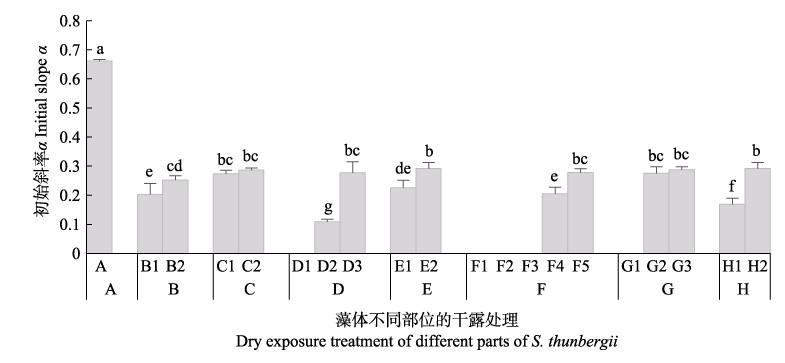
由图 7可知,干露胁迫对鼠尾藻快速光曲线的初始斜率α有显著影响。干露胁迫后,鼠尾藻藻体各部分的快速光曲线初始斜率α均显著降低,且在海水中恢复培养后不能恢复到正常水平。结果表明,干露胁迫后鼠尾藻对光能的利用效率显著降低。
|
图 7 不同干露时间对鼠尾藻初始斜率α的影响 Fig.7 Effect of dehydration on the initial slope α of S. thunbergii A:干露0 h (蓝色);B:干露1 h,B1:顶部和叶片(荧绿色),B2:基部(蓝色);C:干露1 h恢复1 h,C1:顶部和叶片(蓝绿色),C2:基部和柄(蓝色);D:干露3 h,D1:梢部(橙黄色),D2:中部(绿色),D3:基部(蓝色);E:干露3 h恢复3 h,E1:边缘(蓝绿色),E2:基部(蓝色);F:干露6 h,F1:梢部(橙红色),F2:中部(黄绿色),F3:中部(绿色),F4:基部(蓝绿色),F5:基部(蓝色);G:干露6 h恢复3 h,G1:梢部(橙黄色),G2:中部(蓝绿色),G3:基部(蓝色);H:干露6 h恢复24 h,H1:中部(浅蓝绿色),H2:基部(蓝色) A: Control (blue); B: Dehydration for 1h, B1: Top and leaf (fluorescent green), B2: Base (blue); C: Dehydration for 1 h followed by rehydration for 1 h, C1: Top and leaf (blue-green), C2: Base and handle (blue); D: Dehydration for 3 h, D1: Slightly (orange-yellow), D2: Central (green), D3: Base (blue); E: Dehydration for 3 h followed by rehydration for 3 h, E1: Edge (blue-green), E2: Base (blue); F: Dehydration for 6 h, F1: Slightly (orange red), F2: Central (yellow-green), F3: Central (green), F4: Base (blue-green), F5: Base (blue); G: Dehydration for 6 h followed by rehydration for 3 h, G1: Slightly (orange-yellow), G2: Central (blue-green), G3: Base (blue); H: Dehydration for 6 h followed by rehydration for 24 h, H1: Middle (light blue-green), H2: Base (blue) |
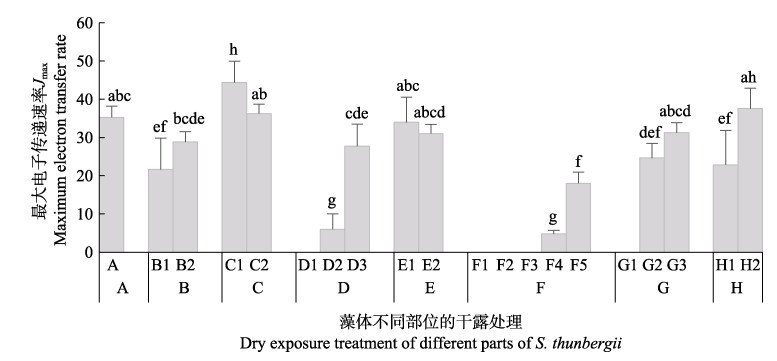
由图 8可知,干露胁迫对鼠尾藻藻体不同部位的光系统最大电子传递速率(Jmax)影响显著。干露1 h后,鼠尾藻的Jmax降低,恢复1 h后可恢复正常。干露3 h后,鼠尾藻的Jmax显著降低,恢复培养3 h后,可恢复正常。干露6 h时,鼠尾藻的Jmax显著低于对照组,干露对小藻体和大藻体的基部、梢部伤害较大,不能恢复正常,而基部可恢复正常。
|
图 8 不同干露时间对鼠尾藻最大电子传递速率(Jmax)的影响 Fig.8 Effect of dehydration on the maximum electron transfer rate of S. thunbergii A:干露0 h (蓝色);B:干露1 h,B1:顶部和叶片(荧绿色),B2:基部(蓝色);C:干露1 h恢复1 h,C1:顶部和叶片(蓝绿色),C2:基部和柄(蓝色);D:干露3 h,D1:梢部(橙黄色),D2:中部(绿色),D3:基部(蓝色);E:干露3 h恢复3 h,E1:边缘(蓝绿色),E2:基部(蓝色);F:干露6 h,F1:梢部(橙红色),F2:中部(黄绿色),F3:中部(绿色),F4:基部(蓝绿色),F5:基部(蓝色);G:干露6 h恢复3 h,G1:梢部(橙黄色),G2:中部(蓝绿色),G3:基部(蓝色);H:干露6 h恢复24 h,H1:中部(浅蓝绿色),H2:基部(蓝色)A: Control (blue); B: dehydration for 1h, B1: Top and leaf (fluorescent green), B2: Base (blue); C: dehydration for 1 h followed by rehydration for 1 h, C1: Top and leaf (blue-green), C2: Base and handle (blue), D: dehydration for 3 h, D1: Slightly (orange-yellow), D2: Central (green), D3: Base (blue); E: Dehydration for 3 h followed by rehydration for 3 h, E1: Edge (blue-green), E2: Base (blue), F: Dehydration for 6 h, F1: Slightly (orange red), F2: Central (yellow-green), F3: Central (green), F4: Base (blue-green), F5: Base (blue); G: Dehydration for 6 h followed by rehydration for 3 h, G1: Slightly (orange-yellow), G2: Central (blue-green), G3: Base (blue); H: Dehydration for 6 h followed by rehydration for 24 h, H1: Middle (light blue-green), H2: Base (blue) |
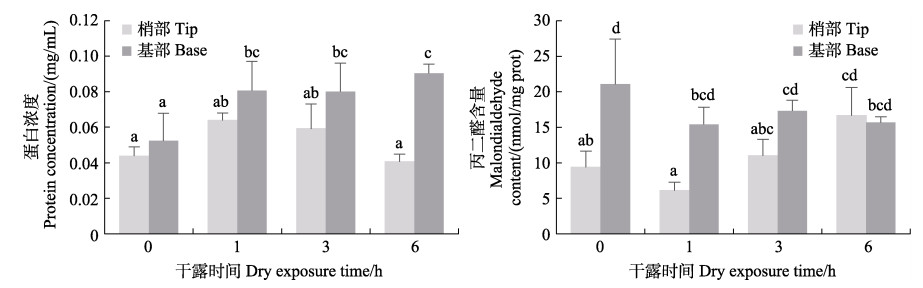
从图 9可以看出,干露处理显著影响鼠尾藻的蛋白浓度和MDA含量。干露处理时,鼠尾藻藻体梢部的蛋白含量先增高后降低,但各组间无显著性差异,藻体基部蛋白含量随着干露时间的增加逐渐增高,且均显著高于无胁迫组。藻体梢部干露处理6 h的MDA含量显著高于其他处理组,0 h时藻体基部的MDA含量显著高于其他干露组,其他组间无显著性差异。
|
图 9 鼠尾藻在不同干露时间下的蛋白浓度和MDA含量 Fig.9 Protein concentration and malondialdehyde content of S. thunbergii under dehydration durations |
从图 10可以看出,干露处理不同时间对藻体的叶绿素a和可溶性糖含量无显著影响。随着干露时间的增加,藻体梢部叶绿素a含量呈逐渐升高的趋势,基部的叶绿素a含量无显著变化,但藻体基部叶绿素a含量均高于藻体梢部。随干露时间的增加,藻体梢部和基部的可溶性糖含量呈逐渐升高的趋势,且藻体基部可溶性糖含量均高于藻体梢部。

|
图 10 鼠尾藻在不同干露时间下的叶绿素a和可溶性糖含量 Fig.10 Chlorophyll a and soluble sugar content of S. thunbergii under dehydration durations |
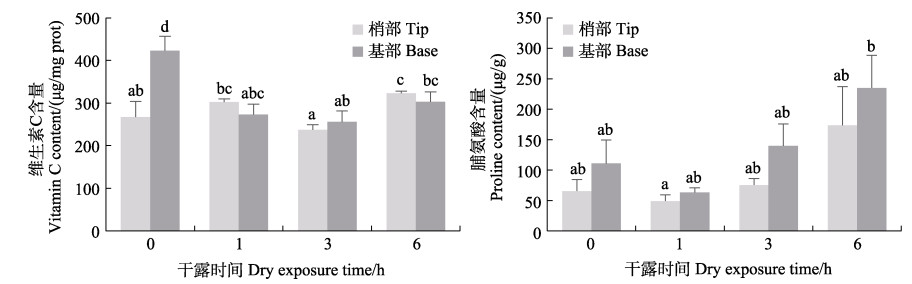
从图 11可以看出,干露处理显著影响鼠尾藻的维生素C和脯氨酸含量。干露处理时,藻体梢部的维生素C含量在6 h时最高,藻体基部在0 h时最高,显著高于其他处理组。藻体梢部和基部的脯氨酸含量随干露时间的增加呈逐渐增高的趋势,干露胁迫6 h后,基部含量增加到1 h处理组的约5倍,梢部含量比干露1 h时增加3倍多,且在实验时间内,基部脯氨酸含量均高于梢部,结合基部的耐干露胁迫能力更强,这可能说明脯氨酸在鼠尾藻耐受干露胁迫过程中具有重要作用。
|
图 11 鼠尾藻在不同干露时间下的维生素C和脯氨酸含量 Fig.11 Vitamin C and proline content of S. thunbergii under dehydration durations |
从图 12可以看出,干露处理对鼠尾藻CAT和ASAFR比活力影响较小。藻体基部的CAT比活力在0 h时最高,显著高于其他处理组,各处理组梢部CAT比活力无显著变化。藻体梢部的ASAFR活性随干露时间的增加呈增高的趋势,干露6 h后显著高于其他处理组,藻体基部ASAFR在1 h时最低,显著低于3 h处理组,其他组间无显著性差异。
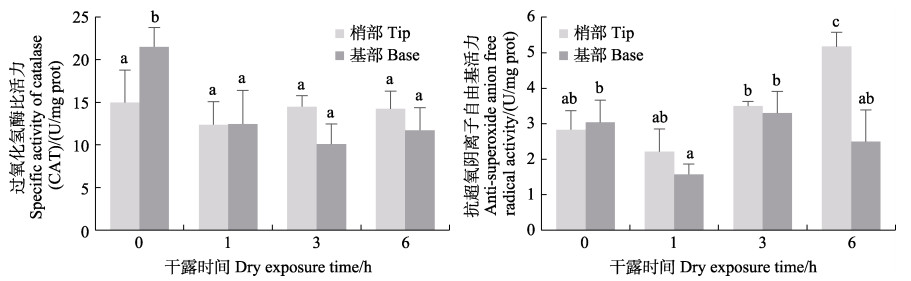
|
图 12 鼠尾藻在不同干露时间下的CAT比活力和抗超氧阴离子自由基(ASAFR)活性 Fig.12 CAT specific activity and anti-superoxide anion free radical (ASAFR) activity under dehydration durations of S. thunbergii |
从图 13可以看出,干露处理不同时间对藻体的POD比活力无显著影响,藻体梢部的POD比活力高于基部。干露处理显著影响藻体的SOD比活力,藻体梢部SOD比活力随干露时间的增加呈逐渐增高的趋势,干露6 h后显著高于其他处理组,藻体基部SOD比活力在1 h时最低,显著低于0 h和3 h处理组,其他组间无显著性差异。

|
图 13 鼠尾藻在不同干露条件下的过氧化物酶(POD)和超氧化物歧化酶(SOD)比活力 Fig.13 Specific activities of peroxidase (POD) and superoxide dismutase (SOD) in S. thunbergii under different dehydration conditions |
随着潮汐作用,潮间带海藻生长于2种不同环境中:低潮时暴露于空气中,高潮时沉没于海水中。在这2种环境条件下,海藻光合能力和作用机理不同。沉没于海水时,海藻利用水体中的溶解性无机碳进行光合作用;在干露状态下,主要利用空气中的CO2进行光合作用(邹定辉等, 2001)。鼠尾藻是分布在潮间带及潮下带礁石上的一种重要的经济海藻,伴随着淹没和露空2种环境动态交替出现(Helmuth et al, 2002; Martínez et al, 2012),不同潮间带的海藻对环境胁迫的耐受能力和恢复能力不同(Davison et al, 1996; Zardi et al, 2011; Colvard et al, 2014)。生长环境的不同会引起鼠尾藻生态结构和生化组分的差异,低潮时的高温、强光和失水是对潮间带固着型的鼠尾藻形成严重环境胁迫的主要因子,会引起鼠尾藻出现显著的生理学变化。
本研究发现,鼠尾藻耐干露的能力与干露时间和藻体大小显著相关。干露1 h时,大、小藻体失水率约为16%,无显著性差异,此时,水分的减少多为藻体叶片外部的水分蒸发。鼠尾藻多于潮间带岩礁固着生长,幼期鳞片状小叶密密的排列在主干上,初生分枝的幼期也覆盖螺旋状重叠的鳞片叶,其后,次生分枝自鳞片叶腋间生出。低潮时,密集排列的鳞状叶片减少了暴露于空气中的面积,同时叶片间隙可储存少量的海水,短时间的干露胁迫仅使藻体表面水分蒸发,对藻体本身的伤害相对较小。随着藻体干露时间逐渐增加,在干露6 h时,小藻体的失水率达到了89%,而相对较大藻体的失水率为65%,随着藻体质量的增加,失水率显著降低,即随着胁迫时间的延长,大藻体鼠尾藻的保水能力高于小藻体。
光合能力的强弱与光合器官PSⅡ活性及电子传递有关,叶绿素荧光参数是反映光合生理状态的重要参数。经过充足暗适应后,测出的Fv/Fm反映了当时所有的PSⅡ反应中心均处于开放态时的量子产量,即植物的潜在最大光合能力,是衡量植物光能转换效率的指标(张守仁, 1999)。随着鼠尾藻失水程度的加大,Fv/Fm显著降低,推测是失水抑制了光合链的电子传递和相关酶的活性。由于植物组织分化的空间差异性,经同一种胁迫条件产生的伤害在同一藻体不同部位也可能是不同的;随着干露胁迫时间增加,同一藻体不同部位呈现不同的荧光值,叶缘荧光值下降,而叶片中部仍呈现规律性的光合活性。随着干露时间增加,胁迫程度增强,鼠尾藻藻体的Y(Ⅱ)、Y(NPQ)、初始斜率α、Isat和Jmax均逐渐降低,Y(NO)值逐渐升高,说明水分缺失使藻体的光能转换效率和光保护能力显著降低,光合损伤程度较高。胁迫下的藻体由调节性的光保护机制转换到非调节性能量耗散机制,恢复适宜条件培养后,荧光值上调,藻体可恢复调节性光保护机制。这些结果表明,干露胁迫会降低鼠尾藻对光照的适应性,自然界中,干露和强光的协同作用可能对鼠尾藻造成严重胁迫。另外,本研究还发现,鼠尾藻不同部位对干露胁迫的响应不同,梢部的光合生理状态更容易受到干露胁迫的影响。
抗氧化系统包括还原物质和抗氧化酶类,在经历失水和复水的过程中,海藻体内不同氧化还原物质的含量与活性的变化也不尽相同。植物体内可溶性蛋白质大多是酶类,参与细胞的各种代谢活动,其含量是反映植物体总代谢的一个重要指标(詹冬梅等, 2013)。干露胁迫时,鼠尾藻藻体边缘和中部表现出不同的蛋白含量变化。轻度胁迫(即1~3 h)时,梢部蛋白浓度增高,可能是藻体内的应激蛋白发挥抵抗胁迫作用,随着胁迫强度增大,超出鼠尾藻的主动调节范围,导致蛋白浓度降低,可能失去了活性。藻体基部叶片生长状态较好,承受干露胁迫的能力也较大,干露胁迫1~6 h时,蛋白浓度呈升高的趋势,可能是藻体内的应激蛋白一直在发挥作用。
植物体内活性氧(ROS)的积累会导致氧化胁迫,MDA是膜脂过氧化的主要产物之一,常被作为判断膜脂过氧化的一种主要指标(杜秀敏等, 2001)。本研究发现,随着胁迫时间延长,梢部的MDA含量不断积累、上升,而基部的MDA含量无显著性变化,说明,梢部遭受ROS的氧化作用更严重。藻体内抗氧化酶系统如ASAFR、CAT、SOD和POD等对于清除ROS至关重要。干露胁迫下鼠尾藻梢部的ASAFR、SOD活性显著升高,积极消除ROS的毒害作用,重建氧化还原平衡以维持生理代谢活动,而基部的抗氧化酶类在干露胁迫下活性变化较小,这说明梢部通过调用抗氧化酶系统来清除ROS,而基部可能通过非抗氧化酶类来调控ROS。对脯氨酸含量变化的研究结果验证了该推测。如图 10所示,鼠尾藻藻体梢部和基部的脯氨酸含量随干露时间的增加呈逐渐增高的趋势,且在实验时间内基部脯氨酸含量均高于梢部。脯氨酸是水溶性最大的氨基酸,脯氨酸的积累是藻类及高等动植物在胁迫下常见的生理反应,其可行使渗透调节、抗氧化和分子伴侣等生理功能(Delauney et al, 1993; Szabados et al, 2010)。鼠尾藻在干露胁迫时,可溶性糖和脯氨酸含量显著增加,在耐受能力弱的梢部和抗氧化酶系统共同发挥作用抵抗胁迫,在耐受能力较好的藻体基部主要是上调脯氨酸等抗氧化物质含量抵抗胁迫,保持藻体活性。糖是参与调节渗透胁迫的小分子物质,在植物对水分胁迫的适应性调节中是增加渗透性溶质的重要组成成分。本研究表明,干露胁迫条件下,鼠尾藻的可溶性糖含量上升。这些可溶性糖一部分作为渗透调节物质起作用,另一部分为新蛋白质的合成提供了碳架,还有一些可间接转化为脯氨酸,这样又促进了植物中脯氨酸含量的增加(史玉炜等, 2007)。
BAO J, TIAN X L, DONG S L, et al. Effect of temperature, salinity and light intensity on nitrogen and phosphorus uptake by Sargassum thunbergii. Journal of Fishery Sciences of China, 2008, 15(2): 293-300 [包杰, 田相利, 董双林, 等. 温度、盐度和光照强度对鼠尾藻氮、磷吸收的影响. 中国水产科学, 2008, 15(2): 293-300 DOI:10.3321/j.issn:1005-8737.2008.02.013] |
CHOUHAN A, IQBAL S, MAHESHWARI R S, et al. Study of effect of air pollution on photosynthetic pigment of some plants growing in Pithampur industrial area, Dhar (M. P.). International Journal of Current Scientific Research, 2011, 1(4): 187-190 |
CHU S H, ZHANG Q S, LIU S K, et al. Tolerance of Sargassum thunbergii germlings to thermal, osmotic and desiccation stress. Aquatic Botany, 2012a, 96(1): 1-6 DOI:10.1016/j.aquabot.2011.09.002 |
CHU S H, ZHANG Q S, YONG Z T, et al. High tolerance to fluctuating salinity allows Sargassum thunbergii germlings to survive and grow in artificial habitat of full immersion in intertidal zone. Journal of Experimental Marine Biology and Ecology, 2012b, 412: 66-71 DOI:10.1016/j.jembe.2011.10.025 |
COLVARD N B, CARRINGTON E, HELMUTH B. Temperature- dependent photosynthesis in the intertidal alga Fucus gardneri and sensitivity to ongoing climate change. Journal of Experimental Marine Biology and Ecology, 2014, 458: 6-12 DOI:10.1016/j.jembe.2014.05.001 |
CUI D L, DENG Y B, MA Y X, et al. Study on heavy metal lead ion adsorption by Sargassum thunbergii. Marine Environmental Science, 2013, 32(6): 893-895 [崔大练, 邓一兵, 马玉心, 等. 鼠尾藻吸附水体重金属铅离子的研究. 海洋环境科学, 2013, 32(6): 893-895] |
DAVISON I R, PEARSON G A. Stress tolerance in intertidal seaweeds. Journal of Phycology, 1996, 32(2): 197-211 DOI:10.1111/j.0022-3646.1996.00197.x |
DELAUNEY A J, HU C A, KISHOR P B, et al. Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. Journal of Biological Chemistry, 1993, 268(25): 18673-18678 DOI:10.1016/S0021-9258(17)46682-8 |
DU X M, YIN W X, ZHAO Y X, et al. The production and scavenging of reactive oxygen species in plants. Chinese Journal of Biotechnology, 2001, 17(2): 121-125 [杜秀敏, 殷文璇, 赵彦修, 等. 植物中活性氧的产生及清除机制. 生物工程学报, 2001, 17(2): 121-125 DOI:10.3321/j.issn:1000-3061.2001.02.003] |
FAN C C. The biological adsorption effect research of rat tail algae on heavy metal lead, copper, zinc, cadmium in water. Master´s Thesis of Zhejiang Ocean University, 2013 [范彩彩. 鼠尾藻对水体重金属铅、铜、锌、镉的生物吸附效应研究. 浙江海洋学院硕士研究生学位论文, 2013]
|
GUO Q, WEI Y X, YIN B Z, et al. Study on the antimicrobial activity of different fractions of phlorotannins from Sargassum thunbergii kuntze. Progress in Fishery Sciences, 2010, 31(1): 117-121 [郭奇, 魏玉西, 殷邦忠, 等. 鼠尾藻多酚分级组分的抑菌活性研究. 渔业科学进展, 2010, 31(1): 117-121 DOI:10.3969/j.issn.1000-7075.2010.01.019] |
HAN X D, LI L P. Characteristics and utilization of Sargassum thunbergii. Special Economic Animals and Plants, 2005, 8(1): 27 [韩晓弟, 李岚萍. 鼠尾藻特征特性与利用. 特种经济动植物, 2005, 8(1): 27 DOI:10.3969/j.issn.1001-4713.2005.01.027] |
HE P, XU W D, WANG L M. Research status and development trend of Sargassum thunbergii. Journal of Shanghai Ocean University, 2011, 20(3): 363-367 [何平, 许伟定, 王丽梅. 鼠尾藻研究现状及发展趋势. 上海海洋大学学报, 2011, 20(3): 363-367] |
HELMUTH B, HARLEY C D G, HALPIN P M, et al. Climate change and latitudinal patterns of intertidal thermal stress. Science, 2002, 298(5595): 1015-1017 DOI:10.1126/science.1076814 |
LI J, WEI Y X, DU G C, et al. Anticoagulant activities of phlorotannins from Sargassum thunbeergii Kuntze. Traditional Chinese Drug Research and Clinical Pharmacology, 2007, 18(3): 191-194 [李敬, 魏玉西, 杜桂彩, 等. 鼠尾藻多酚的抗凝血活性研究. 中药新药与临床药理, 2007, 18(3): 191-194 DOI:10.3321/j.issn:1003-9783.2007.03.007] |
LIU F L, WANG F J, SUN X T, et al. Preliminary study on the response of gene expression to desiccation in Sargassum thunbergii. Journal of Fisheries of China, 2014, 38(2): 282-288 [刘福利, 王飞久, 孙修涛, 等. 鼠尾藻基因表达对干露胁迫响应的初步研究. 水产学报, 2014, 38(2): 282-288] |
MARTÍNEZ B, ARENAS F, RUBAL M, et al. Physical factors driving intertidal macroalgae distribution: Physiological stress of a dominant fucoid at its southern limit. Oecologia, 2012, 170(2): 341-353 DOI:10.1007/s00442-012-2324-x |
NAN G N, ZHANG Q S, SHENG Z T, et al. Coordination between xanthophyll cycle and antioxidant system in Sargassum thunbergii (Sargassaceae, Phaeophyta) in response to high light and dehydration stresses. Journal of Applied Phycology, 2016, 28(4): 2587-2596 DOI:10.1007/s10811-016-0791-x |
PARK P J, HEO S J, PARK E J, et al. Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. Journal of Agricultural and Food Chemistry, 2005, 53(17): 6666-6672 DOI:10.1021/jf050582+ |
SHENG Z T, NAN G N, ZHANG D, et al. Comparison of photosynthesis physiology among different Sargassum thunbergii populations in Shandong Peninsula. Journal of Yantai University (Natural Science and Engineering), 2017, 30(1): 17-25 [盛子彤, 南国宁, 张迪, 等. 山东半岛鼠尾藻不同地理种群的光合生理学比较. 烟台大学学报(自然科学与工程版), 2017, 30(1): 17-25] |
SHI Y W, WANG Y L, LI W B, et al. Effects of water stress on soluble protein, soluble sugar and proline content in Tamarix hispida. Journal of Xinjiang Agricultural University, 2007, 30(2): 5-8 [史玉炜, 王燕凌, 李文兵, 等. 水分胁迫对刚毛柽柳可溶性蛋白、可溶性糖和脯氨酸含量变化的影响. 新疆农业大学学报, 2007, 30(2): 5-8 DOI:10.3969/j.issn.1007-8614.2007.02.002] |
SZABADOS L, SAVOURÉ A. Proline: A multifunctional amino acid. Trends in Plant Science, 2010, 15(2): 89-97 DOI:10.1016/j.tplants.2009.11.009 |
TAKAHASHI S, BADGER M R. Photoprotection in plants: A new light on photosystem Ⅱ damage. Trends in Plant Science, 2011, 16(1): 53-60 |
TAN J, LI F H, CHEN S Q, et al. Effect of temperature and diet on the reproductive condition of sea cucumber Apostichopus japonicus broodstock. Progress in Fishery Sciences, 2020, 41(1): 96-103 [谭杰, 李凤辉, 陈四清, 等. 不同培育水温和饲料对刺参人工促熟效果的影响. 渔业科学进展, 2020, 41(1): 96-103] |
WANG J Q, SUI X N, GU G M, et al. Effects of combination of various feeds and feeding rates on growth and survival of juvenile sea cucumber Apostichopus japonicus. Fisheries Science, 2008, 27(2): 55-58 [王吉桥, 隋晓楠, 顾公明, 等. 不同饲料搭配及投喂量对仿刺参稚、幼参生长和成活的影响. 水产科学, 2008, 27(2): 55-58] |
WANG L M, LI S G, CHAI Y, et al. Indoor culture and sexual reproduction synchronization of Sargassum thunbergii young seedlings. Journal of Fisheries of China, 2011, 35(3): 395-404 [王丽梅, 李世国, 柴雨, 等. 鼠尾藻幼苗的室内培养及有性生殖同步化. 水产学报, 2011, 35(3): 395-404] |
WANG X Y, ZAN D M, LI M Z, et al. Preliminary studies on the nitrogen and phosphorus absorption capability of macroalgae. Progress in Fishery Sciences, 2011, 32(4): 67-71 [王翔宇, 詹冬梅, 李美真, 等. 大型海藻吸收氮磷营养盐能力的初步研究. 渔业科学进展, 2011, 32(4): 67-71] |
WU H Y, LIU H J, ZHAN D M, et al. Research present situation of Sargassum thunbergii. Territory and Natural Resources Study, 2010a(1): 95-96 [吴海一, 刘洪军, 詹冬梅, 等. 鼠尾藻研究与利用现状. 国土与自然资源研究, 2010a(1): 95-96] |
WU H Y, ZHAN D M, LIU H J, et al. Study on accumulation and degradation of heavy metals by the brown alga Sargassum thunbergii. Marine Sciences, 2010b, 34(1): 69-74 [吴海一, 詹冬梅, 刘洪军, 等. 鼠尾藻对重金属锌、镉富集及排放作用的研究. 海洋科学, 2010b, 34(1): 69-74] |
YANG C H. A Study on heavy metal concentration and adsorption by macroalgae in south coastal area of Zhejiang Province. Master´s Thesis of Wenzhou Medical College, 2012 [杨承虎. 浙南海区大型海藻富集和吸附重金属研究. 温州医学院硕士研究生学位论文, 2012]
|
YE Z P, SUGGETT D J, ROBAKOWSKI P, et al. A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of photosystem Ⅱ in C3 and C4 species. New Phytologist, 2013, 199(1): 110-120 |
YU Y Q, ZHANG Q S, TANG Y Z, et al. Diurnal changes of photosynthetic quantum yield in the intertidal macroalga Sargassum thunbergii under simulated tidal emersion conditions. Journal of Sea Research, 2013, 80(l): 50-57 |
ZARDI G I, NICASTRO K R, CANOVAS F, et al. Adaptive traits are maintained on steep selective gradients despite gene flow and hybridization in the intertidal zone. PLoS One, 2011, 6(6): e19402 |
ZENG C K . Seaweed in the Yellow Sea and Bohai Seas of China. Beijing: Science Press, 2009: 395 [曾呈奎. 中国黄渤海海藻. 北京: 科学出版社, 2009: 395]
|
ZHAN D M, WU H Y, LIU M X, et al. Impact of irradiances on growth and biochemical composition of Sargassum muticum. Progress in Fishery Sciences, 2013, 34(1): 140-144 [詹冬梅, 吴海一, 刘梦侠, 等. 光照强度对海黍子生长及部分生化指标的影响. 渔业科学进展, 2013, 34(1): 140-144] |
ZHANG S R. A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chinese Bulletin of Botany, 1999, 16(4): 444-448 [张守仁. 叶绿素荧光动力学参数的意义及讨论. 植物学通报, 1999, 16(4): 444-448] |
ZHOU W, ZHANG H J, LI Z D, et al. Effects of dietary protein levels on growth in juvenile sea cucumber Apostichopus japonicus. Journal of Dalian Ocean University, 2010, 25(4): 359-364 [周玮, 张慧君, 李赞东, 等. 不同饲料蛋白水平对仿刺参生长的影响. 大连海洋大学学报, 2010, 25(4): 359-364] |
ZOU D H, GAO K S. Progress in studies on photosynthetic inorganic carbon utilization in marine macroalgae. Marine Science Bulletin, 2001, 20(5): 83-90 [邹定辉, 高坤山. 大型海藻类光合无机碳利用研究进展. 海洋通报, 2001, 20(5): 83-90] |



